Abstract
The continuous infusion of the opioid peptide β-endorphin prolongs skin allograft survival in mice, while the opiate receptor antagonist naloxone, administered together with the opioid at the time of transplantation, abolishes the effect of the opioid. Consistently, naloxone, when given alone at the time of transplantation, but not later, accelerates graft rejection and increases splenocyte IL-2 and interferon-gamma (IFN-γ) production. Splenocyte β-endorphin concentrations are lower in transplanted animals. The effects of exogenous β-endorphin and naloxone suggest that the endogenous opioid peptide β-endorphin exerts a tonic inhibitory effect over early events of T cell-mediated immune responses in vivo. The effects of β-endorphin and naloxone are consistent with the previously shown role of the opioid system in the modulation of the Th1/Th2 cytokine pattern.
Keywords: β-endorphin, naloxone, allograft, rejection, cytokines, opioids
INTRODUCTION
β-endorphin (BE) is a 31 amino acid opioid peptide known in the last 20 years for its action in the central nervous system (CNS). It is produced as a larger precursor molecule, proopiomelanocortin (POMC). The POMC precursor molecule is post-translationally processed by a series of enzymatic events, which give rise to a number of peptide hormones, e.g. ACTH, βLPH, αMSH and BE. In the CNS, BE is synthesized mainly in the arcuate nucleus of the hypothalamus, and projects in several brain areas. In the periphery the main source of BE is the intermediate pituitary or its vestige [1].
More recently, it has been demonstrated by a number of different laboratories that also the cells of the immune system can express the POMC gene, and synthesize and secrete many POMC-derived peptides such as BE and ACTH [2–4]. BE was first identified in lymphocytes or splenocytes infected by the Newcastle virus or stimulated with lipopolysaccharide (LPS), but it soon became evident that it was a constitutive element of several cells of the immune system [5]; both human and rodent T lymphocytes, B lymphocytes and monocytes are able upon appropriate stimulation to produce, and release, BE [4–6].
It was also shown that BE concentrations in peripheral blood mononuclear cells (PBMC) are under the control of neuropeptides, e.g. CRH, and neurotransmitters, e.g. dopamine, GABA, serotonin, and its modulation is similar to that of CNS opioid [7].
Besides producing BE, immunocytes express on their surface functional opioid receptors. Central opioid receptor binding sites, defined by pharmacological, biochemical, physiological and molecular techniques, are categorized into three predominant classes including μ, δ and κ [8]. Consistently, these same receptors have been identified on murine splenocytes as well on human mononuclear cells and on several cell lines [9,10]. BE binds mostly to μ-opioid receptors, and can therefore participate in the modulation of the immune response.
BE exerts a tonic inhibitory effect on immune responses [11,12], and its participation in the shift from the Th1 to the Th2 cytokine pattern has been hypothesized [13]. We previously showed that administration to the human or the experimental animal of the opiate receptor antagonists naloxone or naltrexone increases the proliferative response of T lymphocytes [11], IL-2 and interferon-gamma (IFN-γ) production, while it decreases the production of IL-4 [13]. BE exerts its tonic inhibitory effect at both central and peripheral sites of action, since the intracerebroventricular and the intravenous administration of an anti-BE antiserum, that does not cross the blood–brain barrier either way, is equally effective in increasing proliferative and natural killer (NK) activity of rat splenocytes and PBMC. Moreover, the effect is still present when the antiserum is administered to hypophysectomized rats, i.e. in the absence of the main peripheral source of the circulating opioid [12]. The latter observation suggests that BE synthesized by PBMC could play a major role in the tonic modulation of the immune system induced by the opioid.
Consistent with the data reported above, an increase of PBMC BE concentrations has been observed in conditions when immunosuppression and the Th2 cytokine pattern prevail, e.g. in cells obtained from HIV+ subjects, in a human cell line after infection with the virus, or after stress [14,15]. BE concentrations in PBMC decrease in Th1-related immune pathologies such as multiple sclerosis, rheumatoid arthritis, Crohn's disease [16–18], and in experimental models such as experimental allergic encephalomyelitis, Freund's adjuvant arthritis, and chicken thyroiditis [13].
Recently, the Th1/Th2 paradigm has been involved also in immune responses to organ transplantation. Under certain experimental conditions in fact, graft rejection has been associated with the presence of Th1 cytokines, while Th2 cytokines have been linked to graft survival [19,20]. In the light of all previous considerations, in the present study we investigate the effect of BE and the opioid receptor antagonist naloxone on the onset of allograft skin rejection in mice, and on the production of IL-2, IFN-γ and IL-4 during the development of the rejection. Moreover, we also measure the splenocyte BE concentrations of transplanted mice.
MATERIALS AND METHODS
Animals and surgical procedures
Female C57Bl/6N and C3H/HeN mice (Charles River, Calco, Italy), aged 7–8 weeks and 20–24 g body weight, were used as skin recipients and donors, respectively. The animals were kept for at least 1 week before each experiment in standard animal facilities at 20 ± 2°C with a 10:14 dark:light cycle, and dry pellets and water ad libitum. Each experimental group consisted of eight animals.
Skin grafting was carried out in accordance with the procedure described by Billingham & Medawar [21] slightly modified as previously described [22]. Each graft consisted of a disk 1 cm in diameter comprising the full thickness of the skin; recipient areas for grafts were prepared in the skin of the lateral thoracic wall of the host. Donor mice were killed by an excess pentobarbital, while the same anaesthetic at the dose of 60 mg/kg was used for surgery on recipients.
Protective bandages were removed on day 5 and grafts were controlled daily by an independent observer ignoring the type of treatment (water, naloxone or BE). The rejection was evaluated considering, for each mouse, the days when 50% (50% graft rejection) and thereafter > 95% (survival time) of the graft surface was necrosed. On the rejection day, or when indicated, animals were killed by cervical dislocation, and spleens removed for BE and cytokine analysis.
Drug treatments
A first study was directed to the evaluation of the effects of BE or naloxone on graft survival. In this study, all substances were diluted in sterile distilled water and administered as continuous infusion using Alzet mini osmotic pumps (Model 2002; Alza Corp., Palo Alto, CA), implanted subcutaneously at the time of transplantation. Pumps were filled with Camel BE (Sigma, St Louis, MO) solution (10 μm) so that every mouse received daily an amount of BE equal to 140 μg/kg (i.e. 3.36 μg/mouse per day), continuously until the day of sacrifice. The volume of the pumps allowed for a continuous release up to 15 days. Camel BE was used since it presents an homology with mouse BE higher than human BE, and it is commonly used for physiological studies in the rodent. Naloxone (SALARS, Como, Italy) was released continuously from Alzet mini pumps at the dose of 5 mg/kg (0.5 mg/mouse per day). When indicated, BE and naloxone were loaded together in the same pump. Two naloxone alone groups were set up: one receiving the pump at the time of grafting, another receiving the pump on day 5 after surgery. Control mice were given one pump filled with sterile water. In these groups of animals, the days when both 50% and > 95% of graft surface were necrosed were evaluated.
A second study was directed to the evaluation of the role of endogenous opioids in the modulation of cytokine patterns at different times from grafting. In this set of experiments, one group of animals received a single s.c. injection of naloxone (5 mg/kg) at the moment of skin grafting, and a second group of animals was injected twice daily from the day of transplantation until the day of rejection. In these experiments the day of rejection refers to the day when 50% of the graft surface was necrosed (see previous section).
As control, a group of untreated transplanted animals was killed before the onset of rejection, i.e. 6 days after surgery, corresponding to the mean time of rejection in naloxone-treated animals. A group of control animals, without treatment and/or surgery, was also included as naive controls.
Splenocyte cultures and measurement of IL-2, IL-4 and IFN-γ
Animals were killed by decapitation and spleens were aseptically removed. Cells were teased from the spleens using 20 G sterile needles through an incision made in the spleen cuticle [6], centrifuged and washed twice in Hanks' balanced salt solution (HBSS). Cells were suspended in RPMI supplemented with 10% fetal calf serum (FCS), 1% glutamine, 2% antibiotics, 10 mm HEPES and 50 μm 2-mercaptoethanol (2-ME) (all from Sigma), and plated at 4 × 106 cells in 24-well plates, containing a final concentration of 2.5 μg concanavalin A (Con A) in a total volume of 1 ml. Plates were incubated at 37°C in 5% CO2 and 95% air. Supernatants were collected after 24 h and 48 h in culture and stored frozen at −80°C for cytokine analysis.
The concentrations of IL-2 in 24-h supernatants were determined by the ELISA protocol standardized by Pharmingen (San Diego, CA). Briefly, the anti-IL-2 capture MoAb (1 μg/ml) was absorbed on a polystyrene 96-well plate and the IL-2 present in the sample was bound to the antibody-coated wells. The biotinylated anti-IL-2 detecting MoAb (0.5 μg/ml) was added to bind the IL-2 captured by the first antibody. After washing, avidin-peroxidase (Sigma) was added to the wells to detect the biotinylated detecting antibody and finally 2,2′-azino-bis (3-ethylbenthiazoline-6-sulphonic acid) (ABTS; Sigma) substrate was added and a coloured product was formed in proportion to the amount of IL-2 present in the sample, which was measured at optical density (OD) 405 nm.
IL-4 production was measured in 48-h supernatants using the ELISA protocol outlined above with MoAb anti-IL-4 at the same concentrations used for IL-2. IFN-γ was also evaluated in 48-h supernatants, with the same ELISA protocol except using anti-IFN-γ capture and detecting antibody at 2 μg/ml and 1 μg/ml, respectively (all MoAbs were from Pharmingen).
Measurement of splenocyte BE
Mice splenocytes were diluted to 10 × 106, and aprotinine (1000 kU/ml) was added to all samples in order to inhibit enzymatic degradation of the peptide during further processing. Pelleted cell samples were homogenized in 0.1 n acetic acid, sonicated, centrifuged at 10 000 g and supernatants frozen at −20°C for radioimmunoassay (RIA) [2,7].
BE concentrations were measured by RIA with procedures previously described and validated [2]. A specific antibody obtained in our laboratory against human BE was used. Sensitivity of the method is 10 pg/tube and intra-assay and interassay variation coefficients are 8% and 11%, respectively.
Statistical analysis
Statistical analysis of results was performed by the one way analysis of variance, followed by Bonferroni t-test for multiple comparisons.
RESULTS
Effect of drug treatments on graft survival
Figure 1 shows the days at which 50% of the graft surface was rejected in the different treatment groups. The administration of Camel BE significantly (P < 0.01) delayed rejection of skin grafts (control value 9.05 ± 0.4 days (mean ± s.e.m.)). In the same figure it is also shown that concomitant treatment with the opiate receptor antagonist naloxone completely blocked the effect of the opioid. Naloxone alone significantly (P < 0.01) accelerated graft rejection when administered starting at the time of grafting, while it was not effective when administered 5 days after surgery.
Fig. 1.
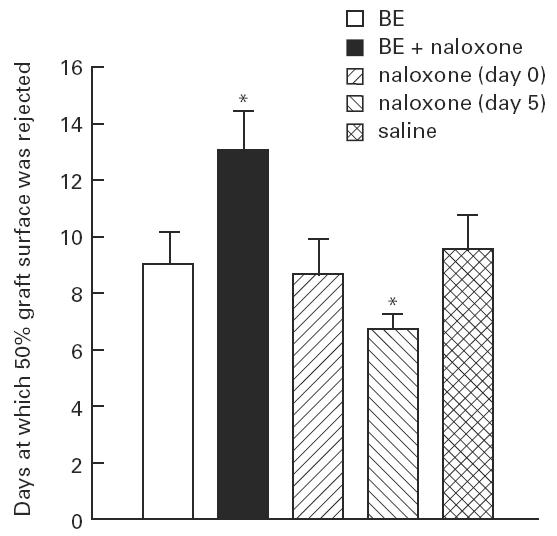
Effect of β-endorphin (BE) or naloxone treatment on skin graft rejection. Protective bandages were removed on day 5, and grafts were scored daily until 50% of the grafted surface was necrosed: this day was scored as rejection day. Results are the mean ± s.e.m. of groups of eight animals. *P < 0.01versus saline.
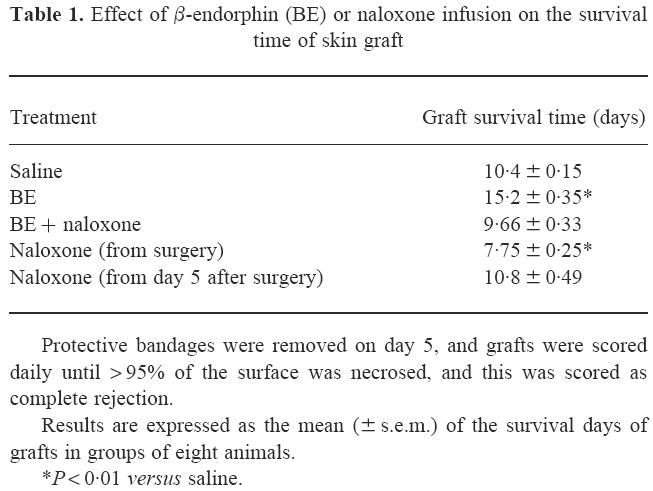
Similar results were obtained also considering the total survival time of the grafts, i.e. the day at which > 95% of the graft surface was necrosed. As reported in Table 1, in fact, the survival time was significantly longer (P < 0.01) than controls in BE-treated animals, while the continuous administration of naloxone significantly (P < 0.01) reduced it.
Table 1.
Effect of β-endorphin (BE) or naloxone infusion on the survival time of skin graft
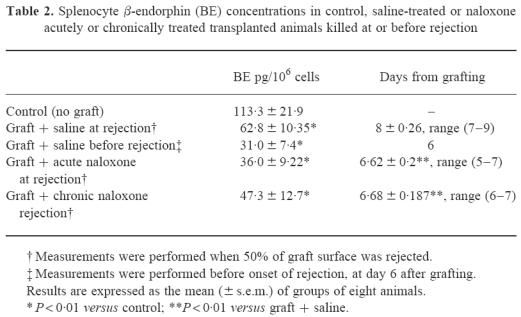
BE splenocyte concentrations
Table 2 shows BE concentrations measured in splenocytes from different experimental groups: naive non-transplanted mice; saline-treated transplanted mice killed when 50% of graft surface was rejected; saline-treated transplanted mice killed before the onset of rejection, i.e. 6 days after transplantation; chronically and acutely naloxone-treated animals killed the day when 50% of graft surface was rejected. In the same table the days after transplantation at which the animals were killed are also reported. Consistent with the results presented above, both acute and chronic naloxone treatments significantly accelerated graft rejection, evaluated as the day at which 50% of graft was necrosed. BE concentrations were significantly lower in all transplanted mice than in naive animals, independently of treatment with naloxone. However, although lower than in naive controls, BE concentrations in mice at the moment of rejection showed a trend towards higher concentrations than in transplanted mice before the onset of rejection.
Table 2.
Splenocyte β-endorphin (BE) concentrations in control, saline-treated or naloxone acutely or chronically treated transplanted animals killed at or before rejection
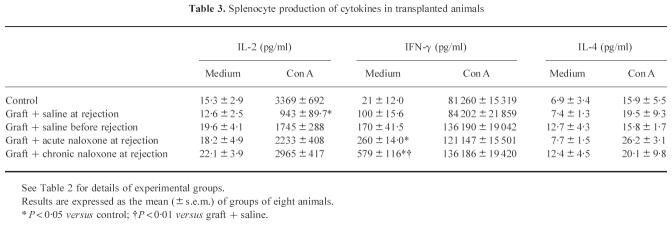
Cytokine concentrations
In Table 3 cytokine concentrations, assayed in resting and Con A-stimulated splenocyte cultures, are reported. The experimental groups and the days after grafting at which cytokines were evaluated are the same as in Table 2. Neither transplantation nor naloxone treatment affected IL-2 concentrations in unstimulated cultures. In contrast, a significant effect of rejection was observed in Con A-stimulated IL-2 production. IL-2 concentrations were lower in cultures of splenocytes obtained from mice at the moment of rejection, but not in mice killed before the rejection had begun, although a not significant trend towards a decrease was present (P = 0.055). A significant effect of both acute and chronic naloxone was present. In fact, in both acutely and chronically naloxone-treated animals, killed at the moment of rejection, IL-2 levels did not differ from naive controls, or from transplanted mice that had not yet started to reject the graft. IL-4 and IFN-γ were assayed in 48-h culture supernatant. IL-4 secretion was not affected by transplantation or naloxone treatment, but a trend to an increase of spontaneous IFN-γ production was present in transplanted animals in comparison with controls. Both acute and chronic naloxone treatments significantly enhanced spontaneous IFN-γ production. In contrast, no effect of either transplantation or naloxone treatment was present in Con A-stimulated cells.
Table 3.
Splenocyte production of cytokines in transplanted animals
DISCUSSION
Our data show that the opioid receptor antagonist naloxone accelerates the time of graft rejection, probably through the blockade of an immunosuppressive tone exerted by endogenous opioids. Consistently, we show that, in contrast to naloxone, BE delays rejection. These observations are consistent with previous data showing that the blockade of CNS or PBMC BE by the administration of a specific anti-BE antiserum or the administration of naloxone induces an activation of some immune responses [12], and that the physiological increase of BE concentrations, e.g. after stress, induces immunosuppression [15]. The data presented extend our previous demonstration of a tonic effect of BE on immune responses and focus this effect on early events of T lymphocyte activation. We show in fact that naloxone, removing the BE tone, accelerates graft rejection, but also that this effect is present only when the administration of the opiate receptor antagonist starts at the time of grafting, but not at a later time. The latter observation is consistent with the finding of Kavelaars et al. [23], who demonstrated that opioid peptides modulate the phosphorylation of the CD3 γ-chain of T cell antigen receptor function, i.e. the early activation of transduction pathways to the biological effect.
An interesting aspect to be discussed is the source of the BE that exerts the inhibitory effect. In our experimental conditions we were dealing with an effect most probably mediated by the circulating opioid. Circulating BE might derive either from the pituitary, or from a number of immune cells where it is synthesized and from which it is released [2–6]. The latter hypothesis is consistent with our previous observation of an autocrine/paracrine effect of BE, since the stimulating effect exerted by the anti-BE antiserum is present also in hypophysectomized rats, i.e. in animals in which the endocrine effect of the opioid has been excluded [12]. In our experimental setting, we have no clues whether naloxone blocks the CNS, the endocrine or the autocrine/paracrine effect of BE. However, we favour the hypothesis of a blockade of the local mechanism that we consider to prevail. In fact, the relative BE concentrations that can be reached in a paracrine/autocrine model are much higher than those achievable by the small amount of the pituitary-originated peptide that can reach PBMC, also considering the relatively short half life of BE in plasma, and that the PBMC-derived opioid amounts to approximately one third of the pituitary peptide.
In pathological conditions it can be expected that an increase of PBMC concentrations of BE could lead to an inhibition of the immune system, while its decrease could lead to immune activation [13]. Consistent with this hypothesis, BE concentrations are decreased in transplanted mice, and low BE concentrations could be permissive for the immune activation leading to rejection. In fact, by supplementing continuously exogenous BE, the rejection time is significantly delayed, while the blockade of the opioid system accelerates it.
The effects on graft rejection that we observe with naloxone or BE could be due to the role of the opioid in the Th1/Th2 balance [13]. We showed, in fact, that naloxone stimulates the production of IFN-γ and IL-2, while decreasing IL-4 production, thus indicating that BE could have a role in the modulation of Th1/Th2 cells (Sacerdote et al., submitted).
Our data on cytokine production are difficult to analyse. Although very attractive, in fact the possibility that graft rejection is sustained by a Th1 response has not been fully demonstrated. In our hands, IL-4 was not modified in transplanted animals. Spontaneous production of the Th1 cytokine IFN-γ is slightly enhanced in transplanted animals; the effect is lost in Con A-stimulated cells. Moreover, consistent with what we observed in normal healthy animals (Sacerdote et al., submitted), both acute and chronic naloxone treatment further increased spontaneous IFN-γ production, confirming stimulation of Th1 cells by the opioid antagonist. A significant decrease of IL-2 production in the culture supernatants is indeed present when the spleen is removed at the moment of rejection. These low IL-2 concentrations could indicate a very rapid and massive utilization of the cytokine by T lymphocytes. It is possible therefore that naloxone, by stimulating IL-2 production [13], leads to a shortening of the time necessary in order to reach the amount of IL-2 that is critical for the onset of rejection. On the whole, these data seem to confirm that the modulation of graft rejection by BE and naloxone could be due to their effects on cytokine production.
Finally, the role of BE gains new interest from the fact that it is known how to modulate pharmacologically the concentrations of BE in immune cells [7]. This opportunity could become an important therapeutic tool in order to potentiate or decrease the effect of the opioid in immune pathologies where the immune system is over- or under-activated.
References
- 1.Olson GA, Olson RD, Kastin AJ. Endogenous opiates. Peptides. 1995;17:1421–66. doi: 10.1016/s0196-9781(96)00225-2. [DOI] [PubMed] [Google Scholar]
- 2.Sacerdote P, Breda M, Barcellini W, et al. Age-related changes of beta-endorphin and cholecystokinin in human and rats mononuclear cells. Peptides. 1991;12:1353–6. doi: 10.1016/0196-9781(91)90219-f. [DOI] [PubMed] [Google Scholar]
- 3.Lyons P, Blalock JE. Proopiomelanocortin gene expression and protein processing in rat mononuclear leukocytes. J Neuroimmunol. 1997;78:47–56. doi: 10.1016/s0165-5728(97)00081-7. [DOI] [PubMed] [Google Scholar]
- 4.Buzzetti R, Mc Loughlin L, Lavender PM, et al. Expression of pro-opiomelanocortin gene and quantification of adrenocorticotropin hormone-like immunoreactivity in human normal peripheral cells and lymphoid and myeloid malignancies. J Clin Invest. 1997;83:47–56. doi: 10.1172/JCI113940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blalock JE. A molecular basis for bidirectional communication between the immune and neuroendocrine system. Physiol Rev. 1989;69:1–32. doi: 10.1152/physrev.1989.69.1.1. [DOI] [PubMed] [Google Scholar]
- 6.Manfredi B, Clementi E, Sacerdote P, et al. Age related changes in mitogen induced β-endorphin release from human peripheral blood mononuclear cells. Peptides. 1995;16:699–706. doi: 10.1016/0196-9781(95)00030-n. [DOI] [PubMed] [Google Scholar]
- 7.Sacerdote P, Rubboli F, Locatelli L, et al. Pharmacological modulation of neuropeptides in peripheral mononuclear cells. J Neuroimmunol. 1991;32:35–41. doi: 10.1016/0165-5728(91)90069-j. [DOI] [PubMed] [Google Scholar]
- 8.Petersen SJ, Robson LE, Kosterlitz HW. Classification of opioid receptors. Br Med Bull. 1983;39:31–36. doi: 10.1093/oxfordjournals.bmb.a071787. [DOI] [PubMed] [Google Scholar]
- 9.Gaveriaux C, Peluso J, Simonin F, et al. Identification of kappa and delta opioid receptors transcript in immune cells. FEBS Letters. 1995;369:272–6. doi: 10.1016/0014-5793(95)00766-3. [DOI] [PubMed] [Google Scholar]
- 10.Chuang TK, Killam KF, Jr, Chuang LF, et al. Mu opioid receptor gene expression in immune cells. Biochem Biophys Res Commun. 1995;216:922–30. doi: 10.1006/bbrc.1995.2709. [DOI] [PubMed] [Google Scholar]
- 11.Manfredi B, Sacerdote P, Bianchi M, et al. Evidence for an opioid inhibitory tone on T-cell proliferation. J Neuroimmunol. 1993;44:43–48. doi: 10.1016/0165-5728(93)90266-2. [DOI] [PubMed] [Google Scholar]
- 12.Panerai AE, Manfredi B, Granucci F, et al. The β-endorphin inhibition of mitogen-induced splenocyte proliferation is mediated by central and peripheral paracrine/autocrine effects of the opioid. J Neuroimmunol. 1995;58:71–76. doi: 10.1016/0165-5728(94)00189-u. [DOI] [PubMed] [Google Scholar]
- 13.Panerai AE, Sacerdote P. β-endorphin in the immune system: a role at last? Immunol Today. 1997;18:318–9. doi: 10.1016/s0167-5699(97)01045-1. [DOI] [PubMed] [Google Scholar]
- 14.Barcellini W, Sacerdote P, Borghi MO, et al. β-endorphin content in HIV-infected HuT78 cell line and in peripheral lymphocytes from HIV-positive subjects. Peptides. 1994;15:769–75. doi: 10.1016/0196-9781(94)90028-0. [DOI] [PubMed] [Google Scholar]
- 15.Sacerdote P, Manfredi B, Bianchi M, et al. Intermittent but not continuous inescapable footshock stress affects immune responses and immunocyte beta-endorphin concentrations in the rat. Brain Behav Immun. 1994;8:251–60. doi: 10.1006/brbi.1994.1023. [DOI] [PubMed] [Google Scholar]
- 16.Wiedermann CJ, Sacerdote P, Mur E, et al. Decreased immunoreactive beta-endorphin in mononuclear leukocytes from patients with rheumatic diseases. Clin Exp Immunol. 1992;87:178–82. doi: 10.1111/j.1365-2249.1992.tb02971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wiedermann CJ, Sacerdote P, Probst A, et al. Decreased β-endorphin content in peripheral blood mononuclear leukocytes from patients with Crohn's disease. Brain Behav Immun. 1994;8:261–9. doi: 10.1006/brbi.1994.1024. [DOI] [PubMed] [Google Scholar]
- 18.Caputo D, Ferrante P, Saresella M, et al. Decreased beta-endorphin levels in peripheral blood cells of multiple sclerosis patients. J Neuroimmunol. 1991;(Suppl. 1):184. [Google Scholar]
- 19.Nickerson P, Steurer W, Steiger J, et al. Cytokines and the Th1/Th2 paradigm in transplantation. Curr Biol. 1994;4:757–64. doi: 10.1016/0952-7915(94)90081-7. [DOI] [PubMed] [Google Scholar]
- 20.Strom T, Roy-Chaundhury P, Manfro R, et al. The Th1/Th2 paradigm and the allograft response. Curr Opin Immunol. 1996;8:688–93. doi: 10.1016/s0952-7915(96)80087-2. [DOI] [PubMed] [Google Scholar]
- 21.Billingham RE, Medawar PB. The technique of free skin grafting in mammals. J Exp Biol. 1951;28:385–402. [Google Scholar]
- 22.Rosso di San Secondo VE, Aniasi A, Piccolo G, et al. Potentiation of cyclosporine A-induced immunosuppression by a lymphocyte low-molecular weight peptide. Transplant Proc. 1994;26:3221–2. [PubMed] [Google Scholar]
- 23.Kavelaars A, Eggen BJ, De Graan PN, et al. The phosphorylation of the CD3 gamma chain of T lymphocytes is modulated by beta-endorphin. Eur J Immunol. 1990;20:943–5. doi: 10.1002/eji.1830200435. [DOI] [PubMed] [Google Scholar]