Abstract
Most receptors for immunoglobulins exist as multi-subunit complexes, with unique ligand binding α-chains, combined with accessory signalling (γ-, β-, or ζ-) chains. The myeloid class I receptor for IgG (FcγRIa) has been shown to be dependent on the FcR γ-chain for surface expression in vivo. In this study we assess the capacity of FcγRIa–γ-chain complexes expressed in IIA1.6 cells to trigger phagocytosis and ADCC. An intact immunoreceptor tyrosine-based activation motif (ITAM) signalling motif proved essential for triggering of biological function via the FcγRIa receptor complex. Both the FcR γ-chain and the FcγRIIa–ITAM proved active in directing phagocytosis of Staphylococcus aureus and ADCC of erythrocytes, triggered by the FcγRIa complex. The capacity of FcγRIa to trigger phagocytic and cytolytic activity by IIA1.6 cells, both considered ‘professional phagocyte’ functions, motivated us to re-evaluate the cell lineage and developmental stage of IIA1.6 cells. Although originally described as mouse B lymphocytes, the IIA1.6 cells proved positive for non-specific esterase activity and expressed the CD5 antigen. These combined characteristics place the IIA1.6 cells within a unique CD5+ B cell/macrophage lineage, optimally suited for cell biological analyses of phagocyte receptors.
Keywords: FcγRIa, FcR γ-chain, phagocytosis, ADCC
INTRODUCTION
Fc receptors (FcR) provide an essential link between humoral and cellular branches of the immune system and unique FcR have been defined for each immunoglobulin class. FcR for IgG (FcγR), IgE (FcɛR) and IgA (FcαR) trigger functions varying from phagocytosis, cytokine production, degranulation, and antigen presentation to ADCC. Most leucocyte receptors for the Fc region of immunoglobulins exhibit multi-subunit composition. Receptor complexes consist of unique ligand binding α-chains, combined with members of a family of accessory FcR γ-, β- and ζ-chains. The FcR γ-chain subunit associates with a variety of FcR and can, furthermore, complex with TCR CD3 molecules [1]. The FcR γ-chain proved important for surface expression of FcγRIIIa [2], FcɛRI [3] and FcγRIa [4]. This accessory molecule was furthermore shown essential for endocytic activity of guinea pig FcγRIII [5], human FcγRIa- and FcγRIIIa-mediated phagocytosis [2,4], and antigen presentation triggered by FcγRIIIa [6]. Within the FcR γ-chain a unique signalling motif named immunoreceptor tyrosine-based activation motif (ITAM), consisting of two YXXL-boxes spaced by seven amino acids, proved important for its signalling ability (reviewed in [1]).
FcγRI (CD64) is expressed on myeloid progenitors, monocytes, macrophages and dendritic cells, and can be induced on polymorphonuclear neutrophils (PMN) by the cytokines interferon-gamma (IFN-γ) and granulocyte colony-stimulating factor (G-CSF) [1,7]. FcγRIa represents the sole Fcγ receptor with high affinity for monomeric IgG. Three highly homologous genes, FcγRIA, IB and IC, have been identified for this receptor class. The prototypic high-affinity IgG receptor is encoded by the FcγRIA gene and has a mol. wt of 72 kD, with a unique extracellular region consisting of three C2-set immunoglobulin-like domains [1].
We now studied structure–function relationships of FcγRIa–γ-chain complexes in the well-known IIA1.6 cell model. A panel of IIA1.6 cell transfectants was generated and phagocytosis and ADCC triggered by the various receptor complexes was evaluated. Motivated by our results, we re-evaluated the cell lineage and developmental stage of this popular cell biological model. These analyses identified the IIA1.6 cell line as a member of a unique CD5+ B cell/macrophage lineage.
MATERIALS AND METHODS
Cells and constructs
Murine IIA1.6, A20, P815 and P388.D1 cells, as well as human U937 cells, were cultured in RPMI 1640 medium supplemented with 10% heat-inactivated fetal calf serum (FCS). IIA1.6 cells transfected with FcγRIa and various FcR γ-chain constructs were cultured in the presence of 5 μm methotrexate (Pharmachemie, Haarlem, The Netherlands).
Human FcγRIa cDNA [8] was cloned into the pRC-CMV plasmid (Invitrogen, Leek, The Netherlands), and wild-type and mutant FcR γ-chain cDNAs were cloned into the pNUT expression vector [4]. The FcR γ-chain mutant γ:Y65F,Y76F represents a molecule in which both tyrosines within the ITAM signalling motif are mutated into phenylalanines. The FcR γ-chain mutant γ:IIa–ITAM represents a chimeric molecule in which the last 22 amino acids of the murine FcR γ-chain cytoplasmic tail are replaced by 29 amino acids of the tail of FcγRIIa [9]. The γY65F,Y76F and γ:IIa–ITAM mutants were generated by two-step overlap extension polymerase chain reaction (PCR), followed by confirmation through sequence analysis [9]. For each transfection, 10 μg of plasmid DNA and 1 × 107 IIA1.6 cells were used. Electroporation was performed with BioRad (Richmond, CA) equipment set at 250 V, and 960 μF. Expression levels of FcγRIa and various FcR γ-chain constructs (cultured with methotrexate) remained high during the course of experiments described in this study.
Immunofluorescence and reverse transcription-PCR
FcγRIa expression levels of the different transfectants were regularly checked by immunofluorescence, using FITC-labelled CD64 MoAb 22 (Medarex, Annandale, NJ). Absence of endogenous FcR expression was checked with the FITC-labelled CD32 MoAb 2.4G2 (Pharmingen, San Diego, CA). CD5 expression on cells was assessed with a biotin-labelled MoAb specific for murine CD5 (clone 53-7.3, rat IgG2a (PharMingen); kindly provided by Dr F. Kroese, University of Groningen, The Netherlands), and detection by strepavidin–PE (Becton Dickinson, San Jose, CA). The antibody recognizing a mouse antigen included anti-Mac-1: M1/70 (PE-labelled) (Boehringer, Mannheim, Germany). Cells were incubated with MoAb for 30 min at 4°C, washed with PBS (pH 7.4)/1% bovine serum albumin (BSA)/0.1% sodium azide, and analysed on a FACScan (Becton Dickinson). Murine FcR γ-chain, γY65F,Y76F, and γ:IIa–ITAM expression levels were checked by reverse transcription (RT)-PCR. Two FcR γ-chain-specific primers γMp1 (sense: 5′-AGGATGATCTCAGCC-3′) and γMP2 (antisense: 5′-GGTCTCTGGCAGCTT-3′), were used encompassing nucleotides 40–575 of murine FcR γ-chain. FcR γ:IIa–ITAM transcripts were detected using γMP1 and FcγRIIa-specific primer AMP2 (antisense: 5′-GTTTGTAGTTTAAGCAA-3′), yielding a 450-bp fragment [9]. Two CD5-specific primers (CD5-prl: 5′-GGTCAAGTGGAGATCCAGATG-3′; and CD5-pr2: 5′-TCGGTGTAGGGCTCCTTCCAG-3′) encompassing nucleotides 21–451 [10] served to detect CD5 mRNA expression. cDNA quality was confirmed by amplification of HPRT [11].
Phagocytosis assay
Staphylococcus aureus Wood bacteria (deficient in protein A) were FITC-labelled as in [12]. Bacteria were opsonized in Hanks' balanced salt solution (HBSS), containing 15% heat-inactivated pooled human serum by incubation for 30 min at 37°C. Following washing, bacteria were incubated with FcγRIa–γ-chain co-transfected IIA1.6 cells for 45 min at 4°C. After two washing steps, cells were further incubated for 45 min, either at 4°C or at 37°C. Remaining cell surface-bound bacteria were detected by incubation for 30 min at 4°C with (PE)-conjugated goat anti-human IgG κ/λ antiserum (Southern Biotechnology, Birmingham, AL) [12]. FITC- and PE-fluorescence intensities of cells maintained at 4°C throughout served as control for binding of bacteria (no phagocytosis), whereas the decrease of PE-fluorescence intensity upon incubation at 37°C reflected bacterial phagocytosis [12]. Involvement of the cytoskeleton in phagocytosis was assessed by incubation with 300 ng/ml cytochalasin D (Sigma, St Louis, MO) for 30 min at 37°C. Incubation of cells in RPMI 1640 medium alone served as control.
ADCC assay
Rhesus D-positive human erythrocytes were labelled with sodium-51chromate (0.1 μCi/108 erythrocytes) for 1 h at 37°C and subsequently opsonized with either a mouse IgG2a anti-glycophorin A MoAb (CLB, Amsterdam, The Netherlands) or human anti-rhesus D IgG (Merz and Dade, Dudingen, Switzerland) for 1 h [13]. Erythrocytes were washed three times with PBS, and added in various ratios to IIA1.6 transfectants (2.5 × 106 cells per sample) in a total volume of 100 μl RPMI 1640 medium (plus 10% FCS). Plates were centrifuged (5 min at 200 g, to facilitate cell–cell contact) and placed in a CO2 incubator set at 37°C. After 2 h, 100 μl of 17 mm NaCl were added to each well, and plates were centrifuged for 5 min at 900 g. Supernatants (100 μl) were removed from each well, and radioactivity was determined in a gamma counter. The percentage specific cytotoxicity was determined by the following formula: (ct/min of test sample − ct/min of spontaneous 51Cr release)/(ct/min of the maximal 51Cr release − ct/min of spontaneous 51Cr release) × 100. The spontaneous 51Cr release was determined by incubating target cells alone, in the absence of effectors. The maximal 51Cr release was obtained upon incubation of the targets with 20% Zap-oglobin (Coulter, Luton, UK).
The expression of pore-forming perforin in IIA1.6 cells was studied by PCR using specific perforin primers: p1perf (sense: 5′-ATGGCCACGTGCCTGTTC-3′) and p2perf (sense: 5′-CACACAGCCCCCAGACACTT-3′), yielding a 1580-bp fragment. Cytotoxic mouse T cells were used as positive control [14]. The involvement of cytoskeletal rearrangements in ADCC was studied on co-incubation for 3 h at 37°C with 2 μg/ml cytochalasin D. Inhibitors were present throughout the experiments.
Non-specific esterase assay
Cytospins were prepared by spinning cells onto glass slides. Upon drying at room temperature and fixation (4% formalin), cytospins were washed twice in water (for 1 min) and further incubated for 30 min with hexazonium pararosanilin (4% in 2 n HCl). This was followed by washing three times in water, and staining for 5 min with haematoxylin (according to Mayer), washing (for 10 min) in water, dehydration, and embedding in DPX mounting medium (Klinipath, Duiven, The Netherlands). Following this protocol, nuclei stain blue, and cytoplasm of non-specific esterase-positive cells brown/red [15].
Statistical analysis
Statistical analyses were performed with a paired Student's t-test. Significance was accepted at the P < 0.05 level.
RESULTS
Transfectant panel
FcγRIa exists as a multi-subunit receptor complex composed of a unique ligand binding α-chain, and a homodimer of FcR γ-chain [4,16]. In order to assess the capacity of FcγRIa to trigger biological functions, we set up a model system in IIA1.6 cells. These cells derive from the murine A20 B cell lymphoma, lack endogenous FcR (due to a deletion in the 5′ end of the FcγRII gene), and express a functional (surface IgG2a) B cell receptor (BCR) [17,18]. Monoclonal antibody 2.4G2 (reactive with both murine CD32 and CD16) documented the absence of endogenous FcR expression on our IIA1.6 cells (data not shown, n = 5). IIA1.6 cells were transfected with both FcγRIa cDNA, and either a mock vector or wild-type/mutant FcR γ-chain cDNAs. Cells were cultured under selection pressure (methotrexate) in order to keep the α-ligand binding chain expressed. This because of FcγRIa is dependent on the FcR γ-chain for high surface expression [4]. To assess the importance of an intact ITAM in the cytoplasmic tail of FcR γ-chain for FcγRIa signalling, we generated a mutant γ-chain in which the ITAM tyrosines were changed into phenylalanines (γY65F,Y76F). In addition, a chimeric molecule was constructed in which the FcR γ-chain ITAM was swapped for the FcγRIIa–ITAM (γ:IIa–ITAM). The FcR γ-chain ITAM consists of two YXXL-boxes interspaced by seven amino acids, whereas the FcγRIIa tail bears a non-canonical ITAM (with two YXXL-boxes separated by 12 amino acids) [1]. FcγRIa expression of transfectants was regularly checked with a CD64 MoAb, and remained high during the course of experiments (Fig. 1a–d). FcR γ-chain expression in our transfectant panel was checked by RT-PCR, and the characteristic (mutant 450 bp or wild-type 536 bp) fragments were present in the FcγRIa–γ-chain transfectants, whereas γ-chain message was not detectable in FcγRIa/mock cells (Fig. 1e). cDNA quality was confirmed by HPRT amplification (Fig. 1e).
Fig. 1.

Expression of FcγRIa and FcR γ-chain in IIA1.6 transfectants. Cells were incubated with FITC-labelled (CD64) MoAb 22 (solid lines), or immunofluorescence buffer alone (dotted lines). Fluorescence was recorded as arbitrary units on a logarithmic scale. Panels represent IIA1.6 cells co-transfected with FcγRIa cDNA, and with either a mock vector (a), FcR γ-chain (b), FcR γY65F,Y76F (c), or FcR γ:IIa–immunoreceptor tyrosine-based activation motif (ITAM) (d). FcR γ-chain, FcR γY65F,Y76F and FcR γ:IIa–ITAM expression was checked by reverse transcription-polymerase chain reaction (RT-PCR). The characteristic FcR γ-chain and FcR γY65F,Y76F 546 base pair, and the FcR γ:IIa–ITAM 450 base pair bands are indicated by lines. cDNA quality was confirmed by RT-PCR of HPRT. Origins of RT cDNAs are shown above the lanes (e).
FcR γ-chain is essential for phagocytosis via FcγRIa
We first analysed the phagocytic capacity of FcγRIa complexes according to the method described in [12]. IgG-opsonized FITC-labelled S. aureus were incubated with IIA1.6 transfectants. Upon incubation at 4°C (bacteria were allowed to bind), temperature was shifted to 37°C (enabling phagocytosis), followed by detection of bacteria remaining at the cell surface by PE-labelled goat anti-human IgG. FITC fluorescence served to detect the total amount of bound/phagocytosed bacteria, whereas PE staining determined non-phagocytosed S. aureus. Untransfected IIA1.6 cells showed low background binding of opsonized FITC-labelled S. aureus which, however, was also observed with non-opsonized bacteria. This background binding may best be explained by ‘stickiness’ of FITC to IIA1.6 cells. At both temperatures, FcγRIa/mock and FcγRIa/γY65F,Y76F transfectants showed comparable PE-staining profiles, consistent with absent phagocytosis of FITC-labelled bacteria. FcγRIa/γ wild-type or FcγRIa/γ:IIa–ITAM transfectants, however, exhibited a vigorous drop in PE staining upon incubation at 37°C, reflecting bacterial phagocytosis. Both the FcR γ-chain ITAM and the FcγRIIa–ITAM were found critical for initiating phagocytosis (Fig. 2). Additionally, no kinetic/quantitative differences were observed between FcγRIa complexes containing wild-type γ-chain or γ:IIa–ITAM chain (data not shown, n = 8).
Fig. 2.
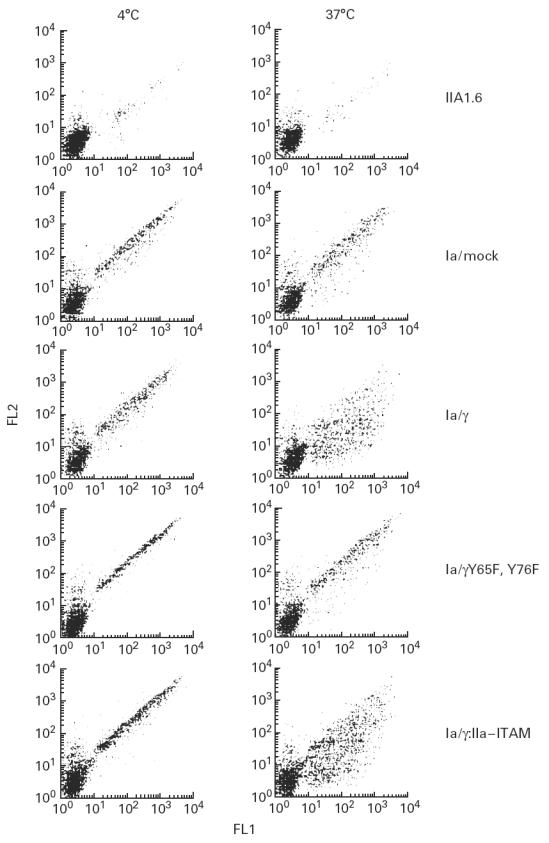
Phagocytosis by FcγRIa-expressing IIA1.6 cells. FITC-labelled IgG-opsonized Staphylococcus aureus were incubated with either non-transfected cells, or FcγRIa complex-transfected cells for 45 min at 4°C. Cells were further incubated for 45 min either at 4°C (left lanes) or 37°C (right lanes). Remaining cell surface-bound bacteria were stained with PE-conjugated goat anti-human antibody. FITC (FL1) and PE (FL2) fluorescence of 3500 cells was quantified by flow cytometry, and dot plot diagrams are shown. Experiments were repeated at least four times yielding almost identical results.
Involvement of the cytoskeleton in phagocytosis, mediated via either FcγRI/γ wild type or FcγRI/γ:IIa–ITAM, was determined by preincubating cells with 300 ng/ml cytochalasin D for 30 min at 37°C. Binding was unchanged, but ingestion of the bacteria was completely blocked, indicating microfilament organization to be critical for uptake of bacteria by FcγRIa-expressing IIA1.6 transfectants (n = 4).
FcγRIa–γ-chain complexes trigger ADCC
Predictably, we observed erythrocytes opsonized with either mouse IgG2a anti-glycophorin A, or human anti-rhesus D IgG (well known ligands for FcγRIa [1,13]) to form EA rosettes with FcγRIa–γ-chain transfectants at 4°C. When temperature was shifted to 37°C and samples were microscopically evaluated during 180 min (at 20-min time points), no phagocytosis was observed (data not shown, n = 4), probably attributable to the size of these opsonized erythrocytes. We next studied the capacity of FcγRIa transfectants to lyse mouse IgG2a- or human anti-rhesus D IgG-opsonized 51Cr-labelled erythrocytes in 3 h 51Cr-release assays. Remarkably, FcγRIa–γ-chain and FcγRIa/γ:IIa–ITAM transfected cells effectively lysed opsonized erythrocytes, in contrast to either FcγRIa/mock, FcγRIa/γY65F,Y76F or non-transfected IIA1.6 cells. Non-opsonized erythrocytes (negative control) were not lysed (Fig. 3a). Optimal lysis occurred at effector:target ratios of 10 for both FcγRIa–γ-chain and FcγRIa/γ:IIa–ITAM transfectants (Fig. 3b) in an antibody dose-dependent way (data not shown, n = 3). Lysis of opsonized erythrocytes was, furthermore, effectively blocked by addition of 25 μg/ml human IgG. Although the IIA1.6 transfectants undoubtedly caused damage to the cell membrane of the erythrocytes, as indicated by the release of 51Cr, we found no evidence for the presence of pore-forming materials such as produced by cytotoxic T lymphocytes (data not shown) [14]. Cytoskeletal rearrangements, however, were involved because addition of 2 μg/ml cytochalasin D (blocking microfilament polymerization) ablated ADCC by the IIA1.6 transfectants (n = 3).
Fig. 3.
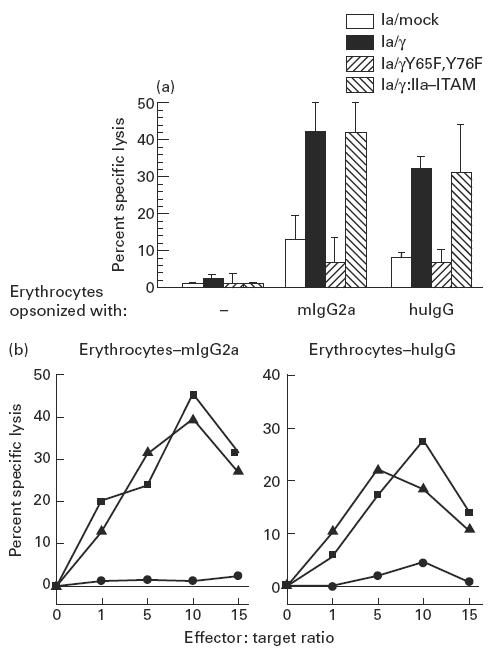
(a) ADCC triggered by FcγRIa-transfected IIA1.6 cells. Lysis of erythrocytes was measured by 51Cr release in supernatants after 3 h coincubation of IIA1.6 transfectants with mouse IgG2a (mIgG2a)- or human IgG (huIgG)-opsonized erythrocytes (effector:target ratio of 10). Non-opsonized erythrocytes (−) served as a control. Data represent mean ± s.e.m. of eight individual experiments. ADCC of (mIgG2a- and huIgG)-opsonized erythrocytes by FcγRIa/γ and FcγRIa/γ:IIa–immunoreceptor tyrosine-based activation motif (ITAM)-transfected cells differed significantly from other transfectants and non-transfected IIA1.6 cells (P < 0.05). (b) Erythrocyte lysis at different effector:target ratios by FcγRIa-transfected IIA1.6 cells. IIA1.6 cells expressing either FcγRIa/mock (•), FcγRIa/γ wild type (▪) or FcγRIa/γ:IIa–ITAM (▴) were incubated at different effector:target ratios (indicated on the abscissas) with either mouse IgG2a-opsonized erythrocytes (erythrocytes–mIgG2a) or human IgG-opsonized erythrocytes (erythrocytes–huIgG). Experiments were repeated at least three times, yielding essentially identical results.
Developmental stage of IIA1.6 cells
The observation that FcγRIa–γ-chain-transfected IIA1.6 cells exhibited both phagocytic and cytolytic capacities (functions considered characteristic for professional phagocytes [19]) motivated us to carefully re-assess the lineage relationships of IIA1.6 cells. During our studies, we also observed IIA1.6 cells to adhere tightly to plastic, another characteristic uncommon to B lymphoid cells [20]. The unusual combination of these ‘phagocytic’ properties led us to check IIA1.6 cells for non-specific esterase activity (NSE), a distinct phagocyte characteristic. All tested IIA1.6 cells stained positively for NSE, using appropriate positive (U937) and negative (HL60) controls (Fig. 4).
Fig. 4.
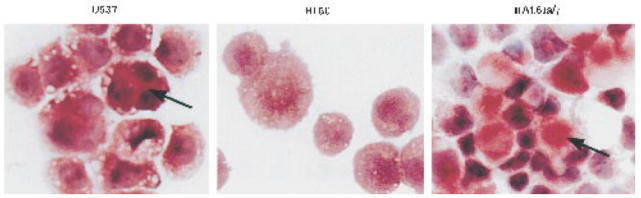
Non-specific esterase activity in IIA1.6 cells. U937, HL-60 and FcγRIa-transfected IIA1.6 cells were stained for non-specific esterase activity, by incubation with hexazonium pararosanilin followed by haematoxylin (see Materials and Methods). This staining method results in blue nuclei and cytoplasm of non-specific esterase-positive cells colouring brown/red (indicated by arrows). This experiment was repeated twice with identical results.
Notably, recent studies documented the existence of a unique B cell/macrophage lineage which is (inter alia) characterized by CD5 expression [21]. We thus assessed CD5 expression at both mRNA and protein levels. Strikingly, both the A20 and IIA1.6 cells (both transfected and untransfected) expressed the CD5 antigen (FACS analysis). P388.D1 cells, pre-B cells with features of malignant B cell lineage switching to macrophage-like cells [22], served as a positive control, whereas human monocytic U937 cells and mouse P815 (mastocytoma) cells served as negative controls (Fig. 5). Consistent with protein data, mRNA for CD5 was also detectable in IIA1.6 transfectants (data not shown). In addition, IIA1.6 transfectants were tested for expression of a monocytic/macrophage marker using anti-Mac-1 MoAb, but appeared to stain negative compared with control P388.D1 cells (data not shown, n = 3).
Fig. 5.
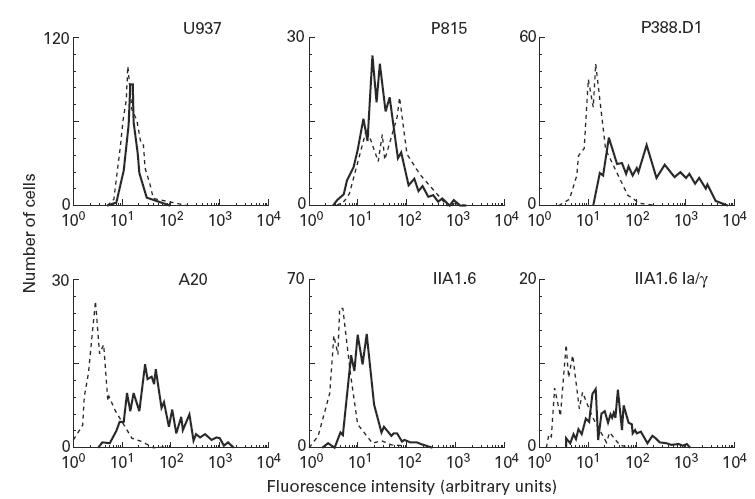
CD5 expression levels on human and murine cell lines. Human monocytic (U937), murine mastocytoma (P815), murine B cell-derived macrophage (P388.D1), murine lymphoid (A20), murine Fc receptor-deficient (IIA1.6), and FcγRIa–γ-chain transfected IIA1.6 cells were stained for murine CD5. Cells were incubated for 30 min with a biotin-labelled CD5 antibody, and subsequently with streptavidin–PE for 30 min (solid lines). A biotin-labelled isotype-matched antibody specific for a mouse mast cell marker (4D11) served as a negative control (dotted line). Experiments were performed at least three times, giving essentially identical results.
DISCUSSION
In this study we evaluated the importance of the FcR γ-chain for functioning of the high-affinity receptor for IgG, FcγRIa (CD64). Our data document efficient phagocytosis of IgG-opsonized S. aureus to require an intact ITAM. These data confirm and extend earlier studies, documenting FcγRIa–γ-chain phagocytosis, in COS cells [23,24]. Assessment of phagocytosis in our panel of FcγRIa transfectants yielded data that confirmed phagocytosis data in literature, which motivated us to analyse the role of the FcR γ-chain for ADCC triggering via FcγRIa. Although no defined signalling domains are reported in the cytoplasmic tail of the α-chain, isolated reports showed the α-ligand binding chain, in absence of the FcR γ-chain, capable of endocytosis [23] and triggering increase in intracellular free calcium [25]. Notably, we show for the first time that the α-chain of FcγRIa is insufficient to trigger ADCC. We document the FcR γ-chain, containing a functional ITAM, to be indispensable for directing efficient lysis of IgG-opsonized erythrocytes by FcγRIa transfectants (Fig. 3).
FcγRIa–γ-chain complex activation has been documented to involve p72 syk [26] and the Src kinases Lyn [27] and Hck [28] and MAP kinase [29]. Furthermore, serine/threonine phosphorylation of the FcR γ subunit, and in particular Raf 1 activation, couples upstream protein tyrosine kinase activation to downstream signalling events [30]. The mechanism of erythrocyte lysis, which clearly involved the γ-chain ITAM (Fig. 3), remains unclear. Although IIA1.6 cells transfected with FcγRIa–γ-chain complexes were able to phagocytose opsonized S. aureus, no uptake of opsonized erythrocytes could be detected within the time span of our ADCC experiments. Because cytoskeletal rearrangements were involved (supported by inhibition with cytochalasin D), we hypothesize lysosomal vesicles to rearrange upon activation of IIA1.6 FcγRIa–γ-chain transfectants, leading to lysis of erythrocytes. IIA1.6 cells were originally described as mouse B lymphocytes [17], cells that are not considered ADCC effectors [31]. Earlier reports documented select B lymphocytes capable of mediating ADCC [32,33]. These latter papers, however, reported that these specific B lymphocytes require an ‘activated’ state, caused by either a viral or oncogenic transformation, in order to become cytotoxic, and thus are distinct from the cells tested in this study. The observed initiation of erythrocyte ADCC via the FcγRIa receptor complex in IIA1.6 cells is therefore novel.
The capacity of IIA1.6 cells to trigger both phagocytic and cytolytic activities motivated us to re-evaluate their cell lineage. Borello & Phipps [21] reviewed the arguments for the existence of a CD5+ B/macrophage cell lineage. In this latter review examples of cell lines are given, which were originally described as monocytic cells lines, but appeared to be derived from the B cell lineage, or pre-B cells undergoing macrophage differentiation. The features of B cell/macrophage lines ranged from expression of monocytic markers (P388.D1) to non-specific esterase activity (KLM-2). A crucial requirement, however, was expression of CD5 [21]. Monocytic/macrophage markers were not expressed by the IIA1.6 transfectants. The fact that IIA1.6 cell transfectants are phagocytic, express the CD5 antigen (Fig. 5), and stain positive for non-specific esterase activity (Fig. 4) documents this popular cell line [34,35] to belong to a distinct subset of CD5+ B cell/macrophage cells.
Various models have been employed to characterize FcR function, including P815 cells for signalling [36], RBL cells for endocytosis and signalling [37], and COS cells, P388D1 cells and 3T6 cells for endocytosis and phagocytosis (provided that p72 syk is expressed) [38–40]. However, none of these latter cell models seems suitable for studying the total array of cell functions. This study and earlier work [9,12,34] establish IIA1.6 cells therefore to represent a good cell biological model to assess structure/function relationships of (myeloid) FcR complexes.
Acknowledgments
The authors thank Dr Jeanette Leusen for assistance with PCR analyses, and Dr Aldwin Vriesema for critically reading the manuscript. This work is supported by a Fellowship from the Netherlands Organization for Scientific Research (901-12-174).
References
- 1.Van de Winkel JGJ, Capel PJA. In: Human IgG Fc receptors. Austin Texas, Landes R.G., editors. 1996. [Google Scholar]
- 2.Hibbs ML, Selvaraj P, Carpen O, Springer TA, Kuster H, Jouvin M-H, Kinet J-P. Mechanisms for regulating expression of membrane isoforms of FcγRIII (CD16) Science. 1989;246:1608–11. doi: 10.1126/science.2531918. [DOI] [PubMed] [Google Scholar]
- 3.Blank U, Ra C, Miller L, White K, Metger H, Kinet J-P. Complete structure and expression in transfected cells of high affinity IgE receptor. Nature. 1989;337:187–9. doi: 10.1038/337187a0. [DOI] [PubMed] [Google Scholar]
- 4.Van Vugt MJ, Heijnen Iafm, Capel PJA, Park SY, Ra C, Saito T, Verbeek JS, Van de Winkel JGJ. FcR γ-chain is essential for both surface expression and function of human FcγRI (CD64) in vivo. Blood. 1996;87:3593–9. [PubMed] [Google Scholar]
- 5.Isashi Y, Yamashita T, Nagasawa S, Murakami M, Uede T. Molecular cloning and characterization of guinea pig FcγRIII: expression but not function is independent of the γ chain of FcɛRI. Int Immunol. 1996;8:1335–46. doi: 10.1093/intimm/8.9.1335. [DOI] [PubMed] [Google Scholar]
- 6.Amigorena S, Salamero J, Davoust J, Fridman WH, Bonnerot C. Tyrosine-containing motif that transduces cell activation signals also determines internalization and antigen presentation via type III receptors for IgG. Nature. 1992;358:337–41. doi: 10.1038/358337a0. [DOI] [PubMed] [Google Scholar]
- 7.Olweus J, Lund-Johansen F, Terstappen Lwmm. CD64/FcγRI is a granulo-monocytic lineage marker on CD34+ hematopoietic progenitor cells. Blood. 1995;85:2402–13. [PubMed] [Google Scholar]
- 8.Van de Winkel JGJ, Ernst LK, Anderson CL, Chiu I-M. Gene organization of the human high affinity receptor for IgG, FcγRI (CD64) J Biol Chem. 1991;226:13449–55. [PubMed] [Google Scholar]
- 9.Van den Herik IE, Ter Bekke MWH, Tempelman MJ, Capel PJA, Van de Winkel JGJ. Functional differences between two Fc receptor ITAM signalling motifs. Blood. 1995;86:3302–7. [PubMed] [Google Scholar]
- 10.Huang H-JS, Jones NH, Strominger JL, Herzenberg LA. Molecular cloning of Ly-1, a membrane glycoprotein of mouse T-lymphocytes and a subset of B-cells: molecular homology to its human counterpart Leu-1/T1 (CD5) Proc Natl Acad Sci USA. 1987;84:204–8. doi: 10.1073/pnas.84.1.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vrieling H, Simons JW, Zeeland AA. Nucleotide sequence determination of point mutations at the mouse HPRT locus, using in vitro amplifications of HPRT mRNA sequences. Mutat Res. 1988;198:107–13. doi: 10.1016/0027-5107(88)90046-2. [DOI] [PubMed] [Google Scholar]
- 12.Van den Herik-Oudijk IE, Capel PJA, Van der Bruggen T, Van de Winkel JGJ. Identification of signalling motifs within human FcγRIIa and FcγRIIb isoforms. Blood. 1995;85:2202–11. [PubMed] [Google Scholar]
- 13.Van de Winkel JGJ, Tax WJM, Van Bruggen MCJ, Van Roozendaal CEP, Willems HW, Vlug A, Capel PJA, Koene RAP. Characterization of two Fc receptors for mouse immunoglobulins on human monocytes and cell lines. Scand J Immunol. 1987;26:663–72. doi: 10.1111/j.1365-3083.1987.tb02302.x. [DOI] [PubMed] [Google Scholar]
- 14.Lowin B, Peitsch MC, Tschopp J. Perforin and granzymes: crucial effector molecules in cytolytic T lymphocyte and natural killer cell-mediated cytotoxicity. Curr Top Microbiol Immunol. 1995;198:1–24. doi: 10.1007/978-3-642-79414-8_1. [DOI] [PubMed] [Google Scholar]
- 15.Davis BJ, Ornstein L. High resolution enzyme localization with a new diazo reagent “Hexazonium pararosaniline”. J Histochem Cytochem. 1959;7:297–301. [Google Scholar]
- 16.Ernst LK, Duchemin A-M, Andersonl CL. Association of the high-affinity receptor for IgG (FcγRI) with the γ subunit of the IgE receptor. Proc Natl Acad Sci USA. 1993;90:6023–7. doi: 10.1073/pnas.90.13.6023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones B, Tite JP, Janeway CA. Different phenotypic variants of the mouse B-cell tumour A20/2J are selected by antigen- and mitogen-triggered cytotoxicity of L3T4-positive, IA-restricted T cell clones. J Immunol. 1986;136:348–56. [PubMed] [Google Scholar]
- 18.Lewis VA, Kock T, Plutner H, Mellman I. A complementary DNA clone for a macrophage-lymphocyte Fc receptor. Nature. 1986;324:372–5. doi: 10.1038/324372a0. [DOI] [PubMed] [Google Scholar]
- 19.Greenberg S, Silverstein SC. Fundamental immunology. New York: Raven Press Ltd; 1993. pp. 941–58. [Google Scholar]
- 20.Hayakawaa K, Hardy RR, Herzenberg LA. Peritoneal Ly-1 B cells: genetic control, autoantibody production, increased lamda light chain reaction. Eur J Immunol. 1986;16:450–6. doi: 10.1002/eji.1830160423. [DOI] [PubMed] [Google Scholar]
- 21.Borello MA, Phipps RP. The B/macrophage cell: an elusive link between CD5+ B lymphocytes and macrophages. Immunol Today. 1996;17:471–5. doi: 10.1016/0167-5699(96)20031-b. [DOI] [PubMed] [Google Scholar]
- 22.Bauer SR, Holmes KL, Morse HC, Potter M. Clonal relationship of the lymphoblastic cell line P388 to the macrophage cell line P388D1 as evidenced by immunoglobulin gene rearrangements and expression of cell surface antigens. J Immunol. 1986;136:4695–9. [PubMed] [Google Scholar]
- 23.Davis W, Harrison PT, Hutchinson MJ, Allen JM. Two distinct regions of FcγRI initiate separate signalling pathways involved in endocytosis and phagocytosis. EMBO J. 1995;14:432–41. doi: 10.1002/j.1460-2075.1995.tb07019.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Indik ZK, Hunter S, Huang MM, et al. The high affinity Fcγ receptor (CD64) induces phagocytosis in the absence of its cytoplasmic tail: the γ subunit of FcγRIIIA imparts phagocytic function of FcγRI. Exp Hematol. 1994;22:599–606. [PubMed] [Google Scholar]
- 25.Indik ZK, Chien P, Levinson AI, Schreiber AD. Calcium signalling by the high affinity macrophage Fcγ receptor requires the cytosolic domain. Immunobiol. 1992;185:183–92. doi: 10.1016/s0171-2985(11)80640-6. [DOI] [PubMed] [Google Scholar]
- 26.Kiener PA, Rankin BM, Burkhardt AL, Schieven GL, Gilliland LK, Rowley RB, Bolen JB, Ledbetter JA. Cross-linking of FcγRI (FcγRI) and receptor II (FcγRII) on monocytic cells activates a signal transduction pathway common to both Fc receptors that involves the stimulation of p72 Syk protein tyrosine kinase. J Biol Chem. 1993;268:2442–8. [PubMed] [Google Scholar]
- 27.Duchemin A-M, Anderson CL. Association of non-receptor protein tyrosine kinases with the FcγRI/γ-chain complex in monocytic cells. J Immunol. 1997;158:865–71. [PubMed] [Google Scholar]
- 28.Wang AV, Scholl PR, Geha RS. Physical and functional association of the high affinity immunoglobulin G receptor (FcγRI) with the kinases Hck and Lyn. J Exp Med. 1994;180:1165–70. doi: 10.1084/jem.180.3.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Durden DL, Kim HM, Calore B, Liu Y. The FcγRI receptor signals through the activation of Hck and MAP kinase. J Immunol. 1995;154:4039–47. [PubMed] [Google Scholar]
- 30.Park RK, Liu Y, Durden DL. A role for Shc, Grb2, and Raf-1 in FcγRI signal relay. J Biol Chem. 1996;271:13342–8. doi: 10.1074/jbc.271.23.13342. [DOI] [PubMed] [Google Scholar]
- 31.Deo YM, Graziano RF, Repp R, Van de Winkel JGJ. Clinical significance of IgG Fc receptors and FcγR-directed immunotherapies. Immunol Today. 1997;18:127–35. doi: 10.1016/s0167-5699(97)01007-4. [DOI] [PubMed] [Google Scholar]
- 32.Lopez DM, Blomberg BB, Padmanabhan RR, Bourguignon LYW. Nuclear disintegration of target cells by killer B lymphocytes from tumor-bearing mice. FASEB J. 1989;3:37–43. doi: 10.1096/fasebj.3.1.2783411. [DOI] [PubMed] [Google Scholar]
- 33.Welsch RM, Haspel MV, Parker DC, Holmes KV. Natural cytotoxicity against mouse hepatitis virus-infected cells. J Immunol. 1986;136:1454–8. [PubMed] [Google Scholar]
- 34.Amigorena S, Bonnerot C, Drake JR, et al. Cytoplasmic domain heterogeneity and functions of IgG Fc receptors in B lymphocytes. Science. 1992;256:1808–12. doi: 10.1126/science.1535455. [DOI] [PubMed] [Google Scholar]
- 35.Muta T, Kurosaki T, Misulovin Z, Sanchez M, Nussenzweig MC, Ravetch JV. A 13-amino-acid motif in the cytoplasmic domain of FcγRIIB modulates B-cell receptor signalling. Nature. 1994;368:70–73. doi: 10.1038/368070a0. [DOI] [PubMed] [Google Scholar]
- 36.Alber G, Kent UM, Metzger H. Functional comparison of FcɛRI, FcγRII, and FcγRIII in mast cells. J Immunol. 1992;149:2428–36. [PubMed] [Google Scholar]
- 37.Bonnerot C, Daeron M. Biological activities of murine low-affinity Fc receptors for IgG. Immunomethods. 1994;4:41–47. doi: 10.1006/immu.1994.1006. [DOI] [PubMed] [Google Scholar]
- 38.Indik ZK, Park JG, Pan XQ, Schreiber AD. Induction of phagocytosis by a protein tyrosine kinase. Blood. 1995;85:1175–80. [PubMed] [Google Scholar]
- 39.Odin JA, Edberg JC, Painter CJ, Kimberly RP, Unkeless JC. Regulation of phagocytosis and [Ca2+]i flux by distinct regions of an Fc receptor. Science. 1991;254:1785–8. doi: 10.1126/science.1837175. [DOI] [PubMed] [Google Scholar]
- 40.Tuijnman WB, Capel PJA, Van de Winkel JGJ. Human low-affinity IgG receptor FcγRIIa (CD32) introduced into mouse fibroblasts mediates phagocytosis of sensitized erythrocytes. Blood. 1992;79:1651–6. [PubMed] [Google Scholar]


