Abstract
Autoantibodies to a 64-kD protein and a 40-kD tryptic fragment from pancreatic islets have been detected at high frequency in the sera of patients with insulin-dependent diabetes mellitus (IDDM). IA-2, a newly isolated transmembrane protein tyrosine phosphatase, is a major islet cell autoantigen in IDDM and the precursor of a 40-kD tryptic fragment. To express large quantities of recombinant IA-2 protein and analyse post-translational modifications we expressed full-length human IA-2 in baculovirus-infected Sf-9 cells. IA-2 expression was analysed by Western blot and by immunoprecipitation of 35S-methionine-radiolabelled proteins with rabbit antisera or IDDM sera. A 120-kD IA-2 protein was detected during the early, but not the late, phase of the infection. Pulse-chase experiments showed that the 120-kD protein was processed into fragments of 64 kD and smaller fragments of ≈ 50 kD, 38 kD and 32 kD. The 64-kD fragment appeared as a doublet. Tunicamycin and PNGase F treatment down-shifted the 120-kD protein and the 64-kD doublet into lower molecular weight bands, suggesting that both were glycosylated. Trypsin treatment converted the 120-kD protein and the 64-kD doublet into a 40-kD fragment. Baculovirus-expressed IA-2 was as sensitive or slightly more sensitive than in vitro translated IA-2 in detecting autoantibodies to IA-2: 66% of sera from newly diagnosed IDDM patients reacted with baculovirus-expressed IA-2 compared with 59% of the same sera which reacted with in vitro translated IA-2. It is concluded that baculovirus-expressed IA-2 is a good source of autoantigen and that a number of lower molecular weight fragments with which IDDM autoantibodies react are derived from the 120-kD full-length IA-2 molecule.
Keywords: IA-2, protein tyrosine phosphatase, post-translational modification, baculovirus expression, autoantibodies
INTRODUCTION
Insulin-dependent diabetes mellitus (IDDM) is characterized by gradual destruction of pancreatic islets [1]. Autoantibodies to islet cells (ICA) are found many years before the onset of clinical diabetes and serve as predictive markers [1–5]. One of the autoantigens to which ICA react is IA-2, a transmembrane protein tyrosine phosphatase (PTP) [6–9]. A high percentage of prediabetics and diabetics have autoantibodies to IA-2 and these autoantibodies are useful markers for predicting and monitoring IDDM [7–14]. Full-length IA-2 cDNA encodes a protein of 979 amino acids with a molecular mass of 105 847 [6]. The protein has an intracellular, transmembrane and extracellular domain with a signal peptide. IA-2 is expressed primarily in neuroendocrine cells including the pancreatic islets [6,15], and appears to be an intrinsic membrane protein of neuroendocrine secretory granules [16]. Trypsin treatment and blocking experiments revealed that IA-2 is the precursor of a 40-kD fragment which is immunoreactive with IDDM sera [10,12,13]. Studies with deletion mutants showed that the autoantibodies in IDDM sera are directed to the intracellular and not extracellular domain of IA-2 and primarily to the COOH-terminus of the intracellular domain [17,18]. Immunoprecipitation with IDDM sera of cell lysates expressing IA-2, however, failed to detect autoantigens in the expected molecular weight range of the full-length IA-2 molecule (106 kD) [12,19,20]. Instead, autoantigens in the 64-kD and lower ranges were found.
The present experiments, in the baculovirus system, were initiated to obtain large amounts of full-length IA-2 protein, examine the possibility that the full-length molecule was processed into smaller fragments within the cell, determine if these fragments could be detected by IDDM autoantibodies and compare the immunoreactivity of baculovirus-expressed IA-2 with in vitro translated IA-2.
MATERIALS AND METHODS
Expression of human full-length IA-2 autoantigen in baculovirus-infected Sf-9 insect cells
An IA-2 recombinant transfer vector, designated pAc-hIA-2, was constructed by inserting a 3.2-kb SacI-KpnI fragment of full-length human IA-2 cDNA into the pAcSG-His NTA transfer vector (PharMingen, San Diego, CA). The reading frame of cloning sites was verified by sequence analysis. IA-2 recombinant transfer vector, pAc-hIA-2, was co-transfected into Sf-9 insect cells with BaculoGold baculovirus DNA using the calcium phosphate transfection method. After 6 days of transfection, the supernatant from the transfection plate was collected for plaque assay. In total, 15 plaques were selected for human IA-2 expression by Western blot analysis. The recombinant baculovirus, named Bac-hIA-2, was prepared through three cycles of infection of Sf-9 cells. Sf-9 cells were cultured as a monolayer in TN-MFH medium supplemented with 10% fetal bovine serum (FBS) at 27°C.
Antiserum and immunostaining
The intracellular domain of IA-2 protein was expressed in bacteria with glutathione S-transferase in pGEX vector (Pharmacia, Arlington Heights, IL). The GST-IA-2 fusion protein was induced with 0.1 mm isopropyl-β-D-thiogalactopyranoside (IPTG) and purified through a glutathione-agarose affinity column [21]. Recombinant IA-2 protein was used to immunize rabbits to generate rabbit polyclonal antiserum [10]. Preimmune rabbit serum served as the negative control. IDDM sera and age-matched normal control sera were kindly provided by Dr N. K. Maclaren (University of Florida, Gainesville, FL). For immunostaining, Bac-hIA-2 transfected Sf-9 insect cells were fixed at 4°C in acetone for 15 min, dried, and rehydrated in PBS. Cells were blocked with 10% normal goat serum for 30 min. Hyperimmune rabbit serum (1:400) or pre-immune serum was then added to the cells at room temperature for 1 h. Fluorescence-conjugated goat anti-rabbit secondary antibody (1:500 dilution) was used for immunostaining.
Western blot analysis
Sf-9 cells were infected with Bac-hIA-2 recombinant virus (5–10 plaque-forming units (PFU)/cell) or wild-type virus at 27°C for 24–96 h. Whole cell lysate was prepared by extraction of Sf-9 cells with lysis buffer (20 mm Tris pH 8.0, 150 mm NaCl, 1% Triton X-100, 5 mm EDTA, 1 mm PMSF, 16 μg/ml benzamidine, 10 μg/ml phenanthroline, 10 μg/ml aprotinin, 10 μg/ml leupeptin, and 10 μg/ml pepstatin A). Lysates were centrifuged for 30 min at 4°C and supernatants collected for analysis. The protein concentration was determined by BCA protein assay reagent (Pierce, Rockford, IL). Lysates (20 μg) were subjected to SDS–PAGE and transferred to a nitrocellulose membrane. Western blot analysis was carried out by blocking the membrane with 5% non-fat dry milk in PBS for 1 h, incubation with primary antibody for 1 h (rabbit antiserum 1:500 dilution), and then horseradish peroxidase (HRP)-conjugated goat anti-rabbit secondary antibody (1:2000 dilution) for an additional hour. The membrane was developed in the ECL system (Amersham).
Metabolic labelling and immunoprecipitation
To prepare 35S-methionine-labelled IA-2 recombinant protein, 2-day infected Sf-9 cells were starved with methionine-free Grace medium for 1 h followed by incubation with 35S-methionine at a concentration of 200 μCi/ml for 5 h. The cells were then washed with cold PBS and solubilized in 1% Triton X-100 lysis buffer. For the pulse-chase experiment, cells were pulsed with 35S-methionine for 30 min, washed three times with cold medium, and then chased for various time intervals. Prior to immunoprecipitation, 10 × 106 ct/min extracts were pre-cleared with 10 μl of protein A-agarose for 1 h, followed by the addition of 5 μl of either anti-human IA-2 rabbit antiserum or IDDM sera at 4°C overnight. Immunocomplexes were precipitated by adding 50 μl of protein A-agarose for 1 h. The beads were washed twice with immunoprecipitation buffer (10 mm Tris pH 8.0, 150 mm NaCl, 1% Triton X-100) and twice with PBS. Immunoprecipitates were separated on 10% SDS–PAGE, fixed, dried, and exposed to x-ray film.
Tunicamycin and peptide: N-glycosidase treatment
Tunicamycin was dissolved in DMSO and added to Sf-9 cells as a final concentration of 10 μg/ml. During tunicamycin treatment, cells were labelled with 35S-methionine for 5 h. Peptide:N-glycosidase F (PNGase F; Boehringer Mannheim, Indianapolis, IN) treatment was performed by washing immunoprecipitation complexes twice with PNGase F buffer containing 20 mm sodium phosphate buffer pH 7, 10 mm EDTA, 1% Triton X-100, 0.2% SDS and 1% 2-mercaptoethanol, followed by treatment with 10 μl of PNGase F (20 U/100 μl) for 20 h at 37°C.
Detection of IA-2 autoantibodies by radioimmunoprecipitation
Immunoprecipitation was performed as described [7]. Either 35S-labelled baculovirus-expressed IA-2 or in vitro translated human IA-2 was incubated at 4°C overnight with 5 μl of sera in 100 μl of precipitation buffer (20 mm Tris–HCl, 1% Triton X-100, 0.15 m NaCl). Fifty microlitres of 50% (v/v) proteinA-agarose (Life Technologies, Rockville, MD) were then added to the reaction mixture for an additional hour. The immunoprecipitation mixture was washed four times with immunoprecipitation buffer and separated on 10% SDS–PAGE gel. The intensity of the IA-2 bands was scored independently by two investigators [7,8].
RESULTS
Expression of Bac-hIA-2 in Sf-9 insect cells
To study the expression and processing of IA-2 in eukaryotic cells, Sf-9 insect cells were infected with the recombinant baculovirus, designated Bac-hIA-2, which contained the IA-2 insert. Cells were then stained with a rabbit polyclonal antiserum raised against bacteria-expressed IA-2 intracellular domain. Figure 1b shows that Bac-hIA-2-infected cells stained brightly with antibody to IA-2 compared with the uninfected Sf-9 cells (Fig. 1a). Pre-immune sera failed to stain infected or uninfected cells.
Fig. 1.
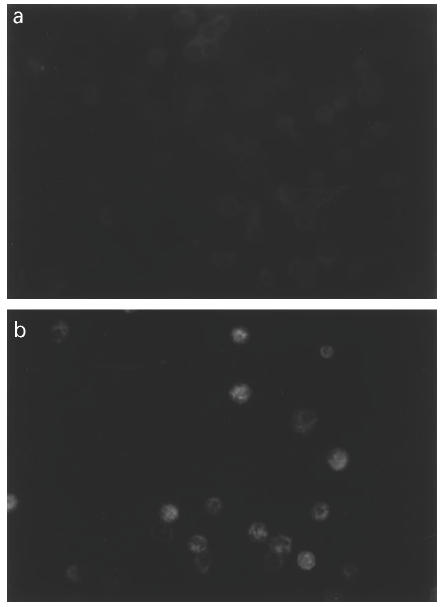
Expression of human IA-2 protein in Bac-hIA-2-infected Sf-9 cells. Uninfected (a) and 2 day-infected (b) Sf-9 cells were stained with rabbit antisera to IA-2 intracellular domain.
To study in greater depth the time course of IA-2 protein expression, lysates from Bac-hIA-2-infected, wild-type-infected and uninfected cells were examined by SDS–PAGE and Western blot at 24, 48, 72 and 96 h after infection. Figure 2 shows a strong 120-kD band at 48 h which gradually decreased in intensity. Beginning at 48 h, bands representing degradation and/or cleavage products of lower molecular weight (e.g. 90, 80 and 64 kD) increased in intensity, peaking at 96 h. At 6 days (data not shown) all of the bands except for the 64-kD band had disappeared.
Fig. 2.
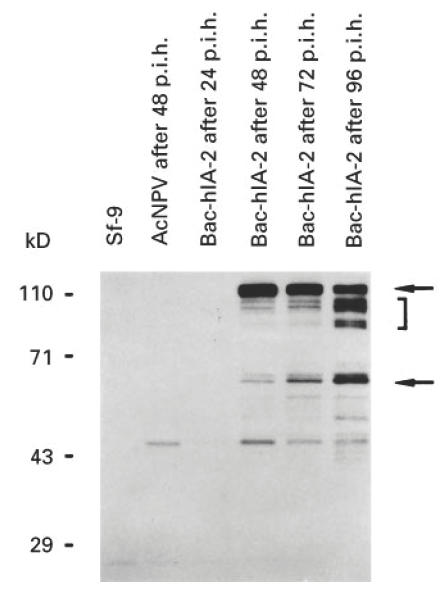
Time course of Bac-hIA-2 expression in Sf-9 cells. At 24, 48, 72 and 96 h post-infection cellular lysates were subjected to 10% SDS–PAGE and immunoblotted with rabbit antiserum to the intracellular domain of human IA-2. The arrows point to the 120-kD full-length human IA-2 protein and the 64-kD IA-2 doublet. The bracket shows degradation products of IA-2. Wild-type baculovirus (AcNPV).
Pulse-chase analysis of Bac-hIA-2
Sf-9 cells, infected with Bac-hIA-2, were pulsed for 30 min with 35S-methionine and chased for 24 h. As seen in Fig. 3, at the end of the 30-min pulse (zero time), a broad 120-kD band was present, probably due to the multiple initiation sites at the 5′ end of the IA-2 cDNA [6]. The intensity of the band increased over the next 4 h. By 9 h the band had markedly decreased in intensity and was absent at 24 h. In contrast, the 64-kD IA-2 band, which appeared as a doublet, was still prominent at 9 and 24 h. Bands representing smaller fragments of ≈ 50, 38 and 32 kD were most pronounced at 2 and 4 h, decreased in intensity by 9 h and were absent by 24 h.
Fig. 3.
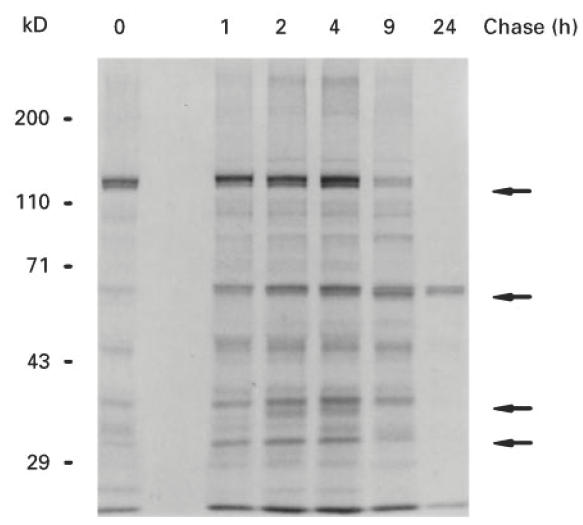
Molecular processing of Bac-hIA-2 protein in Sf-9 cells. Sf-9 cells were pulsed for 30 min with35S-methionine (zero time) and chased for 1, 2, 4, 9 and 24 h. Cellular lysates were then immunoprecipitated with anti-IA-2+ insulin-dependent diabetes mellitus (IDDM) sera and subjected to 10% SDS–PAGE. Arrows point to the 120-kD and 64-kD IA-2 proteins and the various fragments of IA-2 being processed during the chase
Bac-hIA-2 is a glycoprotein
Based on the predicted protein sequence, IA-2 contains two potential N-linked glycosylation sites. IA-2 protein isolated from a rat insulinoma cell line bound wheat germ agglutinin lectin, suggesting that IA-2 is a glycoprotein [12]. To substantiate this possibility, we treated infected Sf-9 cells with tunicamycin which inhibits N-linked glycosylation. As seen in Fig. 4, both the 120-kD and the 64-kD proteins showed a small but reproducible down-shift in their molecular mass, whereas the 50-kD and 38-kD proteins retained the same molecular mass. Similarly, a small down-shift in molecular mass was noted when we treated infected Sf-9 cell lysates with PNGase F, which cleaves N-glycan sugar chains on glycopeptides and glycoproteins [22,23].
Fig. 4.
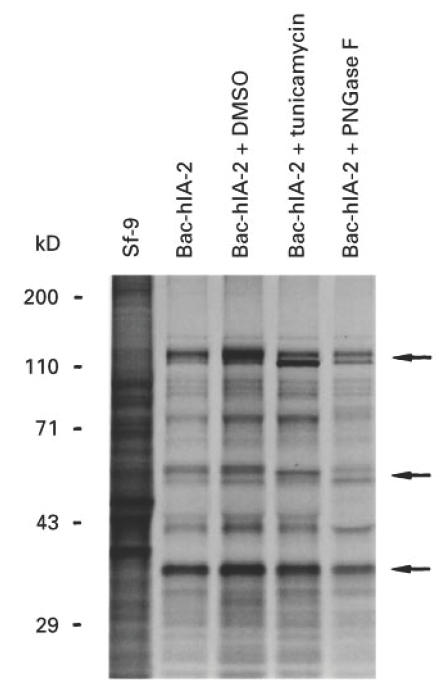
Effect of tunicamycin and peptide:N-glycosidase F (PNGase F) on Bac-hIA-2 protein. Sf-9 cells were treated with 0.1% DMSO or tunicamycin (10 μg/ml) before being infected with Bac-hIA-2 recombinant virus and labelled with 35S-methionine. The cell lysates then were immunoprecipitated with insulin-dependent diabetes mellitus (IDDM) sera and subjected to 10% SDS–PAGE. Immunoprecipitated proteins were treated with PNGase F (lane 5) before being subjected to gel separation. The arrows show the molecular mass and down-shift of the 120- and 64-kD proteins.
Conversion of 120-kD and 64-kD IA-2 proteins into a 40-kD tryptic fragment
As shown in Figs 3 and 4, IA-2 expressed in Bac-hIA-2-infected Sf-9 cells undergoes autoprocessing into multiple fragments (e.g. 64 and 50 kD). To determine whether these fragments are the precursors of the 40-kD tryptic fragment, we treated the infected cell lysates with trypsin. As seen in Fig. 5, trypsin converted the 120-, 64- and 50-kD fragments into a distinct 40-kD tryptic fragment.
Fig. 5.
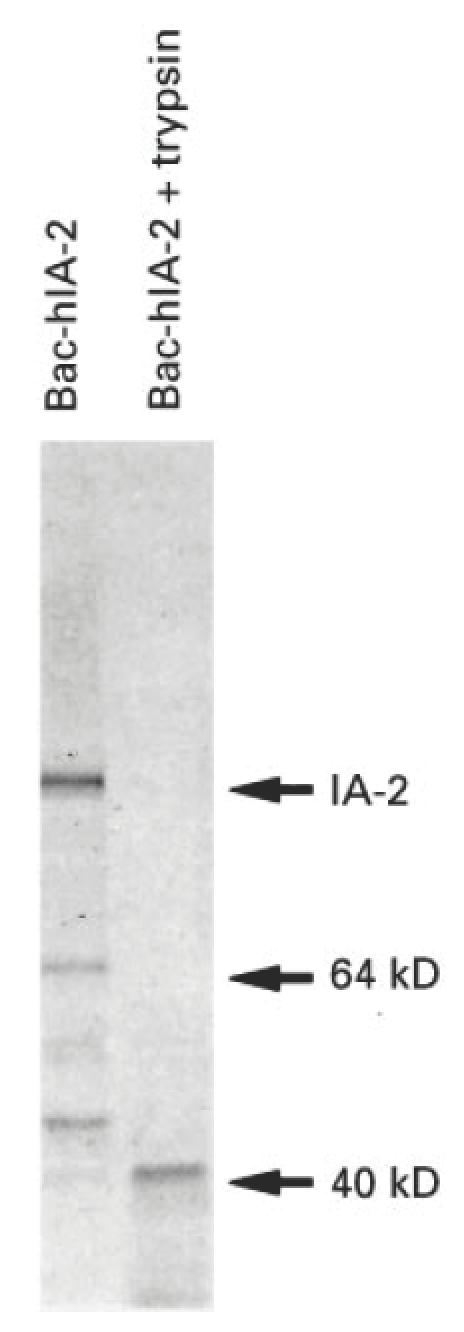
Trypsin treatment of Bac-hIA-2 protein. Infected Sf-9 cell lysates were radiolabelled with 35S-methionine, immunoprecipitated with insulin-dependent diabetes mellitus (IDDM) sera, treated with trypsin (50 μg/ml) for 30 min, and then subjected to 10% SDS–PAGE. The 120-, 64- and 50-kD IA-2 proteins were converted into a 40-kD tryptic fragment.
Radioimmunoprecipitation of baculovirus-expressed human IA-2
To determine whether baculovirus-expressed IA-2 protein reacts with IDDM autoantibodies, radiolabelled cell lysates were precipitated with either IDDM or control sera. Figure 6 shows that a prominent band of 120 kD was precipitated by IDDM sera but not by control sera. A large number of IDDM and control sera were then tested for IA-2 autoantibodies using radiolabelled in vitro translated IA-2 [7] or radiolabelled baculovirus-expressed IA-2. As seen in Fig. 7, 66% of IDDM sera reacted with baculovirus-expressed IA-2, compared with 59% with in vitro translated IA-2. None of the 46 control sera reacted with recombinant IA-2. The correlation coefficient between the assays using baculovirus-expressed IA-2 and in vitro translated IA-2 (Fig. 8) was r = 0.89 (P < 0.0001). Four samples which were negative in the in vitro translated IA-2 assay were positive in the baculovirus-expressed IA-2 assay.
Fig. 6.
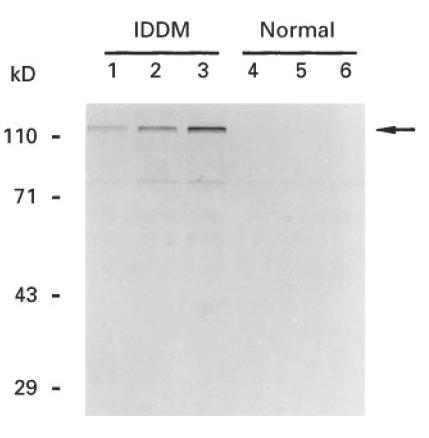
Immunoprecipitation of baculovirus-expressed human IA-2 with insulin-dependent diabetes mellitus (IDDM) serum. Sf-9 cells were labelled with 35S-methionine 48 h after infection with Bac-hIA-2 recombinant virus. Various amounts of labelled cell lysates (lanes 1 and 4, 40 000 ct/min; lanes 2 and 5, 80 000 ct/min; and lanes 3 and 6, 60 000 ct/min) were immunoprecipitated either with IDDM sera (lanes 1–3) or control sera (lanes 4–6) and then subjected to 10% SDS–PAGE.
Fig. 7.
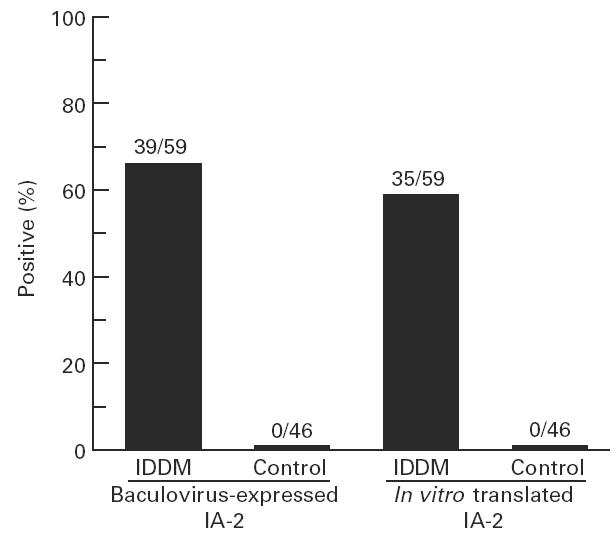
Comparison of the reactivity of baculovirus-expressed IA-2 and in vitro translated IA-2 with insulin-dependent diabetes mellitus (IDDM) sera. Sera from 59 new-onset IDDM patients and 46 healthy controls were tested by radioimmunoprecipitation with either baculovirus-expressed IA-2 (80 000 ct/min) or in vitro translated IA-2 (100 000 ct/min).
Fig. 8.
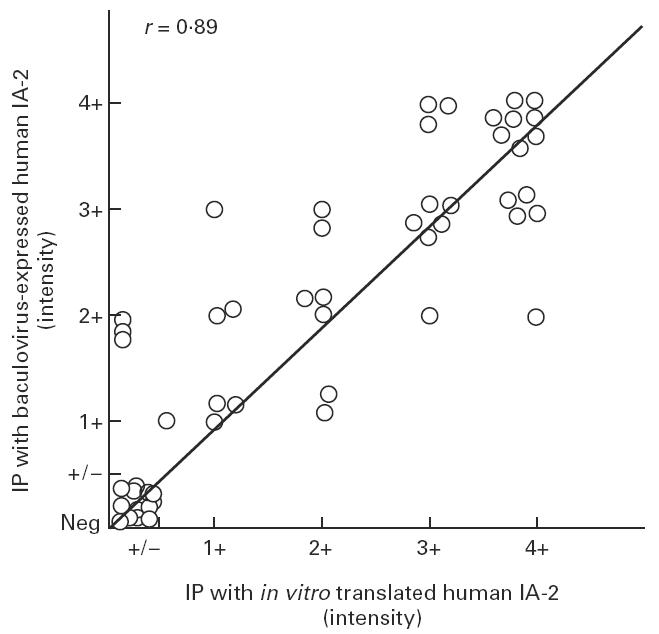
Correlation between the intensity of immunoprecipitated IA-2 bands with baculovirus-expressed IA-2 and in vitro translated IA-2 (r = 0.89, P < 0.0001).
DISCUSSION
In an earlier study we expressed IA-2 in bacteria [10,24]. Baculovirus-infected insect cells, however, have a number of advantages over bacteria. First, they are eukaryotic cells and phosphorylate and glycosylate proteins [25–29]. Second, they correctly process proteins into bioactive peptides and target them to the nucleus or plasma membrane or allow them to be secreted into the media [30–32]. For example, the neuroendocrine protein, synaptophysin, is expressed as an N-glycosylated integral membrane protein in small synaptophysin-rich vesicles in Sf-9 insect cells [30]. In the study reported here, we showed that large amounts of IA-2 were expressed in baculovirus-infected cells, and that the expressed protein retained its ability to bind autoantibodies. Moreover, radioimmunoprecipitation of baculovirus-expressed IA-2 protein with IDDM sera showed that it was as sensitive if not more sensitive than in vitro translated IA-2 protein. Baculovirus-expressed IA-2 should be a good source of antigen for cell-mediated autoimmune studies in patients with IDDM.
The full-length IA-2 molecule was detected early in the course of the infection, but gradually disappeared, being replaced by a 64-kD doublet and a number of fragments of lesser molecular mass. The full-length molecule (approx. 120 kD) was, in fact, larger than that predicted on the basis of its amino acid composition (106 kD). In this context, analysis of the amino acid sequence revealed two potential N-linked glycosylation sites in the extracellular domain of IA-2 [6]. Treatment of the baculovirus-infected cells with tunicamycin or the 120-kD IA-2 molecule with PNGase F down-shifted the molecular mass of IA-2, suggesting that the larger than expected molecular mass was due, at least in part, to glycosylation.
Pulse-chase experiments support the argument that the full-length 120-kD molecule is the precursor of the 64-kD doublet. Dibasic amino acids are a common site for protease cleavage [33] and consecutive lysines are found in IA-2 at amino acid positions 386–387 and 448–449. Cleavage at these sites would result in a doublet in which the two bands would differ from each other by 62 amino acids. Solimena et al. [16] recently isolated a 70-kD fragment from bovine pituitary and showed that the fragment began at position 449 immediately after the dibasic lysines. They did not, however, observe a doublet. The difference in the molecular mass of the IA-2 fragment from baculovirus-infected cells (64 kD) compared with bovine pituitary (70 kD) probably represents a difference in the degree of glycosylation of IA-2 in the two cell types. Similarly, the demonstration of a doublet in baculovirus-infected cells, but not in bovine pituitary, could be due to differences in the expression and abundance of proteases in the different cell types.
Earlier studies, using recombinant IA-2, showed that trypsin treatment of the full-length 106-kD molecule resulted in a 40-kD fragment which retained its immunoreactivity with sera from IDDM patients [8,10,12–14]. The current experiments show that the 120-kD, 64-kD and smaller 50-kD molecules also can be converted, upon treatment with trypsin, into immunoreactive fragments of ≈ 40 kD. From the studies described here (Fig. 9), we conclude that post-translational modification and processing of IA-2 is responsible for the failure to detect the 106-kD or 120-kD autoantigens in the cell types examined thus far, and that the 64-kD fragment is probably the major naturally occurring cleavage product of IA-2 to which autoantibodies in IDDM sera react.
Fig. 9.
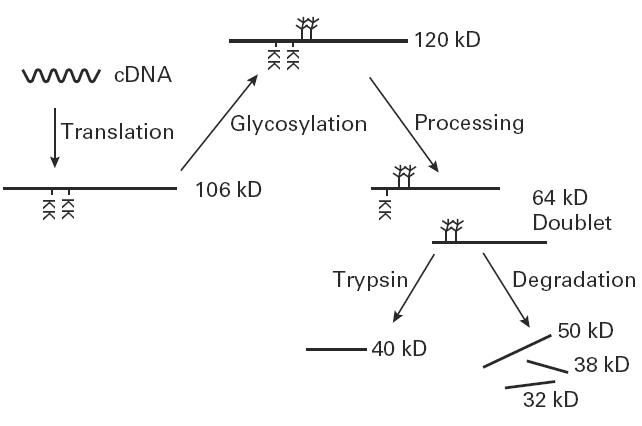
Post-translational modification of IA-2. Full-length IA-2 is processed by glycosylation and protease cleavage. The dibasic amino acids (KK) represent the potential cleavage sites from which the 64-kD doublet is generated. Trypsin treatment converts the 120-kD and 64-kD proteins into a 40-kD fragment.
Acknowledgments
The authors thank Ms Janice Solomon for her excellent editorial assistance.
References
- 1.Atkinson MA, Maclaren NK. The pathogenesis of insulin-dependent diabetes mellitus. N Engl J Med. 1994;331:1427–36. doi: 10.1056/NEJM199411243312107. [DOI] [PubMed] [Google Scholar]
- 2.Schatz D, Krischer J, Home G, et al. Islet cell antibodies predict insulin dependent diabetes in U.S. school age children as powerfully as in unaffected relatives. J Clin Invest. 1994;93:2403–7. doi: 10.1172/JCI117247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonifacio E, Bingley PJ, Shattock M, Dean BM, Dunger D, Gale EAM, Bottazzo GF. Quantification of islet-cell antibodies and prediction of insulin-dependent diabetes. Lancet. 1990;335:147–9. doi: 10.1016/0140-6736(90)90013-u. [DOI] [PubMed] [Google Scholar]
- 4.Christie MR, Tun RY, Lo SS, et al. Antibodies to GAD and tryptic fragments of islet 64K antigen as distinct markers for development of IDDM. Studies with identical twins. Diabetes. 1992;41:782–7. doi: 10.2337/diab.41.7.782. [DOI] [PubMed] [Google Scholar]
- 5.Christie MR, Genovese S, Cassidy D, Bosi E, Brown TJ, Lai M, Bonifacio E, Bottazzo GF. Antibodies to islet 37k antigen, but not to glutamate decarboxylase, discriminate rapid progression to IDDM in endocrine autoimmunity. Diabetes. 1994;43:1254–9. doi: 10.2337/diab.43.10.1254. [DOI] [PubMed] [Google Scholar]
- 6.Lan MS, Lu J, Goto Y, Notkins AL. Molecular cloning and identification of a receptor-type protein tyrosine phosphatase, IA-2, from human insulinoma. DNA Cell Biol. 1994;13:505–14. doi: 10.1089/dna.1994.13.505. [DOI] [PubMed] [Google Scholar]
- 7.Lan MS, Wasserfall C, Maclaren NK, Notkins AL. IA-2, a transmembrane protein of the protein tyrosine phosphatase family, is a major autoantigen in insulin-dependent diabetes mellitus. Proc Natl Acad Sci USA. 1996;93:6367–70. doi: 10.1073/pnas.93.13.6367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Notkins AL, Lu J, Li Q, VanderVegt FP, Wasserfall C, Maclaren NK, Lan MS. IA-2 and IA-2β are major autoantigens in IDDM and the precursors of the 40 kDa and 37 kDa tryptic fragments. J Autoimmun. 1997;9:677–82. doi: 10.1006/jaut.1996.0088. [DOI] [PubMed] [Google Scholar]
- 9.Rabin DU, Pleasic SM, Shapiro JA, Yoo-Warren H, Oles J, Hicks JM, Goldstein DE, Rae PMM. Islet cell antigen 512 is a diabetes-specific islet autoantigen related to protein tyrosine phosphatases. J Immunol. 1994;152:3183–8. [PubMed] [Google Scholar]
- 10.Lu J, Li Q, Xie H, Chen ZJ, Borovitskaya AE, Maclaren NK, Notkins AL, Lan MS. Identification of a second transmembrane protein tyrosine phosphatase, IA-2β, as an autoantigen in insulin-dependent diabetes mellitus: precursor of the 37 kDa tryptic fragment. Proc Natl Acad Sci USA. 1996;93:2307–11. doi: 10.1073/pnas.93.6.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seissler J, Morgenthaler NG, Achenback P, Lampeter EF, Glawe D, Payton M, Christie M, Scherbaum WA the DENIS Study Group. Combined screening for autoantibodies to IA-2 and antibodies to glutamic acid decarboxylase in first degree relatives of patients with IDDM. Diabetelogia. 1996;39:1351–6. doi: 10.1007/s001250050582. [DOI] [PubMed] [Google Scholar]
- 12.Payton MA, Hawkes CJ, Christie MR. Relationship of the 37,000- and 40, 000-Mr tryptic fragments of islet antigens in insulin-dependent diabetes to the protein tyrosine phosphatase-like molecule IA-2 (ICA512) J Clin Invest. 1995;96:1506–11. doi: 10.1172/JCI118188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Passini N, Larigan JD, Genovese S, Appella E, Sinigaglia F, Rogge L. The 37/40 kilodalton autoantigen in insulin-dependent diabetes mellitus is the putative tyrosine phosphatase IA-2. Proc Natl Acad Sci USA. 1995;92:9412–6. doi: 10.1073/pnas.92.20.9412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bonifacio E, Lampasona V, Genovese S, Ferrari M, Bosi E. Identification of protein tyrosine phosphatase-like IA-2 (islet cell antigen 512) as the insulin-dependent diabetes-related 37/40K autoantigen and a target of islet-cell antibodies. J Immunol. 1995;155:5419–26. [PubMed] [Google Scholar]
- 15.Xie H, Notkins AL, Lan MS. IA-2, a transmembrane protein tyrosine phosphatase, is expressed in human lung cancer cell lines with neuroendocrine phenotype. Cancer Res. 1996;56:2742–4. [PubMed] [Google Scholar]
- 16.Solimena M, Dirkx RJ, Hermel JM, Pleasic-Williams S, Shapiro JA, Caron L, Rabin DU. ICA 512, an autoantigen of type 1 diabetes, is an intrinsic membrane protein of neurosecretory granules. EMBO J. 1996;15:2101–14. [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang BW, Lan MS, Notkins AL. Autoantiboodies to IA-2 in IDDM: location of major antigenic determinants. Diabetes. 1997;46:40–43. doi: 10.2337/diab.46.1.40. [DOI] [PubMed] [Google Scholar]
- 18.Lampasona V, Bearzatto M, Genovese S, Bosi E, Ferrari M, Bonifacio E. Autoantibodies in insulin-dependent diabetes recognize distinct cytoplasmic domains of the protein tyrosine phosphatase-like IA-2 autoantigen. J Immunol. 1996;157:2707–11. [PubMed] [Google Scholar]
- 19.Christie MR, Hollands JA, Brown TJ, Michelsen BK, Delovitch TL. Detection of pancreatic islet 64,000 M(r) autoantigens in insulin-dependent diabetes distinct from glutamate decarboxylase. J Clin Invest. 1993;92:240–8. doi: 10.1172/JCI116556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baekkeskov S, Nielsen JH, Marner B, Bilde T, Ludvigsson J, Lernmark A. Autoantibodies in newly diagnosed diabetic children immunoprecipitate human pancreatic islet cell proteins. Nature. 1982;298:167–9. doi: 10.1038/298167a0. [DOI] [PubMed] [Google Scholar]
- 21.Smith DB, Johnson KS. Single-step purification of polypeptide expressed in E. coli as fusions with glutathione S-transferase. Gene. 1988;67:31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- 22.Plummer TH, Elder JH, Alexander S, Phelan AW, Tarentino AL. Demonstration of peptide: N-glycosidase F activity in endo-β-N-acetylgluco-saminidase F preparations. J Biol Chem. 1984;259:10700–4. [PubMed] [Google Scholar]
- 23.Tarentino AL, Gomez CM, Plummer TH. Deglycosylation of asparigine-linked glycans by peptide: N-glycosidase F. Biochem. 1985;24:4665–71. doi: 10.1021/bi00338a028. [DOI] [PubMed] [Google Scholar]
- 24.Lu J, Notkins AL, Lan MS. Isolation, sequence and expression of a novel mouse brain cDNA, mIA-2, and its relatedness to members of the protein tyrosine phosphatase family. Biochem Biophys Res Commun. 1994;204:930–6. doi: 10.1006/bbrc.1994.2549. [DOI] [PubMed] [Google Scholar]
- 25.O'Reilly DR, Miller LK, Luckow VA. Baculovirus expression vectors: a laboratory manual. New York: W.H. Freeman and Co; 1992. [Google Scholar]
- 26.Martin BM, Tsuji S, Lamarca ME, Maysak K, Eliason W, Ginns EI. Glycosylation and processing of high levels of active human glucocerebrosidase in invertebrate cells using a baculovirus expression vector. DNA. 1988;7:99–106. doi: 10.1089/dna.1988.7.99. [DOI] [PubMed] [Google Scholar]
- 27.Jarvis DL, Summers MD. Glycosylation and secretion of human tissue plasminogen activator in recombinant baculovirus-infected insect cells. Mol Cell Biol. 1989;9:214–23. doi: 10.1128/mcb.9.1.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Domingo DL, Trowbridge IS. Characterization of the human transferrin receptor produced in a baculovirus expression system. J Biol Chem. 1988;263:13386–92. [PubMed] [Google Scholar]
- 29.Jarvis DL, Finn EE. Biochemical analysis of the N-glycosylation pathway in baculovirus-infected lepidopteran insect cells. Virology. 1995;212:500–11. doi: 10.1006/viro.1995.1508. [DOI] [PubMed] [Google Scholar]
- 30.Leimer U, Franke WW, Leube RE. Synthesis of the mammalian synaptic vesicle protein synaptophysin in insect cells: a model for vesicle biogenesis. Exp Cell Res. 1996;224:88–95. doi: 10.1006/excr.1996.0114. [DOI] [PubMed] [Google Scholar]
- 31.Lebacq-Verheyden AM, Kasprzyk PG, Raum MG, Coelingh KVW, Lebacq JA, Battey JF. Posttranslational processing of endogenous and of baculovirus-expressed human gastrin-releasing peptide precursor. Mol Cell Biol. 1988;8:3129–35. doi: 10.1128/mcb.8.8.3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grabenhorst E, Hofer B, Nimtz M, Jager V, Conradt HS. Biosynthesis and secretion of human interleukin-2 glycoprotein variants from baculovirus-infected Sf21 cells—characterization of polypeptides and post-translational modifications. Eur J Biochem. 1993;215:189–97. doi: 10.1111/j.1432-1033.1993.tb18022.x. [DOI] [PubMed] [Google Scholar]
- 33.Halban PA, Irminger JC. Sorting and processing of secretory proteins. Biochem J. 1994;299:1–18. doi: 10.1042/bj2990001. [DOI] [PMC free article] [PubMed] [Google Scholar]


