Abstract
TNF-α is involved in infectious and immuno-inflammatory diseases. Different individuals may have different capacities for TNF-α production. This might determine a predisposition to develop some complications or phenotypes of these diseases. The aims of our study were to assess the inter-individual variability of TNF-α production and to correlate this variability to a single base pair polymorphism located at position −308 in TNF gene. We studied 62 healthy individuals. TNF-α production after LPS stimulation was evaluated using a whole blood cell culture model. The TNF gene polymorphism was studied by an allele-specific polymerase chain reaction. Other cytokines produced in the culture, soluble CD14 concentrations and expression of CD14 on blood cells were also measured. Among the 62 individuals, 57 were successfully genotyped. There were 41 TNF1 homozygotes and 16 TNF1/TNF2 heterozygotes. TNF-α production after LPS stimulation of whole blood cell culture was higher among TNF2 carriers than among TNF1 homozygotes (929 pg/ml (480–1473 pg/ml) versus 521 pg/ml (178–1307 pg/ml); P < 0.05). This difference was even more significant after correction of TNF-α production for CD14 expression on blood cells. In conclusion, the single base pair polymorphism at position −308 in the TNF gene may influence TNF-α production in healthy individuals.
Keywords: tumour necrosis factor, gene, whole blood, CD14
INTRODUCTION
TNF-α plays a key role in many infectious and inflammatory diseases. Acute infections and active inflammatory diseases are indeed usually associated with increased production of TNF-α [1–3]. In some illnesses such as septic shock, the role of TNF-α in the induction of the inflammatory cascade has been considered as predominant [4]. Accordingly, anti-TNF therapies have been successfully used in these infectious or inflammatory diseases [5–7].
Variations in the production of TNF-α after various stimuli in healthy subjects might explain predispositions to develop certain complications or phenotypes of infectious and immuno-inflammatory diseases. A low production of TNF-α after endotoxin stimulation was recently associated with a higher risk of fatal meningococcal disease [8]. Differences in the capacity for TNF-α production may be related to the genetic background. Several TNF gene polymorphisms have been described so far [9,10]. Among them the −308 single base pair polymorphism (SBPP) located in the promoter of the gene has been associated with severe cerebral malaria [11], susceptibility to lepromatous leprosy [12], scarring trachoma [13], mucocutaneous leishmaniasis [14] and possibly with some subgroups of Crohn's disease [15]. However, its functional significance remains unclear [16,17].
The whole blood cell culture is an elegant ex vivo technique to study cytokine production, because it keeps the blood microenvironment and avoids the extraction procedure associated with modification of cell ratios and activation [18]. The variation in cytokine production with this technique when individuals are sampled over time is < 15% [19]. It thus represents a technique of choice to investigate inter-individual variations in TNF-α production and its relationship to genetic background.
In vivo as well as in a whole blood assay, many other factors than a genetic polymorphism in the TNF gene may contribute to the difference observed in TNF-α production capacity. After stimulation with endotoxin, TNF-α production may be modulated by the level of expression of the CD14 on immune and inflammatory cells (mainly monocytes) [20], by the level of soluble CD14 in the milieu [21], as well as by the level of other modulatory cytokines [22].
The aims of our study were to assess the intersubject variations in TNF-α production after LPS stimulation in whole blood cell culture and to look for correlations between production rates and the −308 TNF gene polymorphism. We also studied other factors possibly influencing TNF-α production in a whole blood cell culture model including other cytokines produced in the microenvironment, cellular CD14 expression and soluble CD14 concentrations.
MATERIALS AND METHODS
Subjects
Sixty-two healthy subjects, members of the hospital and the laboratory staff, were included (26 men and 36 female; mean (range) age 36 years (22–52 years)). These subjects did not have any chronic illness and were not affected by any acute medical problem at the time of the study.
TNF-α production
To detect early production of TNF-α, that cytokine was measured by a modified one-step culture immunoassay procedure as previously described [23]. Briefly, 25 μl of whole blood or 25 μl of TNF-α standards were incubated in 200 μl RPMI (BioWhittaker, Verviers, Belgium) with or without 100 ng/ml (for the 3-h culture) or 1 ng/ml (for the 24-h culture) LPS (Salmonella enteritidis from Sigma, St Louis, MO) in sterile and pyrogen-free microplates coated with anti-TNF-α MoAbs (Biosource Europe, Fleurus, Belgium). Plates were capped and incubated at 37°C for 3 h or 24 h allowing the immunocapture of the produced cytokine. The caps were then removed and the wells washed intensively to remove unbound TNF-α. A horseradish peroxidase (HRP) anti-TNF-α conjugate MoAb (Biosource Europe) was then added to the plates for 1 h at room temperature with continuous shaking. After washing, 100 μl chromogen solution (TMB) were added to each well and the plates were incubated at room temperature with continuous shaking. Two hundred microlitres stop solution were then added to each well. The colour intensity determined by the absorbance at 450 nm being proportional to the TNF-α concentration in the sample, the exact TNF-α concentration in each sample was then calculated by interpolation from the standard curve.
That procedure was done once in 31 subjects and repeated two to five times at 2–3-week intervals in a subset of 31 subjects (14 male, 17 females, mean age 32 years (22–50 years); two measures, n = 18; three measures, n = 7; four measures, n = 2; five measures, n = 4). When several measures were performed, the result was expressed as the mean value.
IL-1β, IL-6, IL-10 and IL-12 production in whole blood cell culture
The blood was treated as previously described [18]. Blood samples were collected in apyrogenic heparinized tubes (Vacutainer; Becton Dickinson, Mountain View, CA). The blood was processed immediately and diluted with 1/10 RPMI 1640 supplemented with 2 mm glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin (Biowhittaker), and was then distributed in 2-ml wells. RPMI 1640 was tested for apyrogenicity. LPS (endotoxin from S. enteritidis; Sigma) was added at a final concentration of 1 ng/ml or 100 ng/ml. Plates were incubated at 37°C with 5% CO2 for 24 h. The contents of the wells were then collected and centrifuged at 900 g for 10 min. Supernatants were recovered and frozen at −20°C. IL-1β, IL-6, IL-12 and IL-10 concentrations were measured in the culture supernatants with specific immunoassay (EASIA from Biosource Europe). Immunoassays were performed according to the information sheet. The detection limit was 2 pg/ml for IL-1β, 2 pg/ml for IL-6, 3 pg/ml for IL-10, 1.5 pg/ml for IL-12. The measurement of IL-1β, IL-6, IL-12 and IL-10 was performed in 22 out of the 62 subjects.
Soluble CD14 measurement
The soluble CD14 was measured at time 0 before the beginning of the culture with specific immunoassay (EASIA from Biosource Europe). The immunoassay was performed according to the information sheet. The detection limit was 1 ng/ml. Measurement of soluble CD14 was performed in 32 out of the 62 subjects.
Cellular CD14 expression
CD14 expression on blood cells was assessed at time 0, before the beginning of the culture, by immunofluorescence staining. Blood cells were incubated with appropriate dilutions of FITC-conjugated anti-CD14 (anti-Leu-M3) for 40 min at 4°C. The cells were then extensively washed. Erythrocytes were haemolysed and the remaining cells were fixed in 1% paraformaldehyde. Percentage of positive cells and mean fluorescence intensity (MFI) were analysed by a FACScan (Becton Dickinson) gating on the total nucleated cells, as defined by forward and side light scatter. Ten thousand cells were counted. The percentage of CD14+ cells was assessed in 25 subjects and the MFI in 20 subjects.
TNFα−308 genotyping
The genotyping for the SBPP at position −308 in the TNF gene was done as previously described [24]. Briefly, a method involving primers specific for each allele of the G to A polymorphism at residue −308 was used. Four primers were used: the 3′ primer C1 (position −144/−164: 5′-TCTCGGTTTCTTCTCCATCG-3′) was used in combination with either the 5′ primer C2 (position −328/−308G: 5′-ATAGGTTTTGAGGGGCATGG-3′), complementary to the TNF-α 1 allele (TNF1), or the 5′ primer C3 (position − 308/−328A: 5′-ATAGGTTTTGAGGGGCATGA-3′), complementary to the TNF-α 2 allele (TNF2). For each DNA sample, two parallel reactions were performed. The primer pair C1/C2 was used to produce specific amplification of TNF1; C1/C3 were used to amplify the TNF2 allele. As an internal control, primer D (position −675/−655: 5′-GAGTCTCCGGGTCAGAATGA-3′) was added to each reaction. Amplification was carried out using the cycling conditions previously described [25]. The genotyping was performed in the 62 subjects, without information on the result of cytokine production in whole blood cell culture.
Statistical analysis
The reproducibility of TNF-α production over time in a subset of 31 subjects was assessed by the calculation of the coefficient of variation for each subject and expressed as the mean ± s.e.m. The production of TNF-α according to the −308 TNF genotypes was expressed as a median (range) and compared by using a Mann–Whitney test. The correlations between TNF-α production and the production of other cytokines or soluble and cellular CD14 were assessed by Spearman correlation test.
RESULTS
Intersubject and intrasubject variability of TNF-α production
The mean production of TNF-α in a 3-h whole blood cell culture after stimulation with 100 ng LPS was 182.9 pg/ml, with a range from 57 to 341 pg/ml. The mean amount of TNF-α produced in a 24-h whole blood cell culture after stimulation with 1 ng LPS was 720.4 pg/ml, with a range from 178 to 1473 pg/ml. The variability of production is illustrated in the histogram of Fig. 1. In the 31 subjects who had repeated determinations of TNF-α production after 24 h of culture, the mean coefficient of variation was 20.3 ± 2.7%.
Fig. 1.
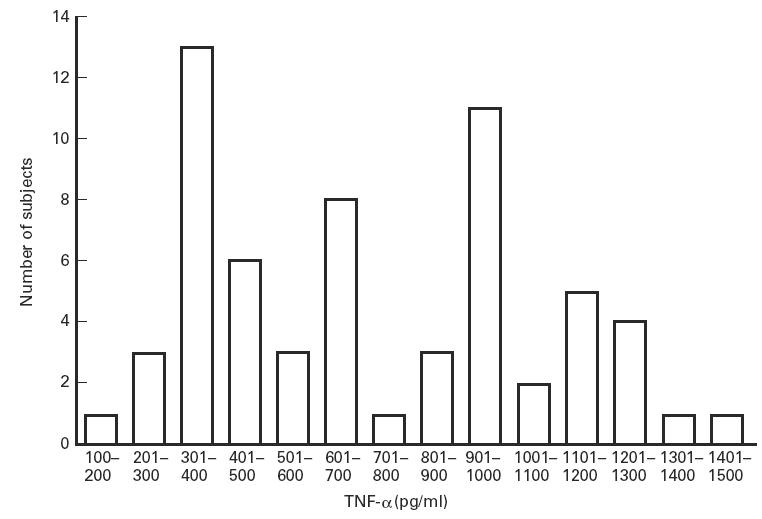
Variations in TNF-α production in healthy subjects. Whole blood cell cultures were stimulated with 1 ng/ml LPS and performed for 24 h. TNF-α production was measured by a one-step culture immuno-assay procedure.
Influence of other cytokines released in the culture medium
No significant correlation was found between TNF-α production and the production of IL-6 (r = 0.02; P = 0.93), IL-1β (r = −0.33; P = 0.34), IL-12 (r = −0.12; P = 0.60) or IL-10 (r = 0.37; P = 0.10) after 24 h (Fig. 2a–d).
Fig. 2.
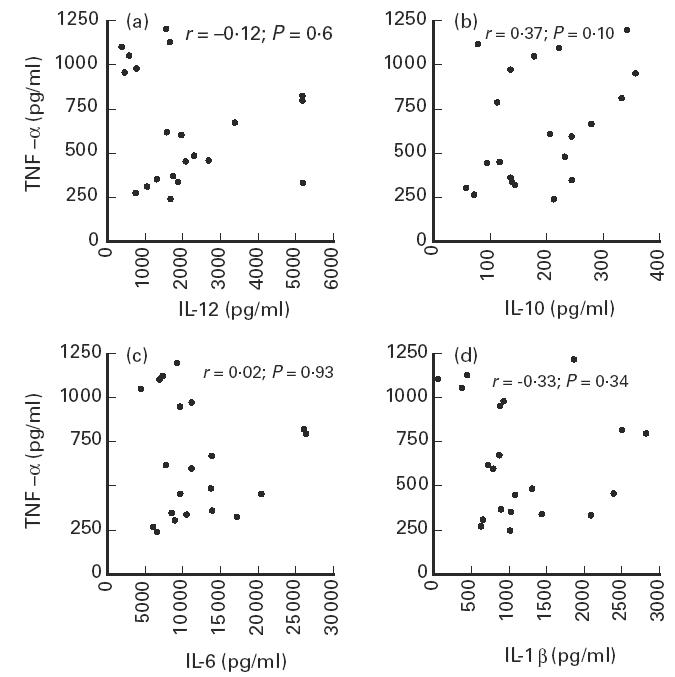
Correlation between TNF-α and IL-12 (a), IL-10 (b), IL-6 (c) and IL-1β (d) production in healthy subjects. Whole blood cell cultures were stimulated with 1 ng/ml (TNF-α, IL-12, IL-6, IL-1β) or 100 ng/ml (IL-10) of LPS and performed for 24 h. TNF-α production was measured by a one-step culture immunoassay procedure. IL-12, IL-10, IL-6 and IL-1β were measured in culture supernatants with specific immunoassays.
Influence of the soluble and cellular CD14
The mean plasma concentration of soluble CD14 in our population was 3243.6 ng/ml (range 1807–6400 ng/ml). No significant correlation was found between TNF-α production after 3 h and 24 h of culture and the level of soluble CD14 in the plasma at the beginning of the culture (r = 0.002, P > 0.05; and r = −0.18, P > 0.05, respectively).
The mean number of CD14+ cells was 0.368 × 109/l (range 0.149 × 109/l − 0.649 × 109/l). The number of CD14+ cells significantly correlated with the levels of TNF-α produced after 24 h (r = 0.59; P < 0.01) (Fig. 3a).
Fig. 3.
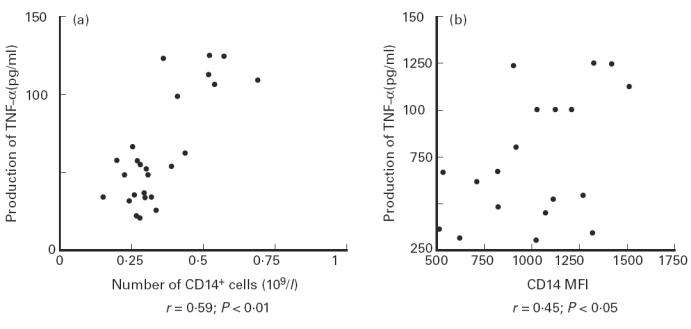
Correlation between TNF-α production and CD14 expression by mononuclear blood cells. Whole blood cell cultures were stimulated with 1 ng/ml LPS and performed for 24 h. TNF-α production was measured by a one-step culture immunoassay procedure. CD14 expression was assessed both by the number of CD14+ cells (a) and the intensity of CD14 expression (mean fluorescence intensity (MFI)) (b) before the beginning of the culture.
The CD14 cellular expression expressed as MFI was 1005.8 (range 517–1512). The MFI for CD14 significantly correlated with TNF-α production after 24 h (r = 0.45; P < 0.05) (Fig. 3b).
Influence of the −308 TNF genotype
Among the 62 subjects, 57 were successfully genotyped. Forty-one were homozygotes for TNF1 while 16 were heterozygotes TNF1/TNF2. There was no TNF2 homozygote. TNF-α production in a 24-h whole blood cell culture after LPS stimulation was significantly higher among TNF2 carriers than among TNF1 homozygotes (929 pg/ml (480–1473 pg/ml) versus 521 pg/ml (178–1307 pg/ml); P < 0.05) (Fig. 4). After 3 h of culture there was already a higher production of TNF-α among TNF2 carriers (203.3 pg/ml (109–341 pg/ml) versus 174.0 pg/ml (57–323 pg/ml); P = 0.16).
Fig. 4.
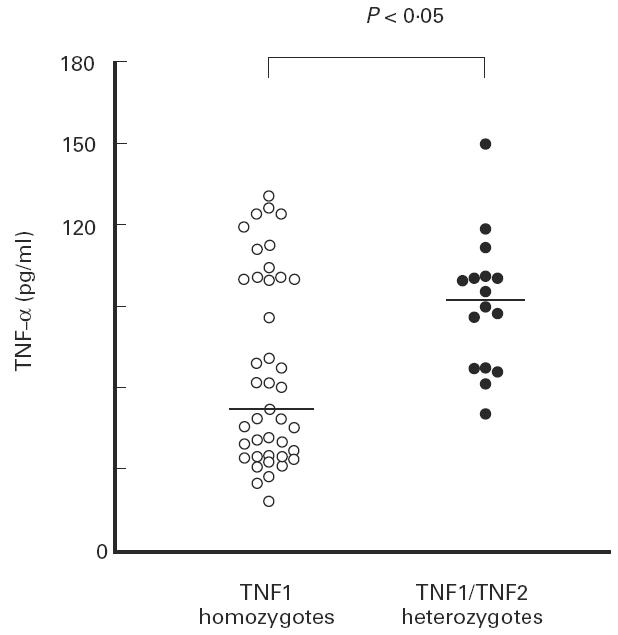
Production of TNF-α in healthy subjects according to the −308 TNF gene polymorphism. Whole blood cell cultures were stimulated with 1 ng/ml LPS and performed for 24 h. TNF-α production was measured by a one-step culture immunoassay procedure.
After correction for CD14 expression (number of CD14+ cells and MFI), TNF-α production was even more significantly higher among the TNF2 carriers than among TNF1 homozygotes (2.8 × 10−3 pg/cell × fluorescence unit (1.6–4.3) versus 1.7 × 10−3 pg/cell × fluorescence unit (0.5–2.6); P < 0.01).
DISCUSSION
We have shown a wide intersubject variability in TNF-α production after LPS stimulation in whole blood cell cultures of healthy subjects. TNF-α production correlated with the number of blood cells expressing CD14, the intensity of CD14 expression and depended on the −308 TNF genotype. The influence of TNF genotype was even more striking after correction of the amount of TNF-α production for the CD14 expression.
An intersubject variability in TNF-α production has been shown in several studies using different methods [26–28]. This has most often been shown on isolated monocytes or peripheral blood mononuclear cells (PBMC). The whole blood cell culture avoids change in cell activation due to extraction procedures, does not modify the lymphocyte/monocyte ratio and preserves cell–cell interactions and concentrations of stimulatory and inhibitory mediators [18]. Thus it is reasonable to think that this model better reflects how cells behave in vivo than the classical PBMC model. Our results show that the whole blood of different healthy subjects exhibits a wide range of TNF-α production after LPS stimulation. Theoretically, this can represent an important element determining the type of immune or inflammatory reaction elicited in vivo by bacterial LPS.
The intersubject variability in such a whole blood cell culture model may be determined by several factors. LPS activates the cells through binding to CD14. CD14 is mainly expressed by monocytes [29] and the level of expression depends on cell maturation [30] and stimulation [31]. Most primary blood monocytes express a high level of CD14 [32]. In the present study, TNF-α production was significantly correlated with the number of CD14+ cells in the blood, confirming thereby previous data from the literature [18]. In addition, we have also shown a significant relationship between the MFI for CD14 cellular expression and TNF-α production. Our observation is in line with a previous study looking at highly purified monocyte subpopulations and showing a higher cytokine production after LPS stimulation from the monocyte subset expressing greater levels of CD14 [33].
Soluble CD14 is shed from cells expressing CD14 [34] and can bind LPS. Its precise physiological or pathological role is still unknown but it might antagonize the effect of LPS stimulation [21]. In our study, however, there was no significant correlation between plasma levels of CD14 and TNF-α production. This could be due to the fact that the neutralizing capacity requires much higher concentrations [35].
Another soluble factor, the lipopolysaccharide binding protein (LBP), plays a key role in the stimulation of CD14+ cells by LPS [36]. By binding the LPS and forming a LBP/LPS complex, it increases the capacity of LPS to stimulate TNF-α production by 1000-fold [36]. LBP is mainly produced by the liver and its concentration may rise by a factor of 10 during acute-phase reactions [37]. It certainly would be interesting in the future to measure this molecule in our whole blood cell culture model.
The other cytokines released in the culture medium may also influence TNF-α production. IL-1β and IL-12 are considered to be strong stimulants of TNF-α production [22,38], while IL-6 and especially IL-10 may have strong inhibitory effects [22,39]. In the present study, however, there was no correlation between production of TNF-α and other cytokines, suggesting that none of those cytokines strongly influenced TNF-α production in our model. However, we can not exclude a slight regulatory effect that does not appear clearly because of the complexity of the factors involved.
Studies on the intersubject variations in TNF-α production have often been associated with HLA [27] or TNF genotyping [17,28]. A possible prominent influence of the genetic background on TNF-α production implies that it remains stable in an individual over time. As already shown, the reproducibility of cytokine production by PBMC of healthy subjects may vary depending on the method of stimulation [40]. The results obtained with whole blood cell culture were shown to be more reproducible [18,19]. In the present study, the 31 subjects who had repeated measurement of TNF-α production in whole blood cell culture after LPS stimulation had a low coefficient of variation, confirming that this model represents a good model to study the influence of genetic background on TNF-α production. The possible functional significance of the −308 SBPP in the TNF gene has been suggested by its position in a non-coding, potentially regulatory area [10], its association with various infectious diseases characterized by high TNF-α production [11–14] and with different gene transcription capacity [16]. In the present study the carriers of the TNF2 allele had a higher production of TNF-α after 24 h of whole blood cell culture. These results are in accordance with the previous clinical [11–14] and transcription [16] studies suggesting an increased capacity of TNF-α production in TNF2 carriers. However, in a more recent study also using whole blood cell cultures [8], this difference related to genotype was not present. This may be due to the complexity of the model, with many other factors influencing the production of TNF-α. As shown in the present study, the difference between groups with different genotypes was more pronounced when a correction was made for some of these other influencing factors, such as CD14 expression.
In conclusion, our results suggest that besides other parameters such as CD14 expression by blood mononuclear cells, the −308 TNF gene polymorphism may influence the magnitude of TNF-α production in whole blood cell culture after LPS stimulation. In healthy subjects, a genetically determined capacity for TNF-α production may predispose to some complications or phenotypes of infectious and immuno-inflammatory diseases. Further studies are required to elucidate these points.
References
- 1.Beutler B, Milsark IW, Cerami AC. Passive immunization against cachectin/tumor necrosis factor protects mice from lethal effect of endotoxin. Science. 1997;229:869–71. doi: 10.1126/science.3895437. [DOI] [PubMed] [Google Scholar]
- 2.Arditi M, Manogue KR, Caplan M, Yogev R. Cerebrospinal fluid cachectin/tumor necrosis factor-α and platelet-activating factor concentrations and severity of bacterial meningitis in children. J Infect Dis. 1990;162:139–47.. doi: 10.1093/infdis/162.1.139. [DOI] [PubMed] [Google Scholar]
- 3.Reinecker HC, Steffen M, Witthoeft T, et al. Enhanced secretion of tumour necrosis factor alpha, IL-6 and IL-1 beta by isolated lamina propria mononuclear cells from patients with ulcerative colitis and Crohn's disease. Clin Exp Immunol. 1993;94:174–81. doi: 10.1111/j.1365-2249.1993.tb05997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Glauser MP, Zanetti G, Baumgartner JD, Cohen J. Septic shock: pathogenesis. Lancet. 1991;338:732–6. doi: 10.1016/0140-6736(91)91452-z. [DOI] [PubMed] [Google Scholar]
- 5.Van Dullemen HM, Van Deventer SJH, Hommes DW, Bijl HA, Jansen J, Tytgat GNJ, Woody J. Treatment of Crohn's disease with anti-tumor necrosis factor monoclonal antibody (cA2) Gastroenterology. 1995;109:129–35. doi: 10.1016/0016-5085(95)90277-5. [DOI] [PubMed] [Google Scholar]
- 6.Elliott MJ, Maini RN, Feldmann M, et al. Randomised double-blind comparison of chimeric monoclonal antibody to tumor necrosis factor α (cA2) versus placebo in rheumatoid arthritis. Lancet. 1994;344:1105–10.. doi: 10.1016/s0140-6736(94)90628-9. [DOI] [PubMed] [Google Scholar]
- 7.Tracey KJ, Fong Y, Hesse DG, Manogue KR, Lee AT, Kuo GC, Lowry SF, Cerami A. Anti-cachectin/TNF monoclonal antibodies prevent septic shock during lethal bacteremia. Nature. 1987;330:662–4.. doi: 10.1038/330662a0. [DOI] [PubMed] [Google Scholar]
- 8.Westendorp RGJ, Langermans JAM, Huizinga TWJ, Elouali AH, Verweij CL, Boomsma DI, Vandenbrouke JP. Genetic influence on cytokine production and fatal meningococcal disease. Lancet. 1997;349:170–3. doi: 10.1016/s0140-6736(96)06413-6. [DOI] [PubMed] [Google Scholar]
- 9.Nedospasov SA, Udalova IA, Kuprash DV, Turetskaya RL. DNA sequence polymorphism at the human tumor necrosis factor (TNF) locus. Numerous TNF/lymphotoxin alleles tagged by two closely linked microsatellites in the upstream region of the lymphotoxin gene. J Immunol. 1991;147:1053–9. [PubMed] [Google Scholar]
- 10.Wilson AG, de Vreis N, Pociot F, di Giovine FS, van der Putte LBA, Duff GW. An allelic polymorphism within the human tumor necrosis factor alpha promoter region is strongly associated with HLA A1, B8, and DR3 alleles. J Exp Med. 1993;177:557–60. doi: 10.1084/jem.177.2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McGuire W, Hill AVS, Allsopp CEM, Greenwood BM, Kwiatkowski D. Variation in the TNF-alpha promoter region associated with susceptibility to cerebral malaria. Nature. 1994;371:508–11. doi: 10.1038/371508a0. [DOI] [PubMed] [Google Scholar]
- 12.Roy S, McGuire W, Mascie-Taylor CGN, Saha B, Hazra SK, Hill AVS, Kwiatowski D. Tumor necrosis factor promoter polymorphism and susceptibility to lepromatous leprosy. J Infect Dis. 1997;176:530–2.. doi: 10.1086/517282. [DOI] [PubMed] [Google Scholar]
- 13.Conway DJ, Holland MJ, Bailey RL, Campbell AE, Mahdi OSM, Jennings R, Mibena E, Mabey DCW. Scarring trachoma is associated with polymorphism in the tumor necrosis factor alpha (TNF-α) gene promoter and with elevated TNF-α levels in tear fluid. Infect Immun. 1997;65:1003–6. doi: 10.1128/iai.65.3.1003-1006.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cabrera M, Shaw MA, Sharples C, Williams H, Castes M, Convit J, Blackwell JM. Polymorphism in tumor necrosis factor genes associated with mucocutaneous leishmaniasis. J Exp Med. 1995;182:1259–64. doi: 10.1084/jem.182.5.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Louis E, Satsangi J, Roussomoustakaki M, Parkes M, Fanning G, Welsh K, Jewell D. Cytokine gene polymorphisms in inflammatory bowel disease. Gut. 1996;39:705–10. doi: 10.1136/gut.39.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilson AG, Symons JA, McDowell TL, McDevitt HO, Duff GW. Effects of a polymorphism in the human tumor necrosis factor α promoter on transcriptional activation. Proc Natl Acad Sci USA. 1997;94:3195–9. doi: 10.1073/pnas.94.7.3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brinkman BMN, Zuijdgeest D, Kaijzel EL, Breedveld FC, Verweij CL. Relevance of the tumor necrosis factor-alpha (TNF-alpha) −308 promoter polymorphism in TNF-alpha gene regulation. J Inflam. 1996;46:32–41. [PubMed] [Google Scholar]
- 18.De Groote D, Zangerle PF, Gevaert Y, et al. Direct stimulation of cytokines (IL-1 beta, TNF-alpha, IL-6, IL-2, IFN-gamma and GM-CSF) in whole blood. I. Comparison with isolated PBMC stimulation. Cytokine. 1992;4:239–48. doi: 10.1016/1043-4666(92)90062-v. [DOI] [PubMed] [Google Scholar]
- 19.Entzian P, Linnemann K, Schlaak M, Zabel P. Obstructive sleep apnea syndrome and circadian rhythms of hormones and cytokines. Am J Respir Crit Care Med. 1996;153:1080–6. doi: 10.1164/ajrccm.153.3.8630548. [DOI] [PubMed] [Google Scholar]
- 20.Ziegler-Heitbrock HWL, Ulevitch RJ. CD14: cell surface receptor and differentiation marker. Immunol Today. 1993;14:121–5. doi: 10.1016/0167-5699(93)90212-4. [DOI] [PubMed] [Google Scholar]
- 21.Schutt C, Schilling T, Grunwald U, Schonfeld W, Kruger C. Endotoxin-neutralizing capacity of soluble CD14. Res Immunol. 1992;143:71–78. doi: 10.1016/0923-2494(92)80082-v. [DOI] [PubMed] [Google Scholar]
- 22.Manogue KR, Van Deventer SJH, Cerami A. Tumor necrosis factor alpha or cachectin. In: Thomson AW, editor. The cytokine handbook. London: Academic Press; 1991. pp. 241–56. [Google Scholar]
- 23.De Groote D, Gevaert Y, Lopez M, et al. Novel method for the measurement of cytokine production by a one-stage procedure. J Immunol Methods. 1993;163:259–67. doi: 10.1016/0022-1759(93)90130-y. [DOI] [PubMed] [Google Scholar]
- 24.Verjans GM, Brinkman BMN, van Doornik CEM, Kijlstra A, Verweij CL. Polymorphism of tumor necrosis factor-alpha at position −308 in relation ankylosing spondylitis. Clin Exp Immunol. 1994;97:45–47.. doi: 10.1111/j.1365-2249.1994.tb06577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bunce M, Taylor CJ, Welsh KI. Rapid HLA-DQB typing by eight PCR amplifications with sequence-specific primers (PCR-SSP) Hum Immunol. 1993;37:201–6. doi: 10.1016/0198-8859(93)90502-r. [DOI] [PubMed] [Google Scholar]
- 26.Jacob CO, Fronek Z, Lewis GD, Koo M, Hansen JA, McDevitt HO. Heritable major histocompatibility complex class II-associated differences in production of tumor necrosis factor alpha: prevalence to genetic predisposition to systemic lupus erythematosus. Proc Natl Acad Sci USA. 1990;87:1233–7. doi: 10.1073/pnas.87.3.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pociot F, Briant L, Jongeneel CV, et al. Association of tumor necrosis factor (TNF) and class II major histocompatibility complex alleles with the secretion of TNF-α and TNF-β by human mononuclear cells: a possible link to insulin-dependent diabetes mellitus. Eur J Immunol. 1993;23:224–31. doi: 10.1002/eji.1830230135. [DOI] [PubMed] [Google Scholar]
- 28.Plevy SE, Targan SR, Rotter JI, Toyoda H. Tumor necrosis factor microsatellite associations within HLADR2+ patients define Crohn's disease and ulcerative colitis specific genotypes. Gastroenterology. 1994;106:A754. [Google Scholar]
- 29.Goyert SM, Ferrero EM, Seremetis SV, Winchester RJ, Silver J, Mattisson AC. Biochemistry and expression of myelomonocytic antigens. J Immunol. 1986;137:3909–14. [PubMed] [Google Scholar]
- 30.Trinchieri G, Rosen M, Perussia B. Induction of differentiation of human myeloid cell lines by tumor necrosis factor in cooperation with 1 alpha, 25-dihydroxyvitamin D3. Cancer Res. 1987;47:2236–42. [PubMed] [Google Scholar]
- 31.Lauener RP, Goyert SM, Geha RS, Vercelli D. Interleukin-4 down-regulates the expression of CD14 in normal human monocytes. Eur J Immunol. 1990;20:2375–81. doi: 10.1002/eji.1830201103. [DOI] [PubMed] [Google Scholar]
- 32.Passlick B, Flieger D, Ziegler-Heitbrock HWL. Identification and characterization of a novel monocyte subpopulation in human peripheral blood. Blood. 1989;74:2527–34. [PubMed] [Google Scholar]
- 33.Ziegler-Heitbrock HWL, Ströbel M, Kieper D, et al. Differential expression of cytokines in human blood monocyte subpopulations. Blood. 1992;79:503–11. [PubMed] [Google Scholar]
- 34.Bazil V, Strominger JL. Shedding as a mechanism of down-modulation of CD14 on stimulated human monocytes. J Immunol. 1991;147:1567–74. [PubMed] [Google Scholar]
- 35.Haziot A, Rong GW, Bazil V, Silver J, Goyert SM. Recombinant soluble CD14 inhibits LPS induced tumor necrosis factor-alpha produced by cells in whole blood. J Immunol. 1994;152:5868–76. [PubMed] [Google Scholar]
- 36.Martin TR, Mathison JC, Tobias PS, Leturcq DJ, Moriarty AM, Maunder RJ, Ulevitch RJ. Lipopolysaccharide binding protein enhances the responsiveness of alveolar macrophages to bacterial lipopolysaccharide. Implication for cytokine production in normal and injured lungs. J Clin Invest. 1992;90:2209–19. doi: 10.1172/JCI116106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tobias PS, Soldau K, Ulevitch RJ. Isolation of a lipopolysaccharide-binding acute phase reactant from rabbit serum. J Exp Med. 1986;164:777–93. doi: 10.1084/jem.164.3.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Trinchieri G. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu Rev Immunol. 1995;13:251–76. doi: 10.1146/annurev.iy.13.040195.001343. [DOI] [PubMed] [Google Scholar]
- 39.de Waal Malefyt R, Abrams J, Bennett B, Figdor CG, de Vries JE. Interleukin-10 (IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med. 1991;174:1209–20. doi: 10.1084/jem.174.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Danis VA, Millington M, Hyland VJ, Grennan D. Cytokine production by normal human monocytes: inter-subject variations and relationship to an IL-1 receptor antagonist (IL-1Ra) gene polymorphism. Clin Exp Immunol. 1995;99:303–10. doi: 10.1111/j.1365-2249.1995.tb05549.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


