Abstract
The present study shows that Langerhans cells of the buccal mucosa and the skin share a similar phenotype, including in situ expression of MHC class II, the mannose receptor DEC-205 and CD11c, and absence of the costimulatory molecules B7.1, B7.2 and CD40 as well as Fas. Application of 2,4-dinitrofluorobenzene (DNFB) onto the buccal mucosa is associated with a rapid migration of dendritic cells (DC) to the epithelium and induction of B7.2 expression on some DC. Buccal sensitization with DNFB elicited a specific contact sensitivity (CS) in response to skin challenge, mediated by class I-restricted CD8+ effector T cells and down-regulated by class II-restricted CD4+ T cells, demonstrated by the lack of priming of class I-deficient mice and the enhanced response of class II-deficient mice, respectively. CS induced by buccal immunization is associated with priming of class I-restricted CD8+ effector T cells endowed with hapten-specific cytotoxic activity. Thus, the buccal epithelium is an inductive site, equivalent to the epidermis, for the generation of CS independent of CD4 help, and of cytotoxic T lymphocyte (CTL) responses mediated by class I-restricted CD8+ T cells. We propose that immunization through the buccal mucosa, which allows antigen presentation by epithelial DC efficient for priming systemic class I-restricted CD8+ CTL, may be a valuable approach for single-dose mucosal vaccination with subunit vaccines.
Keywords: epithelial dendritic cells, buccal mucosa, CD8 T cells, contact sensitivity
INTRODUCTION
The generalized mucosal immune network, comprising mucosal-associated lymphoid tissues (MALT), provides immune responses not only at the site of antigen exposure but also at remote mucosal sites [1–3]. Despite considerable evidence from animal and human studies that antigen delivery at mucosal sites triggers mucosal B cells and humoral responses, verification that MALT serves as an initiation site for T cell-mediated immunity, including contact sensitivity (CS) responses, has been limited by the lack of an appropriate animal model.
The oral mucosa is exposed to a diversity of environmental antigens, including dietary antigens, chemicals and pathogenic organisms, that are ingested or inhaled and thus gain access to the aerodigestive tract. The stratified squamous keratinizing epithelium lining the buccal mucosa exerts a major barrier function, preventing antigen absorption and the outcome of untoward immune responses. However, the presence within the suprabasal epithelial cell layer of a network of MHC class II+ CD1a+ dendritic cells (DC) expressing electron dense lysosomes and Birbeck granules, and analogous to that of Langerhans cells (LC) in the skin [4,5], suggests that the buccal mucosa represents a functional compartment of the MALT. This hypothesis has been supported by our recent observation that the murine buccal mucosa serves as an inductive site for local and remote hapten-specific CS responses [6] and that MHC class II+ DC represent the efficient antigen-presenting cells (APC) of the buccal mucosa [7]. Hence, we observed that within hours after either transepithelial injection of native protein or peptide, or after topical application of haptens onto the buccal epithelium, a substantial number of DC leave the buccal epithelium and migrate to draining cervico-mandibullary lymph nodes, where they acquire the capacity to activate naive or antigen-specific T cells [7]. Furthermore, DC isolated from draining lymph nodes 24 h following buccal sensitization with haptens can adoptively transfer specific skin CS responses to naive recipient mice [4].
The aim of this study was to investigate whether the buccal mucosa represents a potential route of immunization efficient for priming antigen-specific CD8+ T cell-mediated immune responses. We demonstrate that buccal immunization with the hapten 2,4-dinitrofluorobenzene (DNFB) generates a specific CS response mediated by CD8+ effector T cells, independently of CD4 help, and is associated with efficient priming of hapten-specific MHC class I-restricted CD8+ cytotoxic T cells (CTL).
MATERIALS AND METHODS
Mice
Female BALB/c and C57Bl/6 mice purchased from Iffa Credo (l'Arbresle, France) were used at 6–8 weeks of age. MHC class II-deficient mice (Aβ°/°) [8] as well as MHC class I-deficient mice (β2m°/°) [9] and, respectively, heterozygous control mice (Aβ+/° and β2m+/°), all on a C57Bl/6 (H2b) background, were obtained from Diane Mathis and Christophe Benoist (Inserm U184, Strasbourg, France) and bred in the Transgenic Alliance animal facility (Iffa Credo).
Haptens
DNFB, 2,4-dinitrobenzene sulfonate (DNBS), 2,4,6 trinitrochlorobenzene (TNCB) and 2,4,6-trinitrobenzene sulfonate (TNBS) were obtained from Sigma (St Quentin Fallavier, France). Oxazolone (4-ethoxy-methylene-2-phenyloxazol-5-1) (OXA) was purchased from Aldrich (Milwaukee, WI).
Antibodies
The panel of mouse-specific MoAbs used included: a rat anti-MHC class II (CD311) [10], a rat anti-B7.1 (CD80) MoAb (clone 1G10) (ATCC, Rockville, MD), a rat anti-B7.2 (CD86) MoAb (clone GL1), a hamster anti-CD40 MoAb and FITC-conjugated anti-Fas MoAb (all from Pharmingen, Clinisciences, France), the rat anti-DEC-205 MoAb [11] (NLDC-145, a gift from G. Kraal, University, Amsterdam, The Netherlands), an anti-CD11c MoAb (N418) [12] (ATCC), a PE-conjugated anti-CD4 (GK1.5) and FITC-conjugated anti-CD8a MoAbs (YTS 169.4) (both from Caltag, Tebu, France). The anti-CD8 MoAb YTS 169.4 (a gift from C. Benoist, Strasbourg, France) and the anti-CD4 MoAb GK1.5 (a gift from M. Pierres, Marseille, France) were used as hybridoma supernatant for the in vitro depletion of CD8 and CD4 cells, respectively.
Immunohistochemical staining of epithelial sheets and cryostat sections of buccal mucosa
Epithelial sheets. The inner part of the cheeks was excised from the mice and the remaining outer skin and muscle layer was carefully dissected out, as previously described [7]. Briefly, the cheeks were incubated for 2 h at 37°C in PBS supplemented with 20 mm EDTA [13]. Skin epithelial sheets were prepared from ears by a 2-h incubation in 0.25% trypsin solution in PBS as already described [14]. For immunohistochemical analysis, the sheets were incubated overnight at 4°C with specific antibodies or isotype-matched irrelevant antibodies and specific staining was revealed using biotinylated rabbit anti-rat IgG (H + L) (Vector, Biosys, France) or goat F(ab′)2 anti-hamster IgG (Pierce, Interchim, France), followed by streptavidin conjugated to peroxidase (ABC kit; Dako, France). The reaction was developed using AEC substrate and H2O2 (Dako).
Cryostat sections. The cheeks were excised as described above, embedded in OCT compound and frozen in liquid nitrogen. Cryostat sections (5 μm thick) were acetone-fixed and stained for 1 h with either CD311, B7.1 or B7.2 MoAbs (10 μg/ml). Specific binding was revealed by immunoenzymatic staining as described above. The sections were counterstained with Harris haematoxylin.
No staining was observed when the primary antibody was omitted or substituted by an irrelevant rat or hamster IgG antibody.
Skin and buccal sensitizations
Mice were sensitized on day 0 with DNFB applied topically either on the abdominal skin or on the oral mucosa as described [6,15]. Briefly, for skin sensitization, 2 cm2 of shaved abdominal skin was painted with 25 μl of 0.5%, 1% or 2% of DNFB in acetone/olive oil (4:1, v/v). For buccal sensitization, 12.5 μl of 0.5%, 1% or 2% of DNFB in acetone/olive oil (4:1, v/v) were applied onto the inner face of both cheeks.
Elicitation and measure of contact sensitivity
Five days after sensitization, all the mice were challenged with 4 μl of 0.2% DNFB or 0.4% OXA (for hapten specificity controls) in acetone/olive oil applied on each side of the right ear, while the left ear received only the acetone/olive oil. These challenge dose of haptens were selected from the results of dose–response experiments, and were devoid of irritant activity. The ear thickness increment was measured at various time points after challenge using callipers. The results are expressed as mean ear swelling (Δe) calculated as previously described [15], namely: Δe (μm) = increment of right ear — increment of left ear, where ear increment = thickness after challenge — thickness before challenge.
Hapten-specific T cell proliferation assay
T cells were partially purified from the spleen of DNFB-sensitized mice using an anti-immunoglobulin column (Cedarlane, Tebu, France) as previously described [15]. The resulting cell suspensions contained > 90% viable CD3+ Thy-1+ cells from which CD8+ and CD4+ T cells were purified by negative selection using specific MoAbs including, respectively, the anti-CD8a (YTS169.4) and the anti-CD4 (GK1.5) MoAbs and magnetic beads coated with anti-rat antibodies (Dynal, France). This procedure led to the obtention of highly enriched (70–80%) populations of CD4+ and CD8+ T cells, the rest comprising 15–25% granulocytes and < 5% B220+ B cells. T cells (2.5 × 105 cells/well of microculture plates) were co-cultured in RPMI 1640 medium supplemented with 10% fetal calf serum (FCS), 2 mml-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, 1 mm sodium pyruvate, 0.1 mm non-essential amino acids, 10 mm HEPES and 2.10−5 m of 2-mercaptoethanol (2-ME), with mitomycin-treated syngeneic spleen cells previously hapten-derivatized by 20 min incubation at 37°C with 4 mm of either DNBS or TNBS as previously reported [16]. Hapten-specific T cell proliferation was determined on day 3 of culture by 3H-thymidine incorporation (1 μCi/well) during the last 6 h of culture. The results are expressed as ct/min ± s.d.
Hapten-specific CTL assay
Spleen cell suspensions were prepared from DNFB-sensitized mice by carefully teasing the tissue using needles. Cellular debris was eliminated by gentle centrifugation at 1000 rev/min, and total spleen cells (25 × 106) were restimulated in vitro by co-cultivation at 37°C in a tissue culture flask with mitomycin-treated syngeneic spleen cells (25 × 106) either untreated or derivatized with DNBS or TNBS. The viable cells recovered on day 5 were then tested for their cytolytic activity against untreated or haptenated EL-4 cells using the 51Cr-release assay as already described [17]. Briefly, log dilutions of effector cells were plated on round-bottomed microculture plates and the EL-4 target cells (104), previously labelled by a 90-min incubation at 37°C with 50 μCi of Na251CrO4, were added to each well. The plates were then incubated at 37°C for 4 h and the radioactivity released into the supernatant was counted using a gamma counter. The results are expressed as percentage specific lysis ± s.d. calculated as follows: (ct/min test — spontaneous ct/min)/(maximal ct/min — spontaneous ct/min) × 100.
Statistical analysis
Statistical analyses were performed using Student's t-test.
RESULTS
Phenotype of Langerhans cells of the buccal mucosa
Immunohistochemical analysis of buccal epithelial sheets using the anti-MHC class II MoAb (CD311) revealed a network of DC extending their slender processes between adjacent epithelial cells (Fig. 1a), similar to the network of class II-positive epidermal LC of the skin (Fig. 1d). The buccal epithelial DC exhibited a phenotype characteristic of epidermal LC, including expression of the MHC class II molecule (Fig. 1a,d), the CD11c molecule (Fig. 1b,e) as well as the mannose receptor DEC-205 (Fig. 1c,f). Note that conversely to class II expression, CD11c staining of both mucosal and skin DC is more prominent on the dendrites. In contrast, DC present in the skin or buccal mucosal epithelium did not express the costimulatory molecules B7.1 (CD80) and B7.2 (CD86), CD40 or Fas (not shown). These data show that DC of the buccal epithelium have a phenotype of immature DC similar to that of epidermal LC.
Fig. 1.
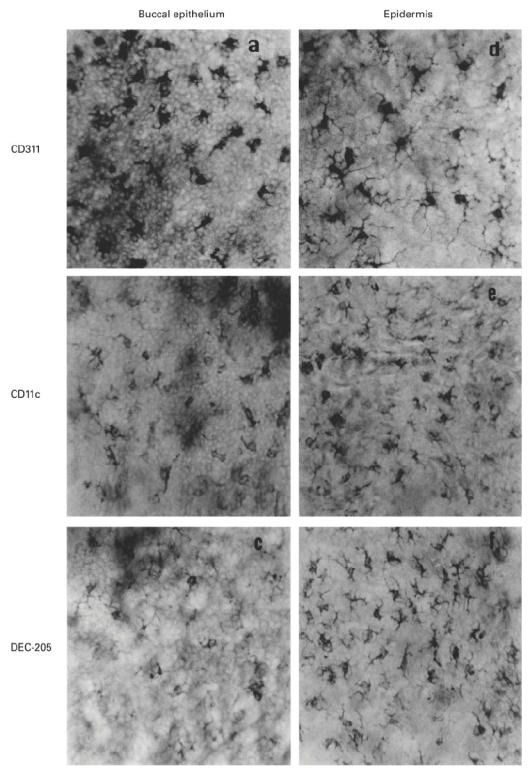
Immunohistochemical analysis of dendritic cells (DC) of the buccal and skin epithelium. Epithelial sheets from the buccal mucosa (a,b,c) and the skin (d,e,f) from BALB/c mice were stained with MoAbs specific for MHC class II (CD311) (a and d), CD11c (N418) (b and e) and the mannose receptor DEC-205 (NLDC-145) (c and f). Staining is exclusively observed on cells with dendritic morphology. (Final mag. × 400.)
Buccal sensitization with DNFB induces DC migration and up-regulation of B7.2 expression on DC
Immunohistochemical analysis of cryostat sections of the buccal mucosa revealed the presence in naive mice of class II+ DC in suprabasal layers of the epithelium, in lamina propria and in the submucosa (Fig. 2a). In contrast, DC expressing a low density of the B7.2 molecule were occasionally found in the lamina propria, but not in the epithelium (Fig. 2b), consistent with the inability to detect them in epithelial sheets. Within 2 h following DNFB application onto the buccal mucosa, we observed a marked increase in the proportion of class II+ DC migrating to the epithelium (Fig. 2c), coincident with an increase of B7.2+ cells in the lamina propria (more rarely in the epithelium), extending slender cytoplasmic processes towards the suprabasal epithelium (Fig. 2d). At 24 h after DNFB application, class II+ DC had left the epithelium and reached the lamina propria and the submucosa, where they were clearly enriched, compared with naive mice (Fig. 2e). A similar dynamic was observed for B7.2+ DC (Fig. 2f). B7.1+ DC were never found in either naive or hapten-painted mice. These data show that DC are activated and recruited into the buccal epithelium, shortly after DNFB application onto the buccal mucosa.
Fig. 2.
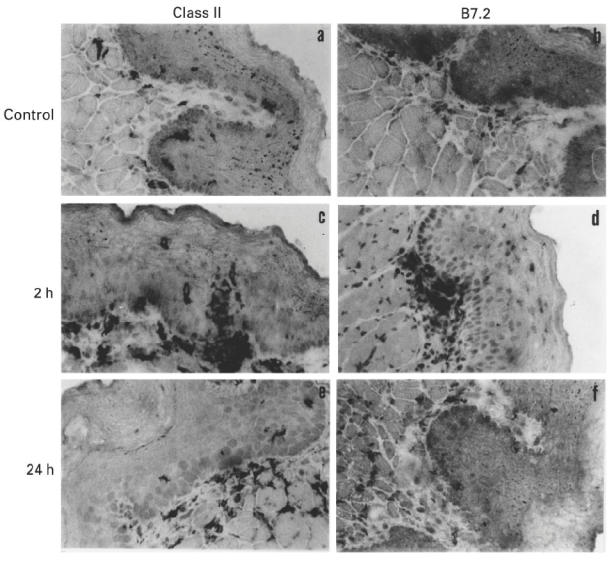
Up-regulation of B7.2 expression on dendritic cells (DC) after 2,4-dinitrofluorobenzene (DNFB) application onto the buccal mucosa. Cryostat sections (5 μm thick) from the buccal mucosa of C57Bl/6 mice either untreated (a,b), or 2 h (c,d) or 24 h (e,f) after buccal sensitization with DNFB were stained for MHC class II (CD311) (a,c,e) or B7.2 (CD86, clone GL1) (b,d,f) molecule expression. Sections were counterstained with Harris haematoxylin. (Final mag. × 400.)
Dose-dependant CS response to DNFB induced by buccal or skin sensitization
We compared the kinetics and dose–response of CS to DNFB induced by either skin or buccal sensitization. The CS induced by epicutaneous application of DNFB in both BALB/c (Fig. 3a) and C57Bl/6 (not shown) mice was optimal at the sensitizing dose of 0.5% (P < 0.05 compared with 1% or 2% DNFB), with maximal ear swelling at 24–48 h following DNFB challenge. Similarly in both BALB/c (Fig. 3b) and C57Bl/6 mice (not shown), buccal immunization with 0.5% DNFB induced the highest DNFB-specific CS response which peaked at 48–72 h (P < 0.05 compared with 1% or 2% DNFB). The DNFB-specific CS response induced by either buccal or skin sensitization was always higher in BALB/c (150 μm) compared with C57Bl/6 mice (70 μm). These data confirm that the buccal mucosa is an inductive site of CS to haptens, and that the inflammatory response generated by the buccal route has a dose–effect and kinetics similar to those induced by cutaneous immunization.
Fig. 3.
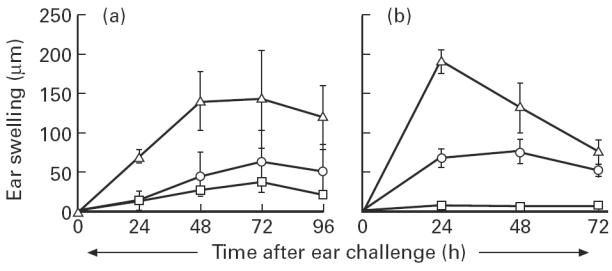
Dose–effect and kinetics of the contact sensitivity (CS) to 2,4-dinitrofluorobenzene (DNFB) generated by buccal or epicutaneous sensitization. BALB/c mice were sensitized by topical application on the buccal mucosa (a) or on abdominal skin (b) of 0.5% (▵), 1% (○) or 2% (□) of DNFB diluted in acetone:olive oil. Five days later all mice were challenged on the right ear with 0.2% DNFB. The skin CS response was evaluated by the increment in ear thickness at various days after challenge. The results (mean ± s.d.) are representative of one experiment out of three using six mice per group. Ear swelling was < 10 μm in unsensitized mice that were ear-challenged with DNFB and in DNFB-sensitized mice that were ear-challenged with the irrelevant hapten oxazolone (not shown).
CD8+ T cells mediate CS induced by buccal immunization with DNFB
We previously reported that CS to DNFB induced by skin immunization of C57Bl/6 mice required the MHC class I/CD8 T cell pathway, and was mediated by CD8+ effector T cells [18]. The buccal mucosa and the epidermis both contain a network of antigen-presenting LC and are endowed with the ability to induce CS in response to skin challenge with haptens. We therefore investigated whether CD8+ T cells are the effector cells mediating CS induced by mucosal sensitization, using β2m°/° mutant mice, which lack MHC class I molecules and (class I-restricted) CD8+ T cells [9]. As shown in Fig. 4a, β2m+/° heterozygous control mice developed a CS response to DNFB following buccal application of the hapten, which was comparable to that observed in haplotype-matched C57Bl/6 mice (not shown). In contrast, no CS response could be induced by mucosal immunization in β2m°/° mice (Fig. 4a) (P < 0.001 compared with β2m+/° mice). These data demonstrate that the skin CS response induced by buccal immunization with DNFB is mediated by class I-restricted CD8+ T cells.
Fig. 4.
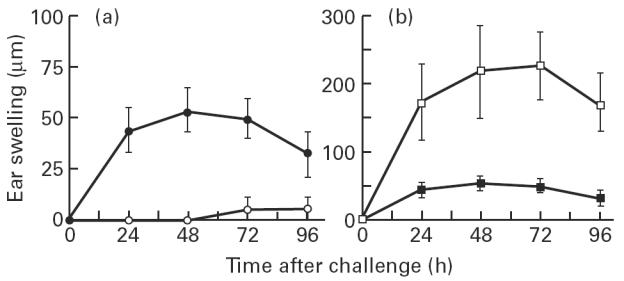
Contact sensitivity (CS) induced by buccal immunization with 2,4-dinitrofluorobenzene (DNFB) is mediated by class I-restricted CD8+ effector T cells, independently of CD4+ T cells. (a) Heterozygote β2m+/° (•) or homozygote β2m°/° (○) mice and (b) heterozygote Aβ+/° (▪) or homozygote Aβ°/° (□) mice were buccally sensitized with 0.5% of DNFB and ear-challenged 5 days later with 0.2% DNFB. Ear swelling, expressed as mean ± s.d. of six mice per group, was determined at various time points after challenge. The data shown are representative of two to three experiments.
CD8+ T cell-mediated CS induced by buccal immunization is independent of CD4+ T cells
In the light of our previous observation that CD4+ T cells can act as regulatory cells of CS to DNFB following skin immunization [18], we next examined whether these cells could similarly regulate the intensity of the DNFB response generated by buccal immunization. As shown in Fig. 4b, Aβ°/° mutant mice, which lack MHC class II molecules and have a drastic reduction in CD4+ T cells [8], developed a CS response to buccal application of DNFB which was four times higher than that of Aβ+/° control mice (P < 0.001). These data show that CD4+ T cells down-regulate the contact hypersensitivity response induced by buccal mucosa sensitization with DNFB.
Buccal sensitization with DNFB induces activation of hapten-specific CD8+ T cells
Our observation that MHC class I-deficient mice, which have a profound defect in CD8+ T cells, are unable to develop a CS response to immunization with DNFB, led us to ask whether hapten-specific CD8+ T cells are induced by buccal immunization with DNFB. We analysed the ability of splenic CD8+ T cells, purified from C57Bl/6 mice on day 5 after immunization, to proliferate in response to DNBS-derivatized syngeneic spleen cells. As shown in Fig. 5, CD8+ T cells from DNFB-primed mice proliferated in response to in vitro stimulation with DNBS. No proliferation was observed in response to either untreated or TNBS-derivated spleen cells. Likewise, T cells from naive mice did not proliferate upon in vitro culture with DNBS or TNBS-derivatized syngeneic spleen cells (not shown). Thus, buccal immunization with DNFB is able to prime hapten-specific CD8+ T cells which are present in the spleen on day 5 after immunization, i.e. at the time of skin challenge with DNFB.
Fig. 5.
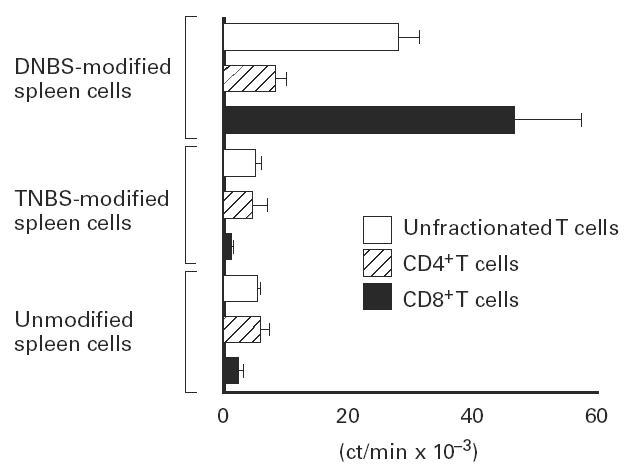
Buccal immunization can prime hapten-specific CD8+ T cells in vivo. Unfractionated T cells, CD4+ or CD8+ T cells were purified from C57Bl/6 mice spleens on day 5 after buccal sensitization with 2,4-dinitrofluorobenzene (DNFB) and restimulated in vitro for 3 days in the presence of either unmodified, 2,4-dinitrobenzene sulfonate (DNBS)-modified or 2,4,6-trinitrobenzene sulfonate (TNBS)-modified syngeneic spleen cells. T cell proliferation was determined by 3H-thymidine uptake over the last 6 h of culture. The result represents mean ± s.d. of quadruplicate wells and is representative of two experiments.
Buccal immunization with DNFB primes for MHC class I-restricted CD8+ CTL
We next examined whether the buccal epithelial mucosa is an inductive site for the hapten-specific CTL response. The presence of hapten-specific CTL activity in the spleen was analysed on day 5 after buccal application of either DNFB or acetone/olive oil, following in vitro restimulation with DNBS-derivatized syngeneic spleen cells. No CTL activity could be detected in control mice, indicating that DNBS stimulation in vitro was unable to prime naive T cells from unimmunized mice (not shown). In contrast, mice immunized by buccal application of DNFB developed a hapten-specific CTL response, as shown by the specific lysis of DNBS-treated EL-4 cells only. No lysis of either TNBS-derivatized or untreated EL-4 cells was observed (Fig. 6a). In order to determine whether this hapten-specific CTL activity was mediated by class I-restricted CD8+ T cells, the CTL response was analysed in MHC class I-deficient mice on day 5 after buccal DNFB sensitization. As shown in Fig. 6b, no CTL activity against DNBS-derivatized EL-4 cells could be induced in β2m°/° mice after buccal DNFB immunization, compared with normal mice. This clearly demonstrates that the hapten-specific CTL generated by buccal immunization is mediated by class I-restricted CD8+ effector CTL.
Fig. 6.
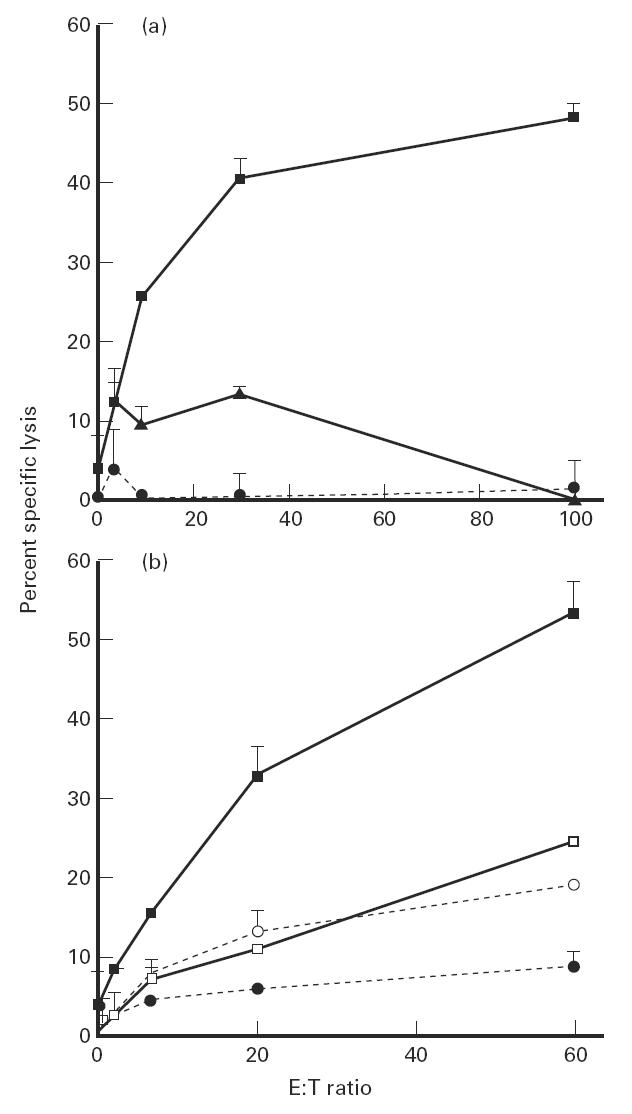
Buccal immunization induces specific class I-restricted CD8+ cytotoxic T lymphocytes (CTL). (a) Pooled spleen cells from either C57Bl/6 mice day 5 after buccal sensitization with 2,4-dinitrofluorobenzene (DNFB) were restimulated for 6 days with syngeneic 2,4-dinitrobenzene sulfonate (DNBS)-modified spleen cells. DNBS-specific CTL activity was determined by testing specific lysis of 51Cr EL-4 target cells that were either unmodified (•), DNBS-modified (▪) or 2,4,6-trinitrobenzene sulfonate (TNBS)-modified (▴). (b) Pooled spleen cells from MHC class I-deficient mice β2m°/° (□,○), or β2m+/° heterozygote control (▪,•) mice were restimulated for 6 days with syngeneic DNBS-modified spleen. DNBS-specific CTL activity was determined by testing lysis of unmodified (○,•) or DNBS-modified (□,▪) 51Cr EL-4 target cells.
DISCUSSION
The oral mucosa represents a unique mucosal site where DC located in the epithelium are directly accessible to foreign antigen and may thus represent a novel immunization route for efficient priming of immune responses against pathogens.
In this study, we observed that the buccal mucosa contains a network of DC present in the suprabasal layers of the buccal epithelium as well as in the lamina propria and in the underlying submucosa. Buccal epithelial DC exhibit a phenotype similar to that of epidermal LC, including expression of high levels of MHC class II molecules, a low density of CD11c and of the mannose receptor DEC-205, but lack expression of the costimulatory molecules B7.1, B7.2, CD40 and Fas (APO-1). Although CD11c has been identified primarily on mature interdigitating DC present in secondary lymphoid organs [11,12], this molecule is also expressed on immature DC localized in the buccal mucosa and skin epithelia (this study) and on DC located in the dome epithelium of Peyer's patches in the intestine [19]. Similarly, DEC-205, a mannose receptor molecule recognized by the MoAb NLDC-145 [20], is present on both mature DC in the T cell area of spleen, lymph nodes and Peyer's patches [11,19] and on epithelial DC of the skin and the buccal mucosa (this study).
Mucosal DC from the respiratory tract [21], the intestine [22] and the oral cavity [5,7], as well as epidermal LC [23,24], have the property to capture antigen and migrate to the paracortical zone of draining lymph nodes, where they represent professional APC efficient for priming naive T cells. The stimulatory property of DC is due to acquisition of costimulatory molecules, including B7.1 and B7.2, during DC maturation upon culture with granulocyte-macrophage colony-stimulating factor (GM-CSF) [25] or initiated by hapten activation and DC transport from epithelial tissues to draining lymph nodes [26]. Although B7.1 and B7.2 are not constitutively expressed on DC present in the buccal or skin epithelium, a limited number of B7.2+ DC could be occasionally detected in the lamina propria of the buccal mucosa. Interestingly, buccal DNFB sensitization resulted 2 h later in the recruitment of class II+ DC infiltrating the epithelium and in the induction of B7.2 molecule on DC, as revealed by the concomitant increase in the number of B7.2+ cells in the lamina propria and the submucosa (and occasionally in the epithelium). Double staining showed that B7.2+ DC co-expressed class II molecules (data not shown). Within 24 h after buccal sensitization, class II+ DC had returned to the suprabasal epithelium and had infiltrated the submucosa. These observations are in line with the up-regulation of B7.2, but not B7.1, described for dermal DC after primary application of DNFB on the skin [26]. Antibody blocking experiments have shown that B7.2 is involved during the afferent phase of CS to DNFB induced by skin sensitization [26–28]. Since buccal DC exhibit phenotypic, dynamic and functional characteristics similar to those of skin LC, it is possible that induction of B7.2 on buccal DC may be involved in T cell priming and then generation of CS by buccal sensitization.
We have recently demonstrated that the buccal epithelium serves as an inductive site for both local and remote CS reactions [6]. We now show that the CS induced by buccal immunization with the hapten DNFB shares similar dose–response, kinetics and mechanisms to those observed after epicutaneous sensitization. The inability of MHC class I-deficient mice to develop a skin CS after buccal immunization with DNFB indicates that CD8+, but not CD4+ T cells, are responsible for the inflammatory response generated by buccal sensitization. Moreover, the observation that the CS induced by buccal DNFB sensitization can develop in MHC class II-deficient mice, which have a defect in CD4+ T cells, demonstrates that the CS response is independent of CD4 help. Alternatively, the increased and prolonged CS response in these mice indicates that class II-restricted CD4+ T cells down-regulate CS induced by buccal immunization. These findings are reminiscent of our previous studies demonstrating that epicutaneous sensitization with DNFB in C57Bl/6 mice induces a specific CS mediated by class I-restricted CD8+ effector T cells, independent of CD4 help, and that the function of CD4+ T cells is largely focused on negatively regulating the magnitude and the duration of the response [18]. More recently, studies confirming and extending these data showed that interferon-gamma (IFN-γ)-producing CD8+ CD44hi T cells could adoptively transfer CS to OXA to naive recipient mice and that the size of the memory CD8+ T cell pool developed independently of CD4+ T cells [29].
The CS generated by buccal immunization with DNFB is associated with priming of hapten-specific CD8+ T cells in secondary lymphoid organs, as shown by the ability of T cells isolated from the spleen on day 5 after buccal sensitization with DNFB (i.e. at the time of the skin challenge) to proliferate in response to hapten-specific in vitro restimulation. This proliferation resulted from in vivo activation of hapten-specific T cells, in as much as no proliferation occurred upon in vitro hapten stimulation of T cells from naive control mice. The higher proliferative response obtained with purified CD8+ T cells compared with either CD4+ T cells or unfractionated T cells, suggests that buccal sensitization favoured the development of memory CD8+ T cells. In addition, the fact that hapten-specific CTL were generated in the spleen on day 5 after buccal sensitization in C57Bl/6, but not in syngeneic MHC class I-deficient mice, demonstrated that CS induced by a single buccal application of DNFB is associated with hapten-specific class I-restricted CD8+ CTL. Whether such hapten-specific CTL activity is involved in the mechanism of CS deserves further investigation.
Buccal epithelial DC have been described to exert a potent accessory cell function, as revealed by their ability to support T cell proliferation in response to concanavalin A (Con A) in vitro [4]. We now show that buccal sensitization efficiently primes class I-restricted CTL responses mediated by CD8+ T cells. That exogenous antigens such as haptens can be presented by MHC class I molecules and activate class I-restricted CD8+ T cells may be explained by several mechanisms. Haptens, including DNFB, are low molecular weight chemicals which bind covalently to discrete amino acid residues of proteins (i.e. lysine in the case of DNFB), and are recognized by the T cell receptor as hapten-modified peptides presented in the groove of MHC class I and class II molecules of specialized APC [30,31]. Thus, DNFB which is liposoluble may gain access to the cytoplasm of buccal DC and bind to self proteins which are processed into class I peptides. Another possibility is that DNFB binds to extracellular proteins and/or cellular debris which are endocytosed by mucosal DC and processed into peptides within phagolysosomes and may gain access to the MHC class I pathway, after either lysis or fusion of phagolysosomes with recycling class I vesicles or regurgitation outside the cell and binding to surface class I molecules [32]. Such cross-priming of CTL responses by exogenous antigens has been described to function in phagocytic cells, including DC [33].
In conclusion, our data demonstrate that the buccal mucosa is an inductive site for the efficient priming of systemic CD8+ T cell-mediated CS and class I-restricted CTL responses in vivo. We propose that immunization through the buccal mucosa, which allows the delivery of antigens to anatomically accessible LC, represents a novel route of immunization which may be valuable for single-dose mucosal vaccination against pathogens.
Acknowledgments
This work was supported by grants from ARC contract 6384, ‘Les amis de l'Institut Pasteur de Lyon’ and the ‘Fondation pour la Recherche Médicale’.
References
- 1.Butcher EC. The regulation of lymphocyte traffic. Curr Top Microbiol Immunol. 1986;128:85–95. doi: 10.1007/978-3-642-71272-2_3. [DOI] [PubMed] [Google Scholar]
- 2.Gowans JL, Knight EJ. The route of re-circulation of lymphocytes in the rat. Proc R Soc Lond B. 1964;159:257–3. doi: 10.1098/rspb.1964.0001. [DOI] [PubMed] [Google Scholar]
- 3.McDermott MR, Bienenstock J. Evidence for a common mucosal immunological system. I. Migration of B immunoblasts into intestinal, respiratory, and genital tissues. J Immunol. 1979;122:1892–7. [PubMed] [Google Scholar]
- 4.Barrett AW, Ross DA, Goodacre JA. Purified human oral Langerhans cells function as accessory cells in vitro. Clin Exp Immunol. 1993;92:158–3. doi: 10.1111/j.1365-2249.1993.tb05963.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van Wilsem EJG, Van Hoogstraten IMW, Brevé J, et al. Dendritic cells of the oral mucosa and the induction of oral tolerance. A local affair. Immunol. 1994;83:128–2. [PMC free article] [PubMed] [Google Scholar]
- 6.Ahlfors E, Czerkinsky C. Contact sensitivity in the murine oral mucosa. I. An experimental model of delayed-type hypersensitivity reactions at mucosal surfaces. Clin Exp Immunol. 1991;86:449–56. doi: 10.1111/j.1365-2249.1991.tb02952.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eriksson K, Ahlfors E, George-Chandy A, et al. Antigen presentation in the murine oral epithelium. Immunol. 1996;88:147–2. doi: 10.1046/j.1365-2567.1996.d01-647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cosgrove D, Gray D, Dierich A, et al. Mice lacking MHC class-II molecules. Cell. 1991;66:1051–6. doi: 10.1016/0092-8674(91)90448-8. [DOI] [PubMed] [Google Scholar]
- 9.Koller BH, Marrack P, Kappler JW, et al. Normal development of mice deficient in β2m, MHC class I proteins, and CD8+ T cells. Science. 1990;248:1227–1. doi: 10.1126/science.2112266. [DOI] [PubMed] [Google Scholar]
- 10.Vidal K, Samarut C, Magaud JP, et al. Unexpected lack of reactivity of allogeneic anti-IA monoclonal antibodies with MHC class II molecules expressed by mouse intestinal epithelial cells. J Immunol. 1993;151:1–9. [PubMed] [Google Scholar]
- 11.Kraal G, Breel M, Janse M, et al. Langerhans cells, veiled cells, and interdigitating cells in the mouse recognized by a monoclonal antibody. J Exp Med. 1986;163:981–7. doi: 10.1084/jem.163.4.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Metlay JP, Witmer-Pack MD, Agger R, et al. The distinct leukocyte integrins of mouse spleen dendritic cells as identified with new hamster monoclonal antibody. J Exp Med. 1990;171:1753–2. doi: 10.1084/jem.171.5.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Juhlin L, Shelley W. New staining techniques for the Langerhans cell. Acta Dermatovener. 1977;57:289–94. [PubMed] [Google Scholar]
- 14.Peguet-Navarro J, Dalbiez-Gauthier C, Dezutter-Dambuyant C, et al. Dissection of human Langerhans cell allostimulatory function: the need for an activation step for full development of accessory function. Eur J Immunol. 1993;23:376–0. doi: 10.1002/eji.1830230212. [DOI] [PubMed] [Google Scholar]
- 15.Galliaerde V, Desvignes C, Peyron E, et al. Oral tolerance to haptens: intestinal epithelial cells from 2,4-dinitrochlorobenzene-fed mice inhibit hapten-specific T cell activation in vitro. Eur J Immunol. 1995;25:1385–0. doi: 10.1002/eji.1830250537. [DOI] [PubMed] [Google Scholar]
- 16.Desvignes C, Bour H, Nicolas JF, et al. Lack of oral tolerance but oral priming for contact sensitivity to dinitrofluorobenzene in major histocompatibility complex class II-deficient mice and in CD4+ T cell-depleted mice. Eur J Immunol. 1996;26:1756–1. doi: 10.1002/eji.1830260814. [DOI] [PubMed] [Google Scholar]
- 17.Etchart N, Wild F, Kaiserlian D. Mucosal and systemic immune responses to measles virus haemagglutinin in mice immunized with a recombinant vaccinia virus. J Gen Virol. 1996;77:2471–8. doi: 10.1099/0022-1317-77-10-2471. [DOI] [PubMed] [Google Scholar]
- 18.Bour H, Peyron E, Gaucherand M, et al. Major histocompatibility complex class I-restricted CD8+ T cells and class II-restricted CD4+ T cells, respectively, mediate and regulate contact sensitivity to dinitrofluorobenzene. Eur J Immunol. 1995;25:3006–0. doi: 10.1002/eji.1830251103. [DOI] [PubMed] [Google Scholar]
- 19.Kelsall BL, Strober W. Distinct populations of dendritic cells are present in the subepithelial dome and T cell regions of the murine Peyer's patch. J Exp Med. 1996;183:237–7. doi: 10.1084/jem.183.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiang W, Swiggard WJ, Heufler C, et al. The receptor DEC-205 expressed by dendritic cells and thymic epithelial cells is involved in antigen processing. Nature. 1995;375:151–5. doi: 10.1038/375151a0. [DOI] [PubMed] [Google Scholar]
- 21.Xia W, Pinto CE, Kradin RL. The antigen-presenting activities of Ia+ dendritic cells shift dynamically from lung to lymph node after an airway challenge with soluble antigen. J Exp Med. 1995;181:1275–3. doi: 10.1084/jem.181.4.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu LM, MacPherson GG. Antigen acquisition by dendritic cells: intestinal dendritic cells acquire antigen administered orally and can prime naive T cells in vivo. J Exp Med. 1993;177:1299–7. doi: 10.1084/jem.177.5.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kripke ML, Munn CG, Jeevan A, et al. Evidence that cutaneous antigen-presenting cells migrate to regional lymph nodes during contact sensitization. J Immunol. 1990;145:2833–8. [PubMed] [Google Scholar]
- 24.Macatonia SE, Knight SC, Edwards AJ, et al. Localization of antigen on lymph node dendritic cells after exposure to the contact sensitizer fluorescein isothiocyanate. J Exp Med. 1987;166:1654–7. doi: 10.1084/jem.166.6.1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Symington FW, Brady W, Linsley PS. Expression and function of B7 on human epidermal Langerhans cells. J Immunol. 1993;150:1286–5. [PubMed] [Google Scholar]
- 26.Nuriya S, Yagita H, Okumura K, et al. The differential role of CD86 and CD80 co-stimulatory molecules in the induction and the effector phases of contact hypersensitivity. Int Immunol. 1996;8:917–26. doi: 10.1093/intimm/8.6.917. [DOI] [PubMed] [Google Scholar]
- 27.Reiser H, Schneeberger EE. Expression and function of B7-1 and B7-2 in hapten-induced contact sensitivity. Eur J Immunol. 1996;26:880–5. doi: 10.1002/eji.1830260424. [DOI] [PubMed] [Google Scholar]
- 28.Xu H, Heeger PS, Fairchild RL. Distinct role for B7-1 and B7-2 determinants during priming of effector CD8+ Tc1 and regulatory CD4+ Th2 cells for contact sensitivity. J Immunol. 1997;159:4217–26. [PubMed] [Google Scholar]
- 29.Xu H, Banerjee A, Dilulio NA, et al. Development of effector CD8+ T cells in contact hypersensitivity occurs independently of CD4+ T cells. J Immunol. 1997;158:4721–8. [PubMed] [Google Scholar]
- 30.Lepoittevin JP, Leblond I. Hapten-peptide-T cell receptor interactions: molecular basis for the recognition of haptens by lymphocytes. Eur J Dermatol. 1997;7:151–4. [Google Scholar]
- 31.Martin S, Weltzien HU. T cell recognition of haptens, a molecular view. Int Arch Allergy Immunol. 1994;104:10–6. doi: 10.1159/000236703. [DOI] [PubMed] [Google Scholar]
- 32.Rock KL. A new foreign policy: MHC class I molecules monitor the outside world. Immunol Today. 1996;17:131–7. doi: 10.1016/0167-5699(96)80605-0. [DOI] [PubMed] [Google Scholar]
- 33.Jondal M, Schirmbeck R, Reimann J. MHC class I-restricted CTL responses to exogenous antigens. Immunity. 1996;5:295–2. doi: 10.1016/s1074-7613(00)80255-1. [DOI] [PubMed] [Google Scholar]


