Abstract
The pathogenesis of thrombocytopenia associated with TAD and the occurrence of overlapping traits between TAD and AITP are still a matter of debate. For this reason, we investigated for the presence and specificity of platelet and thyroid autoantibodies in 18 TAD patients with thrombocytopenia, 19 TAD patients without thrombocytopenia and in 22 patients with primary AITP without clinical signs of TAD. Platelet-associated IgG and/or specific circulating platelet autoantibodies were detected in 83% of patients with TAD and thrombocytopenia, in 10% of patients with TAD without thrombocytopenia and in 86% of patients with primary AITP. The reactivity of serum autoantibodies, assayed by MoAb immobilization of platelet antigens (MAIPA), was directed against platelet glycoproteins Ib and/or IIb/IIIa in 50% of the patients with TAD and thrombocytopenia, as in 46% of the patients with primary AITP. Thyroid autoantibodies were found in 89% of patients with TAD and thrombocytopenia, in 95% of patients with TAD without thrombocytopenia, and in 18% of patients with primary AITP. Thyrotropin (TSH) levels determined in three of four AITP patients with thyroid autoantibodies revealed a subclinical hyperthyroidism in one patient. The present study supports the autoimmune aetiology of thrombocytopenia associated with TAD, since the prevalence and specificity of platelet autoantibodies are similar in TAD and primary AITP. The results indicate also that there exists an overlap between thyroid and platelet autoimmunity with or without clinical manifestations.
Keywords: immune thrombocytopenia, thyroid autoimmune diseases, platelet autoantibodies, platelet glycoproteins, thyroid autoantibodies
INTRODUCTION
AITP is a form of destructive thrombocytopenia which can be either idiopathic, also known as primary AITP, or secondary to other autoimmune disorders. AITP is characterized by an increased platelet destruction mediated by autoantibodies directed against platelet surface antigens [1].
The application of sensitive and specific glycoprotein immobilization assays has led to the finding that autoantibodies from AITP patients are prevalently IgG directed against epitopes located on platelet surface glycoproteins (gp) Ib/IX, IIb/IIIa, IV and Ia/IIa [2–6]. We recently demonstrated that, regardless of the presence or absence of thrombocytopenia, platelet autoantibodies are present in systemic lupus erythematosus (SLE) and in primary antiphospholipid syndrome, and, as in patients with primary AITP, are directed against the same antigens [7]. Thrombocytopenia may also be observed in patients with TAD, especially those with hyperthyroidism [8–18].
It was advanced that in thyroid diseases thrombocytopenia might be secondary to an activation of the reticulo-endothelial phagocyte system [11,19] by thyroid hormones or to a platelet immune destruction [12,15,20].
For a better understanding of the pathogenesis of thrombocytopenia associated with TAD and the frequency of overlapping autoimmune features with AITP, we investigated the presence and specificity of platelet and thyroid autoantibodies in 18 TAD patients with thrombocytopenia, in 19 TAD patients without thrombocytopenia and in 22 patients with primary AITP.
PATIENTS AND METHODS
Patients
We retrospectively analysed the clinical records of the in-patients admitted to our Department during the 1995–96 period. We selected three groups of patients.
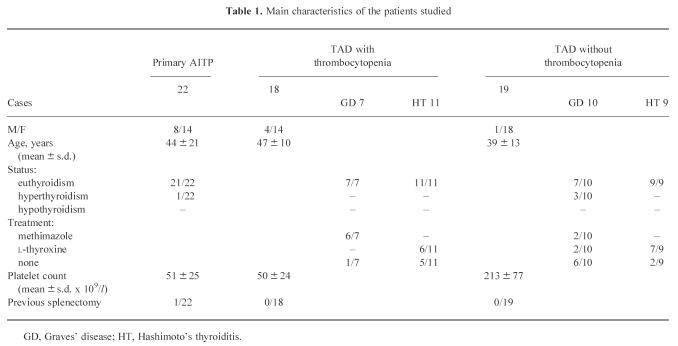
The first group included 18 patients suffering from TAD and thrombocytopenia (platelet counts < 100 × 109/l); seven patients had Graves' disease (GD; two males and five females) and 11 had Hashimoto's thyroiditis (HT; two males and nine females). At the time of the study, six GD patients were on methimazole and T3, T4 and thyrotropin (TSH) levels were in normal range; one patient was in remission without therapy; six HT patients were receiving substitutive doses of l-thyroxine and five were euthyroid without therapy. The second group included 19 patients with TAD without thrombocytopenia; 10 had GD (one male and nine females), and nine had HT (all females). At the time of this study three GD patients were newly diagnosed, three were in remission without therapy, two received methimazole and two received l-thyroxine for hypothyroidism; seven HT patients were receiving substitutive doses of l-thyroxine and two were euthyroid without therapy. The diagnosis of GD was based on the presence of diffuse goitre with hyperthyroidism associated with increased serum levels of thyroid autoantibodies; HT was diagnosed on the basis of diffuse hypoechogenic thyroid goitre and/or the histological finding of lymphocytic infiltration on fine needle aspiration biopsy (FNAB), and the presence of thyroid autoantibodies. The third group included 22 patients with primary AITP diagnosed according to the clinical criteria proposed by Kelton & Gibbons [21]. The main features of all patients studied are reported in Table 1.
Table 1.
Main characteristics of the patients studied
Platelet autoantibodies
Platelet-associated IgG (PAIgG) were assayed by immunofluorescent flow cytometric analysis, as previously described [22].
Specific circulating platelet autoantibodies were detected using the MAIPA assay [23], as previously reported [7]. Briefly, washed platelets were first incubated with patient's serum and then, after washing, with MoAbs against platelet gp Ib (CD42b, clone SZ2) and gp IIb/IIIa complex (CD41a, clone P2), both purchased from Immunotech (Marseille, France). The sensitized platelets were then washed and solubilized in Tris-buffered saline (TBS) pH 7.4, containing 0.5% Nonidet P40 (Sigma Chemical Co, St Louis, MO). After centrifugation, the diluted supernatants were incubated in wells of a microtitre plate (Costar, Cambridge, MA) previously coated with goat anti-mouse IgG (Sigma). Human antibodies bound to platelet glycoproteins were detected using alkaline phosphatase-conjugated goat anti-human IgG (Fc-specific; Sigma) as secondary antibody and p-nitrophenylphosphate as substrate. Absorbance (A) was read at 405 nm (Photometer Titertek-Multiskan; Flow Labs, Irvine, UK). Samples having A > 3 s.d. of the mean value for the normal controls were considered positive; the autoantibody value was expressed as the ratio of the sample absorbance to the mean absorbance of normal controls + 3 s.d. (cut off). Blood samples from 24 healthy adult volunteers from our laboratory staff were used as age- and sex-matched normal controls.
Thyroid autoantibodies
Microsomal and thyroglobulin autoantibodies were assessed by passive haemagglutination test using commercial kits (Thymune M and Thymune T, respectively; Murex Diagnostici Spa, Pomezia, Italy); titres > 1:100 were considered positive. Thyrotropin binding inhibiting immunoglobulins (TBII) were determined by radioimmunoassay (RIA) (Thybia-Assay II; Byk Gulden Italia, Milano, Italy); values > 15% were considered positive. The results obtained in a group of sex- and age-cross-matched normal controls (n = 452, 412 females and 40 males) [24] served as a reference for thyroid autoantibodies. Controls were from normal people without any personal and familial history of autoimmune disease.
Thyroid function
TSH was determined using a commercial immunoradiometric assay (IRMA-mat TSH; Byk Gulden); the normal values ranged from 0.2 to 4 μU/ml.
Statistical analysis
The χ2 test, with continuity correction, was used to compare the relative frequency of antibodies; statistical comparison of the mean level of autoantibodies was performed according to unpaired Student's t-test. P < 0.05 was considered significant.
RESULTS
Thrombocytopenia occurred after the diagnosis of TAD (time range 12–490 months) in 9 of 12 available patients (75%), in two patients (16.6%) preceded the onset of TAD and in one patient (8.4%) occurred at the same time as TAD.
Platelet autoantibodies
The results are summarized in Table 2. In TAD patients with thrombocytopenia, platelet autoantibodies were found in 15/18 (83%). In detail, increased PAIgG or the presence of specific circulating platelet autoantibodies were found in nine patients (50%). The prevalence of increase in PAIgG was higher in HT than in GD (73% versus 14%) but not statistically different (P = 0.053). In contrast, the prevalence of circulating platelet autoantibodies was higher in GD than in HT (71% versus 36%; P = 0.33); in six cases autoantibody (66.6%) recognized gp Ib, in one case (11.1%) gp IIb/IIIa and in two cases (22.2%) both antigens.
Table 2.
Platelet-associated IgG (PAIgG) and specific circulating platelet glycoprotein autoantibodies in the three groups of patients studied
In TAD patients without thrombocytopenia, platelet autoantibodies were found in 2/20 (10%). Both these patients were affected by GD. The first patient had increased PAIgG and circulating platelet autoantibodies, and the other presented only circulating type; these antibodies recognized in one case gp Ib and gp IIb/IIIa in the other.
In patients with primary AITP, platelet autoantibodies were found in 19/22 (86%). Increased PAIgG were present in 14 patients (64%) and circulating platelet autoantibodies in 10 (46%). In four cases (40%), circulating autoantibodies recognized gp Ib, in three cases (30%) gp IIb/IIIa and in three cases (30%) both antigens.
The mean level of anti-gp Ib autoantibodies was statistically higher (P < 0.02) in TAD patients with thrombocytopenia and in patients with primary AITP than in normal controls (Fig. 1); in contrast, the level of autoantibodies against gp IIb/IIIa was significantly increased (P < 0.02) only in TAD patients with thrombocytopenia. However, there was no difference in mean level of specific platelet autoantibodies between thrombocytopenic TAD patients and patients with primary AITP.
Fig. 1.

Platelet autoantibodies against glycoprotein (gp) Ib and gp IIb-IIIa performed by MoAb immobilization of platelet antigens (MAIPA) in the patients. Autoantibody level is expressed as the ratio of the patient absorbance to the cut off (mean absorbance of normal controls + 3 s.d.). A ratio > 1.0 (dashed line) is considered positive. Closed circles are the mean and vertical lines are the s.d. of the different groups. P-AITP, primary autoimmune thrombocytopenic purpura; TP-TAD, thyroid autoimmune disease with thrombocytopenia; NTP-TAD, thyroid autoimmune disease without thrombocytopenia. *P < 0.02 versus normal.
Thyroid autoantibodies
Thyroid autoantibodies were found in 89% of patients with TAD and thrombocytopenia, 95% of patients with TAD without thrombocytopenia and in 18% of patients with primary AITP (Table 3). Two of 22 AITP patients had only microsomal autoantibodies (titre 1:400 and 1:640), and two had autoantibodies reactive against both microsomal (1:6400) and thyroglobulin (1:320 and 1:640). The prevalence of thyroid autoantibodies in primary AITP was higher than in controls (8.6%), but not statistically different (P > 0.05). Three of four patients with AITP and thyroid autoantibodies were evaluated for TSH levels and one had levels under the normal range.
Table 3.
Thyroid (microsomal and thyroglobulin) autoantibodies and thyrotropin binding inhibiting immunoglobulins (TBII) in the three groups of patients and in normal controls
DISCUSSION
The association between thrombocytopenia and TAD, such as GD [8–10,13–15] and HT [6,12,17,18], has long been recognized. Thrombocytopenia has been attributed either to an excess of circulating hormone [10,11,15,16,19] or to an autoimmune mechanism [12–14,20]. On one hand, some studies have shown, as in GD, that platelet count and platelet survival improved after treatment of hyperthyroidism [10,11,15,16,19]; on the other hand, high levels of platelet-bound immunoglobulins have been found in patients with TAD and thrombocytopenia [12,15]. Further evidence of an immune pathogenesis of thrombocytopenia in TAD is that about 13% of patients with primary AITP may develop hyperthyroidism [9,13,25] and that patients with both GD and AITP share common HLA haplotypes [26].
While previous reports supporting the immune hypothesis were anecdotal [13,14,20], we now found in a larger series that 50% of patients with TAD and thrombocytopenia had increased levels of PAIgG. Moreover, we observed that in 50% of such patients circulating autoantibodies were also present, which were directed against epitopes located on platelet gp Ib and IIb/IIIa. To the best of our knowledge this is the first report of such a pattern, which is similar to that observed in patients with primary AITP [2–6] and other systemic autoimmune diseases [7]. The prevalence of anti-gp Ib antibodies was two-fold higher in TAD than in AITP, supporting the hypothesis of a common antigenic target shared by gp Ib and thyroid antigens [28].
While most patients with increased PAIgG levels had HT, circulating anti-platelet antibodies were found mostly in those with GD. This can be explained by a direct effect of methimazole [19] taken by most patients with GD, or by the different immune aetiologies of the two diseases.
PAIgG are a sensitive but non-specific method for the diagnosis of immune thrombocytopenia, while the opposite is true for circulating antibodies [1,2,4], and their usefulness has been improved by measuring elutable [7] or platelet-associated specific autoantibodies [2,4,29]. In this regard, a moderate increase of PAIgG or the presence of circulating platelet autoantibodies have been found in patients with autoimmune diseases regardless of thrombocytopenia [7,12,30,31]. However, by combining the two methods we showed that the presence of circulating and/or platelet-associated autoantibodies was detectable in 83% of patients with TAD and thrombocytopenia, as opposed to only 10% of those without thrombocytopenia. In conclusion, our results support the autoimmune aetiology of thrombocytopenia, since the prevalence and specificity of platelet autoantibodies are similar in TAD and AITP.
Our data seem to rule out an effect of thyroid hormone on platelet count. The discrepancy with other reports in this regard [28] can be explained by the fact that the majority of our thrombocytopenic patients with GD have been studied while in remission; in contrast, most studies supporting the hormonal aetiology considered patients during the active phase of disease [10,15,19] or with only a slight thrombocytopenia [12,16,27]. Therefore, the existence of two separate forms of thrombocytopenia might be surmised in TAD: an acute, hormone-mediated form, and a chronic one caused by an immunological mechanism.
Autoimmune thrombocytopenia seems to represent a complication in the course of TAD; in fact, 75% of our patients suffered from thrombocytopenia 1–4 years after the onset of TAD. Vice versa, we found a 18% prevalence of thyroid autoantibodies in patients with AITP and the presence of a subclinical thyroid disease was observed in one patient. Such findings confirm the existence of an overlap syndrome between AITP and TAD [6,10,18,19,27,28].
TAD can be associated with other endocrine and non-endocrine disorders [32,33], and this association is named polyglandular autoimmune disease (PGAD) type III. We suggest that patients with TAD and thrombocytopenia should also be included in the D subgroup of PGAD type III.
Acknowledgments
This study was supported by grants from C.N.R Rome (Grant 9502257CT04), and from the Veneto Regional Government (Grant 450/0194).
References
- 1.Kiel V, Santoso S, Mueller-Eckhardt C. Serological, biochemical, and molecular aspects of platelet autoantigens. Semin Haematol. 1992;29:26–33. [PubMed] [Google Scholar]
- 2.McMillan R, Tani P, Millard F, Berchtold P, Renshaw L, Woods VL. Platelet-associated and plasma anti-glycoprotein autoantibodies in chronic ITP. Blood. 1987;70:1040–5. [PubMed] [Google Scholar]
- 3.Kiefel V, Santoso S, Kaufmann E, Mueller-Eckhardt C. Autoantibodies against platelet glycoprotein Ib/IX: a frequent finding in autoimmune thrombocytopenic purpura. Br J Haematol. 1991;79:256–62. doi: 10.1111/j.1365-2141.1991.tb04530.x. [DOI] [PubMed] [Google Scholar]
- 4.He R, Reid DM, Jones CE, Shulman R. Spectrum of Ig classes, specificities, and titers of serum antiglycoproteins in chronic idiopathic thrombocytopenic purpura. Blood. 1994;83:1024–32. [PubMed] [Google Scholar]
- 5.Kiefel V, Freitag E, Kroll H, Santoso S, Mueller-Eckhardt C. Platelet autoantibodies (IgG, IgM, IgA) against glycoproteins IIb/IIIa and Ib/IX in patients with thrombocytopenia. Ann Hematol. 1996;72:280–2. doi: 10.1007/s002770050173. [DOI] [PubMed] [Google Scholar]
- 6.Berchtold P, Harris JP, Tani P, Piro L, McMillan R. Autoantibodies to platelet glycoproteins in patients with disease-related immune thrombocytopenia. Br J Haematol. 1989;73:365–8. doi: 10.1111/j.1365-2141.1989.tb07754.x. [DOI] [PubMed] [Google Scholar]
- 7.Cordiano I, Salvan F, Randi ML, et al. Antiplatelet glycoprotein autoantibodies in patients with autoimmune diseases with and without thrombocytopenia. J Clin Immunol. 1996;16:340–7. doi: 10.1007/BF01541670. [DOI] [PubMed] [Google Scholar]
- 8.Adrouny A, Sandler RM, Carmel R. Variable presentation of thrombocytopenia in Graves' disease. Arch Intern Med. 1982;142:1460–4. [PubMed] [Google Scholar]
- 9.Marshall JS, Weisberger AS, Levy RP, Breckenridge RT. Coexistent idiopathic thrombocytopenic purpura and hyperthyroidism. Ann Intern Med. 1967;67:411–4. [Google Scholar]
- 10.Herman J, Resnitzky P, Fink A. Association between thyrotoxicosis and thrombocytopenia. Isr J Med Sci. 1978;14:469–75. [PubMed] [Google Scholar]
- 11.Kurata Y, Nishioeda Y, Tsubasio T, Kitani T. Thrombocytopenia in Graves' disease: effect of T3 on platelet kinetics. Acta Hematol. 1980;63:185–90. doi: 10.1159/000207396. [DOI] [PubMed] [Google Scholar]
- 12.Hymes K, Blum M, Lackner H, Karpatkin S. Easy bruising, thrombocytopenia, and elevated platelet immunoglobulin G in Graves' disease and Hashimoto's thyroiditis. Ann Intern Med. 1981;94:27–30. doi: 10.7326/0003-4819-94-1-27. [DOI] [PubMed] [Google Scholar]
- 13.Valenta LJ, Treadwell T, Berry R, Elias AN. Idiopathic thrombocytopenic purpura and Graves' disease. Am J Hematol. 1982;12:69–72. doi: 10.1002/ajh.2830120110. [DOI] [PubMed] [Google Scholar]
- 14.Dunlap DB, Mcfarland KF, Luchter CL. Graves' disease and idiopathic thrombocytopenic destruction mediated by autoantibodies directed against platelet surface antigens. Am J Med Sci. 1974;268:107–11. doi: 10.1097/00000441-197408000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Jacobs P, Majoos F, Perrotta A. Hyperthyroidism and immune thrombocytopenia. Postgrad Med J. 1984;60:657–61. doi: 10.1136/pgmj.60.708.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Panzer S, Haubenstock A, Minar E. Platelets in hyperthyroidism: studies on platelet counts, mean platelet volume, 111-Indium-labeled platelet kinetics, and platelet-associated immunoglobulins G and M. J Clin Endocrinol Metab. 1990;70:491–6. doi: 10.1210/jcem-70-2-491. [DOI] [PubMed] [Google Scholar]
- 17.Crabtree GR, Lee JC, Cornwell GG. Autoimmune thrombocytopenia purpura and Hashimoto's thyroiditis. Ann Intern Med. 1975;83:371–2. doi: 10.7326/0003-4819-83-3-371. [DOI] [PubMed] [Google Scholar]
- 18.Segal BM, Weitraub MI. Hashimoto thyroiditis, myasthenia gravis, idiopathic thrombocytopenic purpura. Ann Intern Med. 1975;85:761–3. doi: 10.7326/0003-4819-85-6-761. [DOI] [PubMed] [Google Scholar]
- 19.Hofbauer LC, Spitzweg C, Schmauss S, Heufelder AE. Graves' disease associated with autoimmune thrombocytopenic purpura. Arch Intern Med. 1997;157:1033–6. [PubMed] [Google Scholar]
- 20.Verges B, Giroud-Badeydier F, Olsson O, Vaillant G, Brun JM, Putelat R. Association maladie de Basedow et purputa thrombopenique autoimmun: pas d'influence de l'hyperthyroidie sur l'autoimmunitè antiplaquettes. Rev Med Interne. 1989;10:565–9. doi: 10.1016/s0248-8663(89)80079-7. [DOI] [PubMed] [Google Scholar]
- 21.Kelton J, Gibbons S. Autoimmune platelet destruction: ITP. Semin Thromb Hemost. 1982;8:83–104. doi: 10.1055/s-2007-1005045. [DOI] [PubMed] [Google Scholar]
- 22.Fabris F, Cordiano I, Steffan A, Randi ML, Girolami A. Identification of anti-platelet autoantibodies by Western blot in 45 patients with idiopathic thrombocytopenic purpura (ITP) Haematologica. 1992;77:122–6. [PubMed] [Google Scholar]
- 23.Kiefel V, Santoso S, Weisheit M, Mueller-Eckhardt C. Monoclonal antibody-specific immobilization of platelet antigens (MAIPA): a new tool for identification of platelet-reactive antibodies. Blood. 1987;70:1722–6. [PubMed] [Google Scholar]
- 24.Betterle C, Callegari G, Presotto F, et al. Thyroid autoantibodies: a good marker for the study of symptomless autoimmune thyroiditis. Acta Endocrinologica. 1987;114:321–7. doi: 10.1530/acta.0.1140321. [DOI] [PubMed] [Google Scholar]
- 25.Branehog I, Olsson KS, Weinfeld A, Domellof L. Association of hyperthyroidism with idiopathic thrombocytopenic purpura and haemolytic anaemia. Acta Med Scand. 1979;205:125–31. doi: 10.1111/j.0954-6820.1979.tb06017.x. [DOI] [PubMed] [Google Scholar]
- 26.Bizzaro N. Familial association of autoimmune thrombocytopenia and hyperthyroidism. Am J Hematol. 1992;39:294–8. doi: 10.1002/ajh.2830390411. [DOI] [PubMed] [Google Scholar]
- 27.Carré Y, Canivet B, Roul C, et al. Circulating anticoagulant and antiplatelet immunoglobulin in autoimmune thyroid disease. Eur J Med. 1993;2:121–2. [PubMed] [Google Scholar]
- 28.Hofbauer LC, Heufelder AE. Coagulation disorders in thyroid diseases. Eur J Endocrinol. 1997;136:1–7. doi: 10.1530/eje.0.1360001. [DOI] [PubMed] [Google Scholar]
- 29.Berchtold P, Muller D, Beardsley D, et al. International study to compare antigen-specific methods used for the measurement of antiplatelet autoantibodies. Br J Haematol. 1997;96:477–83. doi: 10.1046/j.1365-2141.1997.d01-2064.x. [DOI] [PubMed] [Google Scholar]
- 30.Mulshine J, Lucas FV, Clough JD. Platelet-bound IgG in systemic lupus erythematosus with and without thrombocytopenia. J Immunol Methods. 1981;45:275–81. doi: 10.1016/0022-1759(81)90305-7. [DOI] [PubMed] [Google Scholar]
- 31.Pujol M, Ribera A, Vilardell M, Ordi J, Feliu E. High prevalence of platelet autoantibodies in patients with systemic lupus erythematosus. Br J Haematol. 1995;89:137–41. doi: 10.1111/j.1365-2141.1995.tb08919.x. [DOI] [PubMed] [Google Scholar]
- 32.Doniach D, Bottazzo GF. Polyendocrine autoimmunity. In: Franklin EC, editor. Clinical immunology update. Amsterdam: Elsevier North Holland; 1981. pp. 95–121. [Google Scholar]
- 33.Neufeld M, Blizzard RM. Polyglandular autoimmune disease. In: Pinchera A, Doniach D, Fenzi GF, Baschieri L, editors. Autoimmune aspects of endocrine disorders. New York: Academic Press; 1980. pp. 357–65. [Google Scholar]