Abstract
Complement receptor type 1 (CR1) is an integral membrane protein of many haematopoietic cells and plays an important role in the clearance of complement-associated immune complexes, favouring their transport to liver and spleen macrophages. A small amount of soluble CR1 (sCR1) is also found in plasma and might originate directly from release of leucocytes and other circulating cells. In previous studies, an increase in serum sCR1 level has been observed in liver cirrhosis and end-stage renal failure. High levels have also been found in patients with some haematologic malignancies. sCR1 serum levels were measured using a specific double sandwich ELISA assay. The present study demonstrates the correlation between mean serum sCR1 concentrations and disease severity in patients with chronic liver disease. In patients with liver cirrhosis, grouped according to the Child–Pugh classification, sCR1 rose as liver function decreased. The presence of neoplastic growth in the liver apparently does not play a role in the increase of sCR1. Serum sCR1 was not elevated in other solid malignancies. Since sCR1 accumulates in liver diseases, evaluation of its serum levels could be useful as a liver function test.
Keywords: soluble CR1, liver disease, Child–Pugh, hepatocellular carcinoma
INTRODUCTION
Complement receptor type 1 (CR1, CD35), C3b/C4b receptor, is an intrinsic membrane glycoprotein found on the surface of several circulating blood cells, including erythrocytes, polymorphonuclear neutrophils (PMN), monocytes, B lymphocytes and some T lymphocytes [1]. It is also expressed in renal glomerular podocytes [2]. CR1 binds C3b-coated particles and serves as a cofactor for the degradation of C3b by factor I. On erythrocytes, CR1 contributes to the clearance of circulating immune complexes by transporting them to the liver and spleen macrophages. On phagocytes, CR1 is involved in the initial binding of C3b-coated particles and their subsequent ingestion [3–6]. CR1 acts as a cofactor of I in C3b degradation, and this makes it a potent inhibitor of complement activation. A recombinant soluble form of CR1 (rCR1) was shown to be 100-fold more efficient as a regulator of complement activation than factor H, the physiological cofactor for the inactivation of C3b in plasma, and its possible therapeutic use as an anti-inflammatory agent is at present being investigated [7,8].
More than 10 years ago, a soluble form of CR1 (sCR1) was discovered in plasma. It is composed of most of the extracellular domains of membrane CR1 [9]. According to in vitro studies, sCR1 seems to be released from leucocytes by proteolytic cleavage [10]. Elevated sCR1 levels have been found in liver cirrhosis, end-stage renal failure and some haematologic malignancies [11].
The aim of the present study was to correlate sCR1 changes with liver disease staging, by investigating the relationship between sCR1 levels and some indices of liver function. The results demonstrate a clear positive correlation between sCR1 concentration and disease severity.
PATIENTS AND METHODS
Study population
The patients were divided into three groups: (1) 43 patients with chronic hepatitis (CH); (2) 88 patients with liver cirrhosis (LC) divided into three main subgroups according to the Child–Pugh classification [12], which estimates liver function: (a) 32 patients in Child–Pugh Class A; (b) 26 patients in Class B; (c) 30 patients in Class C; (3) 56 patients with hepatocellular carcinoma (HCC), all associated with cirrhosis.
In most cases the aetiology of cirrhosis was hepatitis C virus (HCV) infection. All cases of CH were HCV-related.
Diagnosis of LC and HCC was based on clinical, biochemical and instrumental criteria and, in selected cases, on liver biopsy; the diagnosis of chronic hepatitis C was based on biochemical and virological criteria and confirmed by liver biopsy.
We also measured sCR1 level in a control group of 30 blood donors. Thirty-one patients with pancreatic cancer, 15 patients with breast cancer and eight with lung cancer were also studied, as controls for HCC.
Serum samples were collected in small aliquots and kept at −20°C until tested.
All patients gave their informed consent.
ELISA for sCR1
Soluble serum CR1 levels were determined with a double sandwich ELISA using two anti-CR1 MoAbs, 3D9 and HB8592 (gifts of J. P. Atkinson, Washington, St Louis, MO), each of which identifies different epitopes of CR1 [11,13–15]. HB8592 was peroxidase-conjugated. Polystyrene microwell plates (Immuno Module Maxisorp, Nalge Nunc International, Glostrup, Denmark) were coated with 100 μl of purified 3D9 in PBS (0.3 μg/ml) and left overnight at 4°C. The plates were washed four times with PBS–Tween 20 (PBS–T) 0.05% v/v. Unbound sites were saturated with PBS–bovine serum albumin (BSA) 1%–Tween 20 0.05% for 1 h at 37°C. The plates were washed four times and appropriate dilutions of samples were added. Serial dilutions (from 20 to 1.25 ng/ml) of rCR1 (T Cell Sciences, Needham, MA) were included as standards for the sCR1 measurement. The plates were incubated for 1 h at 37°C and after four washes the MoAb anti-CR1 HB8592, peroxidase-conjugated, diluted in a solution of fetal bovine serum (FBS; Sigma, St Louis, MO) and PBS (50/50 v/v), was added for 1 h at 37°C. The plates were washed four times before addition of 100 μl of substrate (orthophenylenediamine (OPD) 4%, Sigma; in a citrate-phosphate buffer pH 6, with 37.5 μl of H2O2 35%). The coloured reaction was stopped with H2SO4 1 n after 30 min and absorbance was measured at 492 nm in a microplate reader, Multiscan Bichromatic apparatus (Labsystems, Helsinki, Finland).
Statistical analysis
Student's t-test, the analysis of variance and the Spearman rank correlation were used when appropriate and evaluated through StatView SE and Statistica for Windows.
RESULTS
sCR1 and relation to disease
In all patients with liver disease at different stages, serum concentrations of sCR1 were significantly higher than in the blood donor controls (BD). Mean levels of sCR1 were 33.2 ± 11 ng/ml in the 30 BD, 58.7 ± 18.6 ng/ml in the 43 patients with CH, and 77.20 ± 44.10 ng/ml in the 88 patients with LC. In addition, soluble CR1 levels were 57.7 ± 23.6 ng/ml in Child–Pugh class A patients, 77.1 ± 35.7 ng/ml in class B, 98.1 ± 57 ng/ml in class C. In patients with HCC, serum sCR1 was 86.2 ± 47.7 ng/ml (Fig. 1). No significant difference was found between sCR1 levels of LC patients with or without HCC, belonging to the same functional classes. Soluble CR1 levels in other solid malignancies were similar to those of BD: pancreatic carcinoma (PC) 26.14 ± 8.68 ng/ml; breast and lung carcinoma 31.49 ± 4.73 ng/ml (Fig. 1).
Fig. 1.
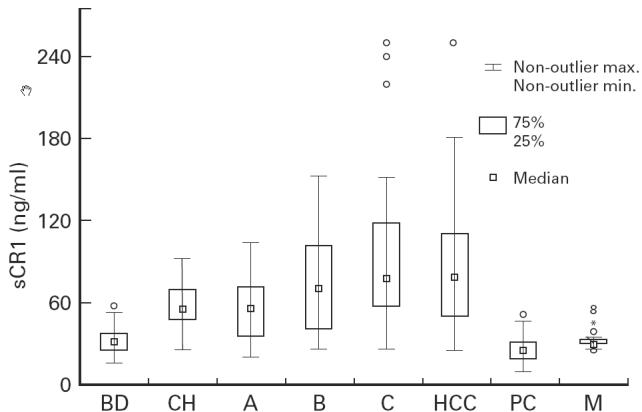
sCR1 serum levels in patients with liver diseases at different stages and in controls. Box and whisker plot (type: median/quart/range). Internal rectangle, median; external rectangle, interquartile range; whiskers, range; open circles, outliers. BD, Blood donors; CH, chronic hepatitis; A,B,C, Child–Pugh classes of liver cirrhosis; HCC, hepatocellular carcinoma; PC, pancreatic carcinoma; M, other malignancies: breast (n = 15) and lung (n = 8) cancer.
Soluble CR1 level and liver function
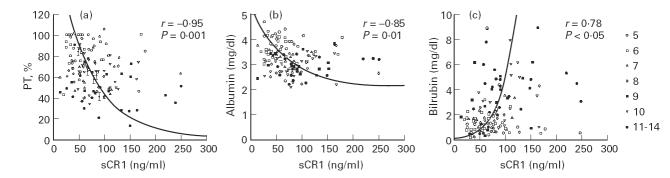
We compared sCR1 serum levels with three standard biochemical parameters of liver function in patients with LC at different stages (Fig. 2a, albumin, Fig. 2b, prothrombin time (PT), and Fig. 2c, bilirubin). When we divided patients according to Child–Pugh classification, a grading score from 5 to 15, which is inversely correlated with liver function, into seven different groups (scores 5, 6, 7, 8, 9, 10 and 11–15 together in the last group) we found a strong inverse correlation for sCR1 serum levels versus PT (r = −0.95) and albumin (r = −0.85) levels for each group. A positive correlation was observed for bilirubin (r = 0.78; Fig. 2a–c). A comparison of sCR1 levels and Child–Pugh score showed that the increase in sCR1 levels paralleled the increasing Child–Pugh score (r = 0.86), suggesting that liver dysfunction was responsible for the increase in sCR1 in plasma (Fig. 3).
Fig. 2.
Correlation between sCR1 serum concentration and prothrombin time (PT) (a), albumin (b), and bilirubin (c). Numbers refer to Child–Pugh groups (5, 6, 7, 8, 9, 10, 11–15). Significant correlation in cirrhotic patients, grouped according to Child–Pugh classification, between sCR1 serum levels and (a) PT (r = − 0.95; P = 0.001), (b) albumin (r = − 0.85; P = 0.01) and (c) bilirubin (r = 0.78; P < 0.05).
Fig. 3.
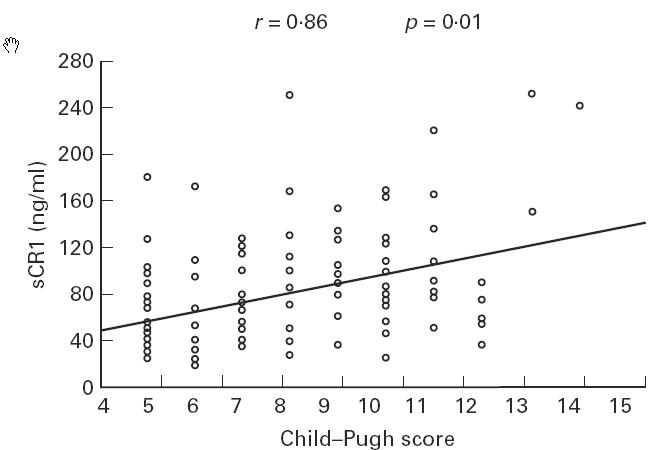
Correlation between sCR1 serum levels and Child–Pugh score. Scatterplot. ○, Scattergram of sCR1 serum levels.
DISCUSSION
Our study confirms that serum sCR1 is increased in chronic liver disease and that this increase is related to the severity of liver dysfunction. We found that sCR1 levels in patients with CH were two times higher than in the BD controls, while a more marked increase was found in patients with cirrhosis, especially those at a more advanced stage of disease. Among cirrhotic patients there was a significant difference between the three functional Child–Pugh classes, with a progressive increase from A to C. We found increased levels of sCR1 in HCC but not in other malignancies (pancreatic, breast and lung carcinoma). The presence of HCC per se probably does not determine high levels of sCR1. Indeed, no significant difference was found between sCR1 levels of cirrhotic patients with or without HCC belonging to the same functional class. The rise in sCR1 in patients with HCC is therefore more likely to have been caused by the underlying liver failure secondary to cirrhosis, rather than by the neoplastic growth.
Both reduced catabolism and increased synthesis may affect sCR1 levels. The negative correlations with the biochemical parameters of protein synthesis, such as PT and albumin, and the positive correlation with bilirubin as well as with Child–Pugh score, emphasized that sCR1 rises as liver function decreases, as previously shown in A. phalloides poisoning and liver transplantation [11,16]. Hence, the increase in sCR1 was probably the result of an accumulation in plasma because of defective hepatic clearance. In patients with LC we cannot exclude an additional cause for the rise in sCR1 in plasma. In histological cirrhosis, leucocyte infiltration and increase in Kupffer cell numbers could be the source of some additional sCR1. Moreover, there may be an increased production of certain cytokines, especially IL-6, able to up-regulate the expression of different cell surface molecules on peripheral blood mononuclear cells (PBMC) such as CR1. This could influence sCR1 serum levels through shedding of CR1 from circulating leucocytes and erythrocytes [17–21]. Thus, high levels of sCR1 in liver diseases may be the result of chronic liver inflammation and they indicate variable, but substantial, liver infiltration by leucocytes and up-regulation by systemic inflammatory response syndrome (SIRS)-related cytokines. sCR1 serum concentration further increases because of impaired liver clearance [18]. In a recent study, an inverse relationship was shown between erythrocyte CR1 and immune complex levels in HCV-infected patients and further decrease in those with severe liver inflammation and cirrhosis [22]. Light still needs to be shed on the precise role or function of sCR1 in liver disease. Recombinant sCR1 is being offered as a potential anti-inflammatory molecule. A similar activity for sCR1 at the observed concentration was not investigated, but it certainly deserves further attention.
In conclusion, evaluation of sCR1 serum levels could be useful as a good liver functional test; the rise observed in chronic hepatitis might be used to detect patients suffering from liver disease, without symptoms or serologic alterations, as is often the case in patients with hepatitis C chronic infection. Prospective studies will probably contribute to a better understanding of the role of sCR1 and clarify its importance in the study of liver disease.
Acknowledgments
We would like to thank Professor Maria Brai for statistical analysis. This work was supported by MURST 40–60%, ISS Progetto Tubercolosi 1995.
REFERENCES
- 1.Fearon DT. Identification of the membrane glycoprotein that is the C3b receptor of the human erythrocyte, polymorphonuclear leucocyte, B lymphocyte, and monocyte. J Exp Med. 1980;152:20–30. doi: 10.1084/jem.152.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pascual M, Steiger G, Sadallah S, Paccaud JP, Carpentier JL, Jamer RJ, Schifferli JA. Identification of membrane-bound CR1 (CD35) in human urine: evidence for its release by glomerular podocytes. J Exp Med. 1994;179:889–99. doi: 10.1084/jem.179.3.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Herbert LA, Cosio FG, Birmingham DJ, Mahan JD. Biological significance of the erythrocyte complement receptor: a primate perquisite. J Lab Clin Med. 1991;118:301–8. [PubMed] [Google Scholar]
- 4.Herbert LA. The clearance of immune complexes from the circulation of man and other primates. Am J Kidney Dis. 1991;3:352–61. doi: 10.1016/s0272-6386(12)80488-4. [DOI] [PubMed] [Google Scholar]
- 5.Medof ME, Iida K, Mold C, Nussenzweig V. Unique role of the complement receptor CR1 in the degradation of C3b associated with immune complexes. J Exp Med. 1982;156:1739–54. doi: 10.1084/jem.156.6.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hourcade D, Holers VM, Atkinson JP. The regulators of complement activation (RCA) gene cluster. Adv Immunol. 1989;45:381–415. doi: 10.1016/s0065-2776(08)60697-5. [DOI] [PubMed] [Google Scholar]
- 7.Weisman HF, Barlow T, Leppo MK, et al. Soluble human complement receptor type 1: in vivo inhibitor of complement suppressing post-ischemic myocardial inflammation and necrosis. Science. 1990;249:146–51. doi: 10.1126/science.2371562. [DOI] [PubMed] [Google Scholar]
- 8.Kalli KL, Hsu P, Bartow TJ, Ahearn JM, Matsumoto AK, Klickstein LB, Fearon DT. Mapping of C3b-binding site of CR1 and construction of a (CR1)2-F(ab′)2 chimeric complement inhibitor. J Exp Med. 1991;174:1451–60. doi: 10.1084/jem.174.6.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yoon SH, Fearon DT. Characterization of a soluble form of C3b/C4b receptor (CR1) in human plasma. J Immunol. 1985;134:3332–7. [PubMed] [Google Scholar]
- 10.Danielsson C, Pascual M, French L, Steiger G, Schifferli JA. Soluble complement receptor type 1 (CD35) is released from leucocytes by surface cleavage. Eur J Immunol. 1994;24:2725–31. doi: 10.1002/eji.1830241123. [DOI] [PubMed] [Google Scholar]
- 11.Pascual M, Duchosal MA, Steiger G, Giostra E, Pechere A, Paccaud JP, Danielsson C, Schifferli JA. Circulating soluble CR1 (CD35) J Immunol. 1993;151:1702–11. [PubMed] [Google Scholar]
- 12.Albers I, Hartman H, Bircher J, Creutzfeldt W. Superiority of the Child–Pugh classification to quantitative liver function tests for assessing prognosis of liver cirrhosis. Scand J Gastroenterol. 1989;24:269–76. doi: 10.3109/00365528909093045. [DOI] [PubMed] [Google Scholar]
- 13.Edberg JC, Kimberly RP, Taylor RP. Functional characterization of non-human primate erythrocyte immune adherence receptors: implications for the uptake of immune complexes by the cells of the mononuclear phagocytic system. Eur J Immunol. 1992;22:1333–9. doi: 10.1002/eji.1830220602. [DOI] [PubMed] [Google Scholar]
- 14.Quadri RA, Schifferli JA. Over-estimation of the number of complement receptor type 1 (CR1) on erythrocytes. Scand J Immunol. 1992;36:125–30. doi: 10.1111/j.1365-3083.1992.tb02948.x. [DOI] [PubMed] [Google Scholar]
- 15.Madi N, Paccaud JP, Steiger G, Schifferli JA. Immune complex binding efficiency of erythrocyte complement receptor 1. Clin Exp Immunol. 1991;84:9–15. doi: 10.1111/j.1365-2249.1991.tb08116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sadallah S, Giostra E, Mentha G, Schifferli JA. Increased levels of soluble complement receptor 1 in serum of patients with liver diseases. Hepatology. 1996;24:118–22. doi: 10.1002/hep.510240120. [DOI] [PubMed] [Google Scholar]
- 17.Deviere J, Content C, Denys C, Vandenbussche P, Schandene L, Wybran J, Dupont E. High interleukin-6 levels and increased production by leucocytes in alcoholic liver cirrhosis. Correlation with IgA serum levels and lymphokine production. Clin Exp Immunol. 1989;77:221–5. [PMC free article] [PubMed] [Google Scholar]
- 18.Rosenbloom AJ, Pinsky MR, Bryant JL, Shin A, Tran T, Whiteside T. Leucocyte activation in the peripheral blood of patients with cirrhosis of the liver and SIRS. JAMA. 1995;247:58–65. [PubMed] [Google Scholar]
- 19.Byl B, Roucloux I, Crusiaux A, Dupont E, Deviere J. Tumor necrosis factor alpha and interleukin-6 plasma levels in infected cirrhotic patients. Gastroenterology. 1993;104:1492–7. doi: 10.1016/0016-5085(93)90361-f. [DOI] [PubMed] [Google Scholar]
- 20.Kuo CH, Changchien CS, Yang CY, Sheen IS, Liaw YF. Bacteremia in patients with cirrhosis of the liver. Liver. 1991;11:334–9. doi: 10.1111/j.1600-0676.1991.tb00539.x. [DOI] [PubMed] [Google Scholar]
- 21.Hinglais N, Kazatchkine MD, Mandet C, Appay MD, Bariety J. Human liver Kupffer cells express CR1, CR2 and CR4 complement receptor antigens. Lab Invest. 1989;61:509–14. [PubMed] [Google Scholar]
- 22.Kanto T, Hayashi N, Takehara T, et al. Low expression of erythrocyte complement receptor type 1 in chronic hepatitis C patients. J Med Virol. 1996;50:126–34. doi: 10.1002/(SICI)1096-9071(199610)50:2<126::AID-JMV5>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]