Abstract
Immune unresponsiveness in HIV-1 infection can result from impaired signals delivered by the costimulatory CD28-B7 pathway and the altered production of immunoregulatory cytokines, in particular IL-10, whose production is altered in HIV-1 infection. In this study we investigate IL-10 regulation in T cells and monocytes from HIV+ individuals, and its association with CD28-mediated T cell proliferation. IL-10 production as analysed in T cell- and monocyte-depleted peripheral blood mononuclear cells (PBMC), and by intracellular staining at the single-cell level, reveals a defect in IL-10 production by CD4+ and CD8+ T cells, whereas monocytes constitute the major IL-10-producing cell type. To investigate the impact of IL-10 on immune responsiveness, CD28-mediated proliferative responses in HIV+ individuals were correlated with PHA-induced IL-10 production. CD4+ T cells expressed CD28, yet exhibited markedly reduced CD28-mediated cell proliferation. This CD28-mediated CD4+ T cell proliferation was found to be inversely associated with the levels of PHA-induced IL-10 production and could be restored, at least in part, by anti-IL-10 antibodies. These results suggest that IL-10 production is differentially regulated in T cells and monocytes of HIV+ individuals, and that IL-10 may have a role in inducing immune unresponsiveness by modulating the CD28-B7 pathway.
Keywords: HIV-1, IL-10, CD28
INTRODUCTION
Infection with HIV-1 produces profound phenotypic and functional disturbances within the cellular immune system eventually resulting in the depletion of CD4+ T cells, severe immunodeficiency and the development of opportunistic infections [1,2]. The functional defects in the CD4+ T cells such as loss of proliferative responses to recall antigens and anti-CD3 antibodies are observed at early stages even before the depletion of CD4+ T cells [3–5]. The mechanism of unresponsiveness observed in both CD4+ and CD8+ T cells has been attributed, at least in part, to the loss of IL-2 production, as proliferative responses to recall antigens can be enhanced by IL-2 [4–7]. Activation and proliferation of CD4+ T cells in response to specific antigens requires two signals. The first signal is provided by interaction of the T cell receptor (TCR) with an antigen presented in association with the MHC receptor complex. The second signal that is necessary for IL-2 production is delivered following interaction of CD28, expressed on T cells, with B7, expressed on antigen-presenting cells (APC) [8]. An imbalance in the signals delivered to T cells via the TCR, the CD4 co-receptor, or accessory molecules can lead to anergy and apoptosis. This has been observed following perturbation of the CD3–TCR interaction [9] and ligation of CD4 by gp120 prior to TCR occupancy [10].
Unresponsiveness in HIV+ individuals may also be due to the impaired signals delivered by the CD28-B7 costimulatory pathway. The expression of CD28 on CD4+ and CD8+ T cells of HIV+ individuals is altered [11,12]. The percentage of CD8+ T cells that express CD28 is significantly decreased and this has been shown to correlate with stages of disease [12]. Expression of CD28 on CD4+ T cells infected in vitro with HIV and CD4+ T cell lines generated from HIV+ individuals is decreased [13,14]. The inability of CD8+CD28+ T cells to produce IL-2 may provide evidence for a disrupted CD28-B7 pathway [11,15]. Modulation of CD28 expression may thus account for the loss of the cytotoxic and proliferative capacity of T cells.
The optimal proliferative response to specific antigens may also depend upon the immunoregulatory cytokines present in the microenvironment that regulate the CD28-B7 signalling pathway. IL-10 assumes significance in the regulation of immune responses as its biological effects are mediated at least in part through the B7 receptors [16] and the MHC class II molecules [17]. IL-10 is produced by T helper 2 (Th2), Th0, CD8+ T cells and monocytes and functions to inhibit cellular immune responses by inhibiting cytokine production by Th1 cells, T cell proliferation and monocyte functions [17–19]. It has been shown to induce immune unresponsiveness both in vitro and in vivo [20,21]. Regarding its role in HIV immunopathogenesis, IL-10 has been shown to inhibit HIV replication in vitro [22,23]. Increased IL-10 production by phytohaemagglutinin (PHA)-stimulated peripheral blood mononuclear cells (PBMC) from HIV+ individuals has been shown in one study to be inversely associated with IL-2 production following stimulation with recall antigens [24]. However, other studies have shown that PHA-stimulated PBMC from HIV+ individuals produced varying levels of IL-10 [25–34]. We hypothesized that varying levels of IL-10 produced in response to antigen/mitogen may influence immune responsiveness by modulating CD28-mediated proliferative signals. The present investigations were therefore conducted to study the regulation of IL-10 by T cells and monocytes of HIV+ individuals. The effect of IL-10 produced in response to PHA on immune responsiveness was evaluated by measuring the CD28-mediated proliferative responses of CD4+ mononuclear cells, and this proliferation was correlated with PHA-induced IL-10 production in HIV-infected individuals.
MATERIALS AND METHODS
Collection of blood samples, PBMC isolation, cell culture and reagents
Blood was obtained for isolation of PBMC from healthy adult volunteers and HIV+ individuals with CD4+ T cell counts < 625 cells/μl. Blood samples were collected following approval of the protocol by the Ethics Review Committee of the Ottawa General Hospital (University of Ottawa, Canada). None of the patients had received therapy with protease inhibitors and they did not have clinical evidence of acute infection during the course of specimen collection. PBMC were isolated by density gradient centrifugation using Ficoll–Hypaque (Pharmacia, Uppsala, Sweden) as described elsewhere [35]. The cell layer consisting mainly of mononuclear cells was collected and washed three times and resuspended in PBS at a concentration of 2 × 106 cells/ml. All cells including PBMC were cultured in complete Iscove's modified Dulbecco's medium (IMDM; Sigma Chemical Co., St Louis, MO) supplemented with 10% fetal calf serum (FCS; Gibco Labs, Grand Island, NY), 100 U/ml penicillin, 100 μg/ml gentamicin, 10 mm HEPES and 2 mm glutamine. Neutralizing anti-IL-10 antibodies were purchased from R&D Systems (Minneapolis, MN). Recombinant human IL-12 was kindly provided by Dr M. Gately (Hoffmann-LaRoche, Nutley, NJ).
Isolation of CD4+ mononuclear cells (T cells and monocytes)
CD4+ mononuclear cells were isolated from PBMC by positive selection using anti-CD4 antibody-coated immunobeads (Dynal, Lake Success, NY), as described previously [26,36]. Briefly, PBMC were incubated with immunobeads at a bead:target cell ratio of 4:1 at 4°C with gentle rotation for 20 min. The cells attached to the immunobeads were washed six times and separated using a magnetic field. CD4+ T cells and monocytes bound to the immunobeads were detached by Detachabeads (Dynal) as described by the manufacturer. Briefly, cells were resuspended at a concentration of 5 × 106/100 μl of culture medium with 1 U of CD4 Detachabeads for 1 h at room temperature. The detached cells were separated from the beads with a magnet followed by washing three times with IMDM containing 1% FCS. The detached cells were subsequently analysed for the presence of CD4+ and CD8+ T cells, monocytes and natural killer (NK) cells and B cells by flow cytometric analysis using FITC-conjugated anti-CD4 antibodies, FITC-conjugated anti-CD8 antibody, FITC-conjugated anti-CD14 antibody and PE-conjugated anti-CD16/anti-CD56 antibodies (all from Becton Dickinson, Mountain View, CA), and Quantum Red-conjugated anti-CD19 antibody (Sigma), respectively. There were < 2% B cells or NK cells in the purified CD4+ mononuclear cell population (data not shown).
Depletion of T cells and monocytes from PBMC
PBMC were depleted of T cells and monocytes by using anti-CD2 antibody- and anti-CD14 antibody-coated immunobeads (Dynal), respectively, as described elsewhere [26,36]. Briefly, PBMC were incubated with the immunobeads at a bead:target cell ratio of 10:1 with gentle rotation for 40 min. The cells attached to the beads were washed once and separated with a magnetic field. The unbound cells were washed and analysed for T cells, B cells and monocytes by flow cytometric analysis with FITC-conjugated anti-CD3 antibody, PE-conjugated anti-CD20 antibody (all from Becton Dickinson), and FITC-conjugated anti-CD15 antibody (Caltag, San Francisco, CA), respectively. T cell-depleted PBMC contained < 2% T cells. Alternatively, monocytes and NK cells were removed by l-leucine-methyl ester (L-LME) as described elsewhere [37]. Briefly, PBMC (3 × 106 cells/ml) were treated with 0.05% L-LME in serum-free medium at room temperature for 30 min. L-LME was neutralized by the addition of FCS at a final concentration of 10%. The cells were washed three times and analysed for monocytes by flow cytometry. L-LME-treated PBMC contained < 2% monocytes.
Measurement of cell proliferation
CD4+ mononuclear cells (2 × 104 cells/ml) were stimulated with anti-CD28 antibodies at a final dilution of 1:100 (CLB-CD28; Research Diagnostics, Inc., Flanders, NJ) and suboptimal concentrations of PHA-M (Gibco; 1:200 final dilution) for 2 days. The cells were pulsed with 0.5 μCi 3H-thymidine (Amersham, Arlington Heights, IL) and cultured for a further 16 h followed by cell harvest and measurement of 3H-thymidine incorporation. Stimulation index (SI) was calculated as the ratio of 3H-thymidine incorporation (ct/min) by cells stimulated in the presence of anti-CD28 antibodies and PHA to that of cells stimulated with PHA alone.
Cell stimulation and collection of culture supernatant
To determine the ability of PBMC from HIV+ individuals to produce IL-10, cells were cultured at a concentration of 2 × 106 cells/ml in 24-well tissue culture plates (Falcon Labware, Oxnard, CA). PBMC were stimulated with either PHA (Gibco) at a final dilution of 1:50 or lipopolysaccharide (LPS; Sigma) at a concentration of 1 μg/ml. The supernatants were harvested after 24, 48 and 72 h and frozen at −70°C. Initial kinetics experiments demonstrated that peak levels of IL-10 were produced after 48 h of cell culture (data not shown), and therefore in subsequent experiments IL-10 production was assayed after 48 h of culture. Supernatants were thawed once at the time of analysis of cytokine production by ELISA. Thawing of supernatants once did not influence measured cytokine concentration.
Measurements of IL-2, IL-10 and IL-12 by ELISA
IL-10 and IL-12 were measured by a sandwich ELISA assay using two different MoAbs that recognize distinct epitopes, as described previously [26,36]. Recombinant human IL-10 (R&D Systems) and IL-12 (Hoffman-LaRoche) were used as standards. The sensitivity of the IL-10 and IL-12 ELISA was 8 pg/ml. IL-10 stimulation index (IL-10 SI) was defined as the ratio of IL-10 levels (pg/ml) produced by PHA-stimulated PBMC to IL-10 levels produced by unstimulated PBMC. IL-2 was measured by ELISA using a commercially available kit obtained from R&D Systems, as described by the manufacturer. The sensitivity of the assay was 16 pg/ml.
Measurement of IL-10 production by CD4+ and CD8+ T cells and monocytes at the single-cell level
Intracellular staining of CD4+, CD8+ T cells and CD15+ monocytes for IL-10 was performed as described [27]. Preliminary experiments suggested that optimal levels of intracellular IL-10 were detected after 36 h of cell stimulation with PHA. PBMC were cultured in medium alone or with PHA (1:50 final dilution) for 36 h at a concentration of 2 × 106 cells/ml. Brefeldin A (Sigma; 5 μg/ml final concentration) was added to the cultures 4 h before staining with anti-IL-10 antibodies to block the intracellular transport processes within the cells. The cells were harvested and washed in staining buffer (PBS without Ca2+ or Mg2+ containing 1% FCS and 0.1% NaN3). Fc receptors were blocked by incubation with aggregated human gamma globulins at 4°C for 15 min. Cells were stained with Quantum Red-labelled anti-CD4 and anti-CD8 antibodies (Sigma), and FITC-labelled anti-CD15 antibodies (Caltag) for 30 min at 4°C followed by washing of cells twice with staining buffer. The cells were fixed with 100 μl of fixation buffer (4% paraformaldehyde in PBS) for 20 min at 4°C followed by two washes in permeabilization buffer (0.1% saponin (Sigma) in staining buffer). The cells were resuspended in the permeabilization buffer and stained with PE-labelled anti-IL-10 antibody (Pharmingen, San Diego, CA) at 4°C for 30 min. The appropriate negative controls were used including isotype-matched PE-labelled antibody of irrelevant specificity, and PE-labelled anti-IL-10 antibody preincubated with purified recombinant IL-10 for 30 min at 4°C. Cells were protected from light throughout the staining procedure and storage. The cells were analysed by three-colour cytometric analysis using a flow cytometer (Coulter Electronics, Hialeah, FL). CD4+, CD8+, IL-10+ T cells and CD15+, IL-10+ monocytes were gated and subjected to histogram analysis. The results from samples obtained and analysed at different times were compared following standardization of the instrument using Standard Brite flow cytometric fluorescence intensity standardization beads, as described by the manufacturer (Coulter).
Analysis of CD28 expression on CD4+ and CD8+ T cells by flow cytometry
Cells were washed once with PBS/0.05% NaN3, distributed into flow cytometry tubes (Sarstedt, Numbrecht, Germany) and stained with 5 μl of FITC-labelled anti-CD28 antibody (Becton Dickinson) and either Quantum Red-conjugated anti-CD4 or anti-CD8 antibodies (Sigma) for two-colour flow cytometric analysis. Autofluorescence and isotype controls (IgG2a for anti-CD28 antibodies (Becton Dickinson) and IgG2b for anti-CD4 antibodies (Pharmingen)) were also included (data not shown). Using a Coulter Flow Cytometer, 5000 events were counted. The data were acquired on an Excel flow cytometer (Coulter Electronics), saved as list modes and the percentages of CD4+ and CD8+ T cells that expressed CD28 were calculated.
Statistical analysis
Means were compared by the two-tailed Student's t-test. The results were expressed as mean ± 1 s.e.m.
RESULTS
IL-10 production by PBMC of HIV+ individuals following PHA stimulation
We have previously demonstrated that PBMC from HIV+ individuals spontaneously produce IL-10 as determined by both semiquantitative reverse transcriptase-polymerase chain reaction (RT-PCR) analysis and ELISA [25]. Similar results were obtained in this study showing high levels of IL-10 production by unstimulated PBMC of HIV+ individuals compared with that produced by HIV− individuals (31.48 ± 2.1 pg/ml versus 10.1 ± 2.5 pg/ml; P < 0.005; Fig. 1a). To determine IL-10 production following mitogen stimulation, PBMC from 37 HIV+ individuals (CD4+ T cells/μl range, 100–625) and 16 HIV− controls were stimulated with PHA for 48 h and the supernatants were analysed for IL-10 production by ELISA. PHA-stimulated PBMC from HIV+ individuals produced significantly lower levels of IL-10 compared with the HIV− controls (334.55 ± 69.02 pg/ml versus 563.18 ± 90.9 pg/ml; P < 0.005). Based on the levels of IL-10 production, two subsets of HIV+ individuals were identified irrespective of their CD4+ T cell numbers. PBMC from one subset of HIV+ individuals, designated as IL-10 producers (n = 19), produced levels of IL-10 (564.1 ± 111.7 pg/ml; mean of IL-10 SI = 23.2) comparable to that produced by PBMC from HIV− controls (563.18 ± 90.9 pg/ml; mean of IL-10 SI = 31.28). In contrast, PBMC from a second subset of HIV+ individuals (n = 18), designated as low IL-10 producers, produced significantly lower levels of IL-10 compared with the HIV− individuals (92.24 ± 7.7 pg/ml; mean of IL-10 SI = 2.3, versus 563.18 ± 90.9 pg/ml; mean of IL-10 SI = 31.28; P < 0.001; Fig. 1a). An arbitrary value of IL-10 SI > 4 was chosen to differentiate IL-10 producers from low IL-10 producers.
Fig. 1.
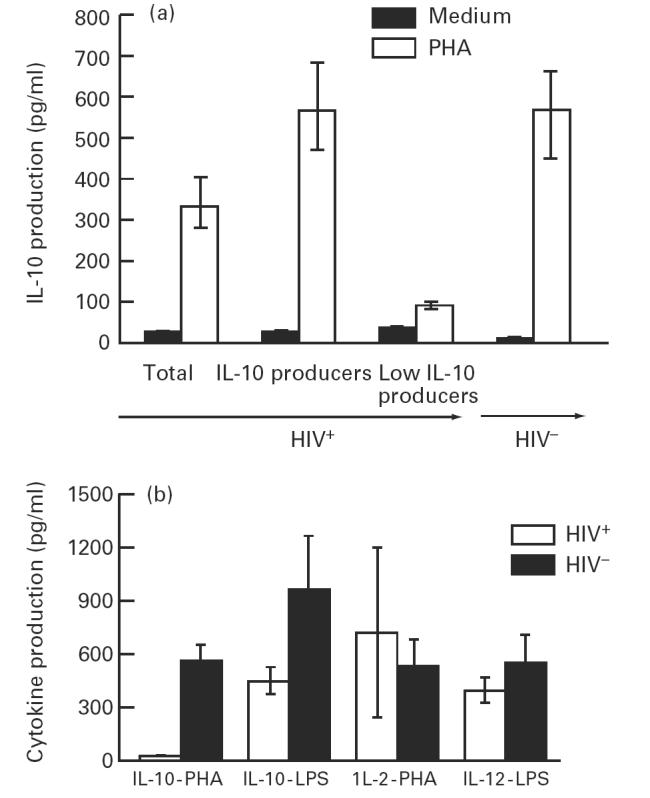
(a). Production of IL-10 by phytohaemagglutinin (PHA)-stimulated peripheral blood mononuclear cells (PBMC) from HIV+ and HIV− individuals. PBMC from 37 HIV+ and 16 HIV− individuals were cultured at a concentration of 2 × 106/ml for 48 h with PHA (1:50 final dilution) and supernatants were collected and assayed for IL-10 production by ELISA. IL-10 levels are reported as pg/ml (mean ± s.e.m.). PBMC from HIV+ individuals were stratified into low IL-10 producers (IL-10 stimulation index (SI) < 4) and IL-10 producers (IL-10 SI > 4). (b) Production of IL-2, IL-10 and IL-12 by PBMC from HIV+ low IL-10 producers and HIV− normal controls. PBMC from 15 HIV+ low IL-10 producers were stimulated with PHA and analysed for IL-10 (IL-10-PHA) and IL-2 production (IL-2-PHA). PBMC from these patients were also stimulated with lipopolysaccharide (LPS) (1 μg/ml) and analysed for IL-10 (IL-10-LPS) and IL-12 (IL-12-LPS) production. The levels of IL-2, IL-10, and IL-12 are reported as pg/ml (mean ± s.e.m.).
Down-regulation of IL-10 production by PHA-stimulated PBMC from the low IL-10 producers may be attributed to the general impairment of T cells and monocytes. This was examined by analysis of IL-2, IL-10 and IL-12 production following stimulation of PBMC with either PHA or LPS. PBMC from 15 HIV+ individuals (CD4+ T cells/μl range, 131–480) that produced very low or undetectable levels of IL-10 (19.27 ± 31.58 pg/ml) produced levels of IL-2 following PHA stimulation (705.81 ± 477.02 pg/ml) and IL-12 following LPS stimulation (379.72 ± 72.2 pg/ml; Fig. 1b) that were comparable to the levels produced by PBMC from HIV− individuals (IL-2, 516.3 ± 154.01 pg/ml; and IL-12, 525.52 ± 156.61 pg/ml; n = 10). However, PBMC from these individuals produced significantly lower levels of IL-10 compared with that produced by HIV− individuals following LPS stimulation (438.3 ± 75.98 pg/ml versus 1451 ± 186.7 pg/ml; P < 0.001; Fig. 1b). In contrast, PBMC from 19 HIV+ IL-10 producers (CD4+ T cells/μl range, 123–492) produced significantly low levels of IL-2 compared with that produced by HIV− individuals (296.9 ± 101.5 pg/ml versus 516.3 ± 154.01 pg/ml; n = 10; P < 0.005) following PHA stimulation. Moreover, PBMC from these individuals produced high levels of IL-10 following LPS stimulation that were comparable to that produced by HIV− individuals (1498.38 ± 306.66 pg/ml versus 1451 ± 186.7 pg/ml; P > 0.05). These results suggest a down-regulation of IL-10 production by T cells of low IL-10 producers, as PHA-stimulated PBMC from these individuals produced IL-2 that was comparable to that produced by HIV− individuals.
Down-regulation of IL-10 production by T cells of HIV+ individuals
To analyse the source of IL-10 production in PBMC from HIV+ individuals showing levels of IL-10 comparable to HIV− controls, PBMC from six HIV+ individuals and six HIV− controls were depleted of either T cells or monocytes and stimulated with PHA. Following T cell depletion, IL-10 production was reduced by 70–80% in HIV− controls, whereas significant differences in IL-10 production were not observed between T cell-depleted and undepleted PBMC of HIV+ individuals. In contrast, monocyte depletion reduced IL-10 production by 70–80% in both HIV− and HIV+ individuals. IL-10 production from PBMC of two representative HIV+ and one HIV− individual following depletion of either monocytes or T cells is shown in Fig. 2a and b, respectively. These results suggest that T cells do not play a critical role in PHA-induced IL-10 production in HIV+ IL-10 producers, while monocytes may represent the major source of IL-10 in this subset of HIV+ individuals. Furthermore, IL-10 production in HIV− individuals required the presence of both T cells and monocytes, whereas monocytes from HIV+ individuals appear to be capable of producing IL-10 in the absence of T cell-mediated signals.
Fig. 2.
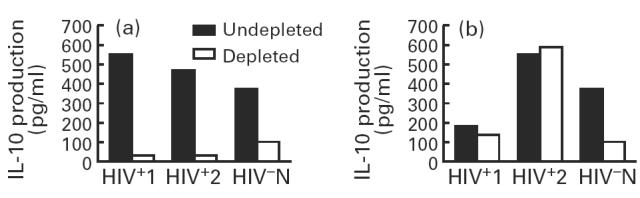
Effect of monocyte (a) and T cell (b) depletion from peripheral blood mononuclear cells (PBMC) of HIV+ and HIV− individuals on IL-10 production following stimulation with phytohaemagglutinin (PHA). PBMC from six HIV+ and six HIV− individuals were depleted of either T cells or monocytes by using anti-CD2 conjugated immunobeads and by l-leucine-methyl ester (L-LME) treatment, respectively. The depleted and undepleted PBMC were stimulated with PHA for 2 days and supernatants were analysed for IL-10 production by ELISA. The results from two representative HIV+ and one HIV− individual are shown.
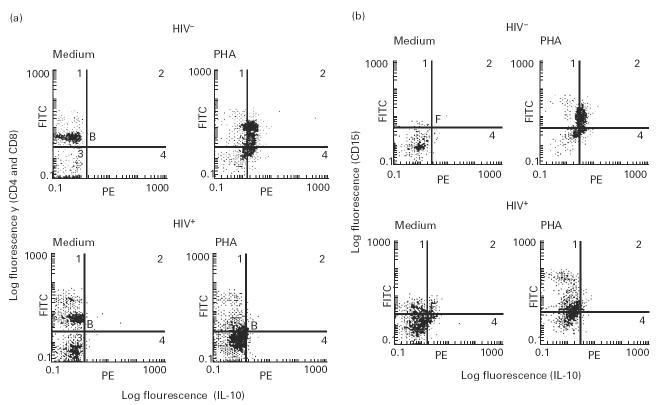
IL-10 expression was directly determined by intracellular staining and multiparameter flow cytometric analysis of individual IL-10-producing CD4+ T cells, CD8+ T cells and CD15+ monocytes within unseparated PBMC populations. PBMC from six HIV+ IL-10 producers and five HIV− individuals were stimulated with PHA for 36 h and stained for CD4, CD8 and CD15 cell surface antigens followed by counterstaining for intracellular IL-10 as described in Materials and Methods. PHA stimulation of PBMC from HIV− individuals induced IL-10 expression in both T cells and monocytes. Similar stimulation of PBMC from HIV+ individuals induced IL-10 expression in monocytes but not in CD4+ and CD8+ T cells. IL-10 expression in T cells and monocytes from one representative HIV− and IL-10-producing HIV+ individual is shown in Fig. 3a and b, respectively. CD15+ monocytes from low IL-10 producers exhibited a defect in IL-10 induction following PHA stimulation (data not shown). A consistent increase in IL-10 expression in CD15+ monocytes of HIV+ individuals following stimulation with LPS was also observed (data not shown). These results suggest that CD4+ and CD8+ T cells from HIV+ individuals exhibited a defect in IL-10 production following activation. CD15+ monocytes from low IL-10 producers also exhibited a defect in IL-10 induction following PHA stimulation, but retained the ability to induce IL-10 expression following LPS stimulation.
Fig. 3.
Analysis of IL-10 expression by T cells and monocytes of HIV+ and HIV− individuals by intracellular staining. Peripheral blood mononuclear cells (PBMC) (2 × 106 cells/ml) from six HIV+ and five HIV− individuals were stimulated with phytohaemagglutinin (PHA) for 36 h and analysed for IL-10 production at the single-cell level by intracellular staining using three-colour flow cytometry as described in Materials and Methods. CD4+ and CD8+ T cells (stained with Quantum Red-labelled anti-CD4 and anti-CD8 antibodies) and CD15+ monocytes (stained with FITC-labelled anti-CD15 antibodies) were analysed for IL-10 expression (counter-stained with PE-labelled anti-IL-10 antibodies). Results of IL-10 expression by CD4+ and CD8+ T cells (a) and CD15+ monocytes (b) of one representative HIV+ and HIV− individual are shown. Medium and PHA indicate unstimulated and PHA-stimulated cells, respectively. Unstimulated cells were also stained with isotype-matched antibody of irrelevant specificity and exhibited similar patterns of staining as for PHA-stimulated cells (data not shown).
Loss of CD28-induced proliferative responses in HIV+ individuals
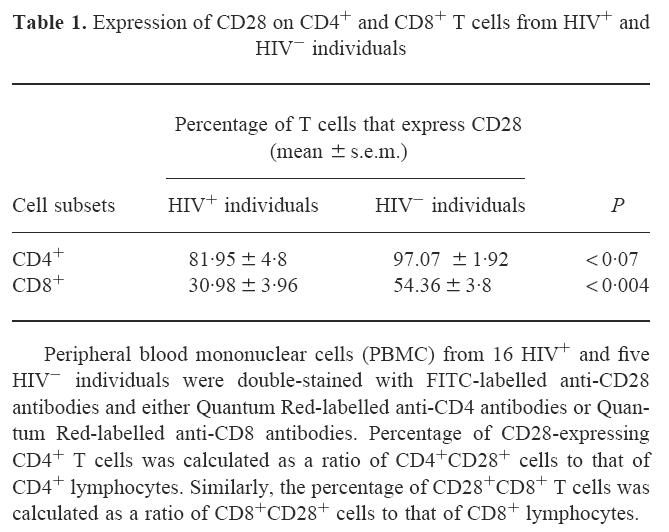
IL-10 production during HIV infection has been shown to be inversely correlated with IL-2 production and T cell proliferation in response to recall antigens, alloantigens and mitogens [24,26]. Since IL-2 production and T cell proliferation require costimulatory signals delivered by the CD28-B7 pathway [8,38], modulation of the expression of CD28 on CD4+ T cells or modulation of CD28-induced signals by immunoregulatory cytokines such as IL-10 may influence T cell proliferation. We therefore undertook studies to investigate CD28 expression on CD4+ and CD8+ T cells, CD28-induced proliferative signals, and to correlate this with IL-10 production. To analyse the expression of CD28 on CD4+ and CD8+ T cells by two-colour flow cytometry, PBMC from 16 HIV+ and five HIV− individuals were double-stained with FITC-labelled anti-CD28 antibodies and either Quantum Red-labelled anti-CD4 or anti-CD8 antibodies. The percentages of CD4+ T cells and CD8+ T cells that expressed CD28 are shown in Table 1. In HIV+ individuals, most CD4+ T cells expressed CD28, though the percentage of CD4+ T cells that expressed CD28 receptors was moderately decreased (81.95 ± 4.80%) compared with HIV− individuals (97.07 ± 1.92%, P < 0.07). Furthermore, there was no difference in the fluorescence intensity (MCF) of CD28 expressed on CD4+ T cells from HIV+ and HIV− individuals (data not shown). In contrast, the percentage of CD8+ T cells that expressed CD28 was significantly decreased in HIV+ individuals compared with HIV− controls (Table 1).
Table 1.
Expression of CD28 on CD4+ and CD8+ T cells from HIV+ and HIV− individuals
Although most CD4+ T cells in HIV+ individuals express CD28, these cells may exhibit defects in the CD28 signalling pathway. To assess CD28-induced proliferative signals in CD4+ T cells, CD4+ mononuclear cells were stimulated with a suboptimal concentration of PHA (1:200 final concentration) in the presence or absence of anti-CD28 antibodies. Since CD28-mediated proliferative signal is a non-specific costimulatory signal, CD4+ T cells must be preactivated for eliciting proliferative responses by anti-CD28 antibodies. Therefore, very low concentrations of PHA were used in these experiments to induce activation of CD4+ T cells without causing proliferation, and to amplify the proliferative effects of anti-CD28 antibodies. Addition of anti-CD28 antibodies alone did not influence T cell proliferation (data not shown). However, addition of anti-CD28 antibodies in the presence of the suboptimal concentration of PHA induced an approx. 50-fold increase in 3H-thymidine incorporation by CD4+ cells compared with cell proliferation by PHA alone in HIV− individuals. Anti-CD28 antibody-induced proliferation of CD4+ cells from HIV+ individuals was markedly impaired compared with the HIV− controls. Figure 4 shows CD28-induced proliferation of CD4+ cells in four representative HIV+ and two HIV− individuals.
Fig. 4.
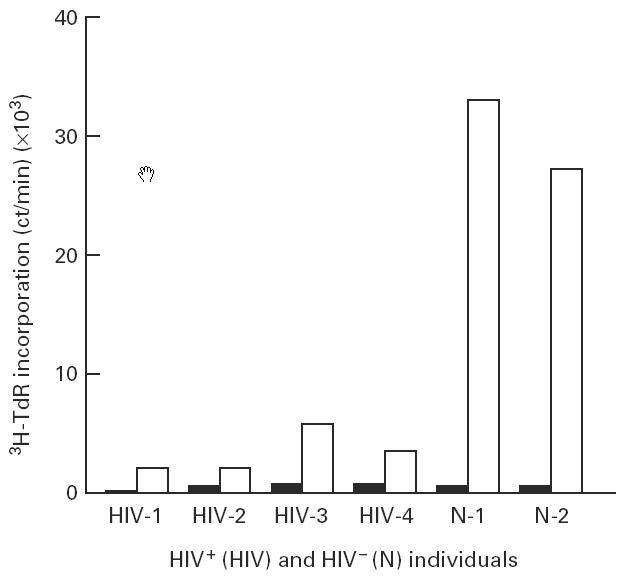
Proliferative response of CD4+ mononuclear cells (CD4+ T cells and monocytes) from peripheral blood mononuclear cells (PBMC) of HIV+ and HIV− individuals in response to suboptimal concentrations of phytohaemagglutinin (PHA) and anti-CD28 antibodies. CD4+ cells from PBMC of four HIV+ (HIV-1 to HIV-4) and two HIV− (N-1 and N-2) individuals were stimulated with a suboptimal concentration of PHA (1:200 final dilution) and anti-CD28 antibodies (1:100 final dilution) followed by measurement of cell proliferation by 3H-thymidine incorporation. ▪, Cells stimulated with a suboptimal concentration of PHA alone; □, cells stimulated with a suboptimal concentration of PHA and anti-CD28 antibodies.
Correlation of CD28-induced proliferation with the levels of IL-10 production
CD4+ cells from a total of 35 HIV+ individuals were stimulated with anti-CD28 antibodies in the presence and absence of suboptimal concentrations of PHA. Simultaneously, PBMC were also stimulated with optimal concentrations of PHA (1:50 final concentration) and the levels of IL-10 in the supernatants were analysed by ELISA. The cell proliferation SI was correlated with the IL-10 SI of HIV+ low IL-10 producers (IL-10 SI < 4) and of IL-10 producers (IL-10 SI > 4). The results show that CD28-induced proliferation of CD4+ cells was significantly reduced in the group of IL-10 producers compared with that of low IL-10 producers (cell proliferation SI 29.4 ± 8.13 in low IL-10 producers versus 7.69 ± 2.77 in IL-10 producers; P < 0.018, Fig. 5). To determine whether endogenously produced IL-10 influences anti-CD28 antibody-induced proliferation, CD4+ cells from five HIV+ low IL-10 producers were stimulated with suboptimal concentrations of PHA and anti-CD28 antibodies in the presence of anti-IL-10 antibodies. Addition of anti-IL-10 antibodies enhanced CD28-induced proliferative responses of CD4+ cells. The isotype-matched antibodies of irrelevant specificity, and anti-IL-4 antibodies used in this assay system did not enhance cell proliferation (data not shown). The results of cell proliferation from two representative HIV+ individuals are shown in Fig. 6. An increase in cell proliferation following addition of anti-IL-10 antibodies is deemed significant, keeping in view the very low numbers of CD4+ cells (2 × 104/well) used in this assay system. Furthermore, anti-IL-10 antibodies did not restore the cell proliferation in HIV+ IL-10 producers (data not shown).
Fig. 5.
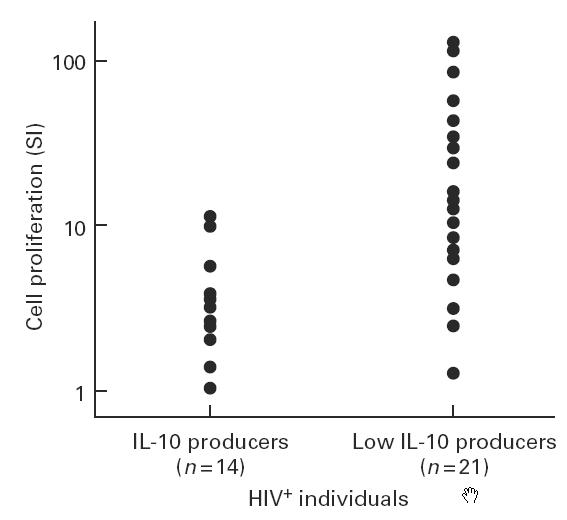
Correlation of IL-10 production by phytohaemagglutinin (PHA)-stimulated peripheral blood mononuclear cells (PBMC) from HIV+ low IL-10 producers and IL-10 producers with proliferative responses of CD4+ mononuclear cells to anti-CD28 antibodies and PHA. CD4+ mononuclear cells (2 × 104/ml) from HIV+ individuals were stimulated with suboptimal concentrations of PHA and anti-CD28 antibodies and cell proliferation was measured as described in the legend to Fig. 6. PBMC from the same HIV-infected individuals were simultaneously stimulated with PHA (1:50 final dilution) for 48 h and supernatants were analysed for IL-10 production. The cell proliferation stimulation index (SI; ratio of 3H-thymidine incorporation in the presence of PHA and anti-CD28 antibodies to that observed in the presence of PHA alone) was correlated with the levels of IL-10 SI (ratio of the levels of IL-10 produced following stimulation with PHA to that produced by unstimulated PBMC) in low IL-10 producers (IL-10 SI < 4) and IL-10 producers (IL-10 SI > 4).
Fig. 6.
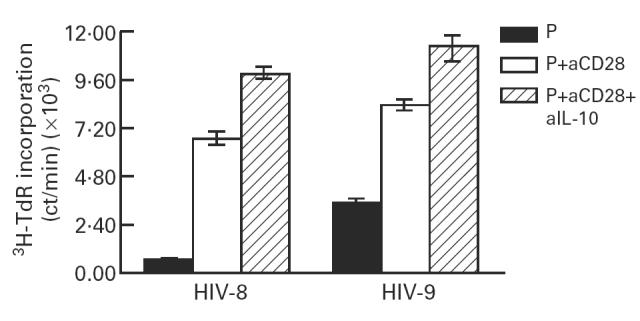
Effect of anti-IL-10 antibodies on the CD28-induced proliferative responses of CD4+ mononuclear cells. CD4+ mononuclear cells from HIV+ low IL-10 producers were stimulated with suboptimal concentrations of phytohaemagglutinin (PHA) and anti-CD28 antibodies in the presence of anti-IL-10 antibodies (10 μg/ml). Cells were pulsed with 3H-thymidine after 48 h of culture followed by measurement of 3H-thymidine incorporation 16 h later. Results from two representative HIV+ individuals are shown.
DISCUSSION
In this study, we demonstrate differential production of IL-10 by T cells and monocytes from HIV+ individuals. CD4+ and CD8+ T cells exhibited a specific defect in IL-10 production, whereas monocytes constitute the major cell type that produce IL-10. The results also suggest that CD4+ T cells from HIV+ individuals deliver impaired CD28-mediated proliferative signals. The majority of CD4+ T cells from HIV+ individuals expressed CD28, yet exhibited significantly reduced CD28-mediated cell proliferation. This CD28-mediated CD4+ T cell proliferation was found to be inversely associated with the levels of IL-10 produced following PHA stimulation and proliferative responses could be restored, at least in part, by anti-IL-10 antibodies.
We confirm our previous observations and those of others which suggest that unstimulated PBMC from HIV+ individuals produce high levels of IL-10 compared with that produced by HIV− individuals [25,28]. However, different investigators have reported variable results for IL-10 secretion following PHA stimulation of PBMC from HIV+ individuals. Shearer and coworkers have shown enhanced IL-10 production [24], whereas other studies have demonstrated that IL-10 production either remains unaltered [29,31,33,34] or is decreased [27,32]. The possible explanations for this discrepancy may include the selection of patients in the study group, use of anti-retroviral therapy, mode of cell activation and the virus strain. Consistent with our earlier results [26], we have stratified HIV+ individuals into low IL-10 producers and IL-10 producers following stimulation of PBMC with PHA. Loss of IL-10 production in low IL-10 producers was not due to the general impairment of T cells and monocytes, as PBMC from these patients produce IL-2 following PHA stimulation. Down-regulation of IL-10 production in T cells was demonstrated by intracellular staining of IL-10 at the single-cell level and in T cell depletion studies. To our knowledge, this is the first study demonstrating markedly reduced IL-10 production by T cells of HIV+ individuals. Similar results showing down-regulation of IL-10 production by CD4+ T cells were obtained in symptomatic rhesus monkeys infected with simian immunodeficiency virus (SIV) [39].
Decrease in IL-10 production by T cells following infection of PBMC from HIV− individuals was not observed by either ELISA or intracellular staining (data not shown). This may be due to the cytopathic effect of the virus on IL-10-producing Th0/Th2 cells [30], as infected PBMC did not survive for a prolonged time. Alternatively, culture conditions of PBMC infected in vitro with HIV may not mimic the in vivo microenvironment, and prolonged exposure to HIV antigens may be necessary to influence IL-10 production. The source of IL-10 production in HIV+ IL-10 producers was within the CD15+ monocytes, as depletion of T cells did not influence IL-10 production. Decreased IL-10 production in low IL-10 producers in response to PHA suggests a defect in IL-10 production by monocytes in this subset of HIV+ individuals. However, stimulation of PBMC with LPS induced IL-10 production, and intracellular staining of CD15+ monocytes for IL-10 demonstrated that monocytes retain the ability to produce IL-10. Consistent with results from other laboratories [23,40,41], in vitro infection of monocytes from HIV− individuals, and of a monocytic cell line with HIV enhanced IL-10 production (data not shown). The mechanism by which IL-10 production following HIV infection is differentially regulated in T cells and monocytes may be attributed to the altered production of immunoregulatory cytokines. It is also likely that HIV antigens tat, nef, gp120 or other HIV antigens may differentially regulate IL-10 in T cells and monocytes. HIV antigens tat and nef down-regulate the expression of several cellular genes including the cytokine genes [42–44]. The role of HIV antigens and/or cytokines in inhibiting IL-10 production by T cells at the transcriptional or post-transcriptional level remains to be determined.
IL-10 has been associated with the immunopathogenesis of a number of diseases, including septic shock, lymphoproliferative disorders, autoimmune diseases and AIDS [17,45,46]. It has been shown to induce immune unresponsiveness both in vitro and in vivo [20,21] and inhibits HIV replication in cells of monocyte lineage [22,23]. High levels of IL-10 produced by unstimulated and antigen/mitogen-stimulated PBMC may, thus, negatively regulate HIV replication. Spontaneous production of IL-10 by unstimulated PBMC from HIV+ individuals may also contribute to the defective antigen/recall responses and immunological unresponsiveness/immune suppression. The mechanism by which IL-10 induces immunosuppression has been attributed in part to its inhibitory effects on the synthesis of cytokines such as IL-2, IL-12 and interferon-gamma (IFN-γ) [17–19,47] and inhibiting B7.2 expression on APC [16,48]. Increased expression of IL-10 [23,40,41] and decreased production of IL-12 by monocytes of HIV+ individuals [28,31] may induce immune unresponsiveness by inhibiting the CD28-B7 pathway, as IL-10 and IL-12 mediate their biological effects by regulating the CD28-B7 pathway [16,49]. The inverse correlation of IL-10 production with proliferative responses to anti-CD28 antibodies and the observation that anti-IL-10 antibodies enhance CD28-mediated proliferation suggest that IL-10 exerts immunosuppressive effects by inhibiting the CD28-B7 pathway in HIV infection. The fact that this enhanced proliferation was not comparable to CD28-induced proliferation of CD4+ T cells of HIV− individuals suggests the involvement of other factors such as altered cytokine production (IL-2, IL-12, etc.) or the expression of their receptors, and apoptosis.
CD28-induced proliferation of CD4+ T cells is reduced in HIV+ individuals, though most CD4+ T cells express CD28, indicating impaired CD28-mediated proliferative signals in CD4+ cells. Whether CD8+ T cells exhibit impaired CD28-induced proliferation remains to be investigated. The mechanism for impaired CD28-induced proliferation in HIV infection is not well understood but may be attributed to the impaired regulation of B7 expression on APC. We have observed that expression of B7 expression on either B cells or monocytes is not altered in HIV+ individuals. However, regulation of B7 expression on monocytes by IL-10 is impaired (Kumar et al., manuscript submitted). The effect of HIV infection on CD28-B7-mediated signalling pathway in T cells remains controversial. A defective CD28-mediated signalling pathway as indicated by altered cellular levels of fyn and lck tyrosine kinases in anti-CD28 antibody-stimulated T cells in HIV infection has been suggested [50]. In contrast, Meyaard et al. have suggested that CD28 costimulation remains normal in HIV infection [51]. Loss of CD28-mediated proliferative signals in T cells may have implications with respect to apoptosis [52], the development of T helper responses [53–55] and virus replication [56,57]. Interfering with the signals delivered by the CD28-B7 pathway results in modulation of T helper responses [53–55,58]. Stimulation of T cells by soluble anti-CD28 antibodies enhances virus replication [57], whereas stimulation by immobilized anti-CD28 antibodies results in synthesis of chemokines and inhibition of virus replication [56]. It is therefore likely that negative correlation of IL-10 production with CD28-mediated T cell responses may have a role in influencing T helper responses and HIV replication.
The status of low IL-10 producers and IL-10 producers with respect to disease progression is not clear at present and could not be distinguished by CD4+ T cell counts. Since a sequential loss of immune responsiveness to recall antigens, alloantigens and PHA has been suggested to be associated with disease progression [5,24], low IL-10 producers that respond to anti-CD28 antibodies may be in an earlier stage of infection and may eventually progress to a more advanced stage with higher levels of IL-10 production and associated immune unresponsiveness. The present cross-sectional study of asymptomatic HIV+ individuals does not specifically address the effect of the virus load on IL-10 production and CD28-induced proliferative responses over time. HIV+ individuals receiving potent anti-retroviral therapy that dramatically reduces the virus load may be the ideal subjects to study these issues. Preliminary results indicate that up-regulation of CD28 expression on CD8+ T cells, enhanced production of IL-2 and IL-12, and increased proliferative responses to recall antigens and mitogens are observed in patients receiving potent anti-retroviral therapy [59]. Studies on the effect of IL-10 on CD28-mediated signals in T cells may provide further insights into the immunopathogenesis of HIV disease.
Acknowledgments
We would like to thank Dr Maurice Gately (Hoffman-LaRoche, Nutley, NJ) for providing us with IL-12 and the reagents for detecting IL-12 by ELISA. We also thank the personnel and patients of the HIV Clinic at the Ottawa General Hospital for providing us with the clinical specimens. Donna Lester is gratefully acknowledged for her secretarial assistance. Drs M. Freedman, K. Wright and M. Kryworuchko are gratefully acknowledged for critically reading the manuscript. This work was supported by grants from the Canadian Foundation for AIDS Research (A.K.) and the Research Institute, Children's Hospital of Eastern Ontario (A.K.).
REFERENCES
- 1.Fauci AS. Host factors and the pathogenesis of HIV-induced disease. Nature. 1996;384:529–34. doi: 10.1038/384529a0. [DOI] [PubMed] [Google Scholar]
- 2.Levy JA. Pathogenesis of human immunodeficiency virus infection. Microbiol Rev. 1993;57:183–289. doi: 10.1128/mr.57.1.183-289.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giorgi JV, Fahey JL, Smith DC, Hultin LE, Cheng HL, Mitsuyasu RT, Detels R. Early effects of HIV on CD4 lymphocytes in vivo. J Immunol. 1987;138:3725–30. [PubMed] [Google Scholar]
- 4.Clerici M, Herkin FT, Venzon DJ, Blatt S, Hendrix GW, Wynn T, Shearer GM. Changes in interleukin-2 and interleukin-4 production in asymptomatic, human immunodeficiency virus-seropositive individuals. J Clin Invest. 1993;91:789–95. doi: 10.1172/JCI116294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clerici M, Stocks NI, Zajac RA, Boswell RN, Lucey DR, Via CS, Shearer GM. Detection of three distinct patterns of T helper cell dysfunction in asymptomatic, human immunodeficiency virus-seropositive patients. Independence of CD4+ cell numbers and clinical staging. J Clin Invest. 1989;84:1892–9. doi: 10.1172/JCI114376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bell SJ, Cooper DA, Kemp BE, Doherty RR, Penny R. Heterogenous effects of exogenous IL-2 on HIV-specific cell mediated immunity (CMI) Clin Exp Immunol. 1992;90:6:–12. doi: 10.1111/j.1365-2249.1992.tb05823.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shearer GM, Clerici M. T helper cell immune dysfunction in asymptomatic, HIV-seropositive individuals: the role of TH1–TH2 cross regulation. Chem Immunol. 1992;54:21–43. [PubMed] [Google Scholar]
- 8.June CH, Bluestone JA, Nadler LM, Thompson CB. The B7 and CD28 receptor families. Immunol Today. 1994;15:321–31. doi: 10.1016/0167-5699(94)90080-9. [DOI] [PubMed] [Google Scholar]
- 9.Linette GP, Hartzman RJ, Ledbetter JA, June CH. HIV-1 infected T cells show a selective signaling defect after perturbation of CD3/antigen receptor. Science. 1988;241:573–6. doi: 10.1126/science.2899908. [DOI] [PubMed] [Google Scholar]
- 10.Banda NK, Bernier J, Kurahara DK, Kurrle R, Haigwood N, Sekaly R-P, Finkel TH. Cross linking CD4 by human immunodeficiency virus gp120 primes T cells for activation induced apoptosis. J Exp Med. 1992;176:1099–106. doi: 10.1084/jem.176.4.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brinchmann JE, Dobloug JH, Heger BH, Haaheim LL, Sannes M, Egeland T. Expression of costimulatory molecule CD28 on T cells in human immunodeficiency virus type 1 infection: functional and clinical correlations. J Infect Dis. 1994;169:730–8. doi: 10.1093/infdis/169.4.730. [DOI] [PubMed] [Google Scholar]
- 12.Roos MThL, Miedema F, De Meinesz AAP, Leeuw Nasm, Pakker NG, Lange JMA, Coutinho RA, Schellekens PThA. Low T cell reactivity to combined CD3 plus CD28 stimulation is predictive for progression to AIDS: correlation with decreased CD28 expression. Clin Exp Immunol. 1996;105:409–15. doi: 10.1046/j.1365-2249.1996.d01-794.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haffar OK, Smithgall MD, Brandshaw J, Brady B, Damle NK, Linsley PS. Costimulation of T-cell activation and virus production by B7 antigen on activated CD4+ T cells from human immunodeficiency virus type-1 infected donors. Proc Natl Acad Sci USA. 1993;90:11094–8. doi: 10.1073/pnas.90.23.11094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haffar OK, Smithgall MD, Wong JGP, Bradshaw J, Linsley PS. Human immunodeficiency virus type 1 infection of CD4+ T cells down regulates the expression of CD28: effect on T cell activation and cytokine production. Clin Immunol Immunopathol. 1995;77:262–70. doi: 10.1006/clin.1995.1152. [DOI] [PubMed] [Google Scholar]
- 15.Zanussi S, Simonelli C, D'Andrea M, Caffau C, Clerici M, Tirelli U, De Paoli P. CD8+ lymphocyte phenotype and cytokine production in long-term non-progressor and in progressor patients with HIV-1 infection. Clin Exp Immunol. 1996;105:220–4. doi: 10.1046/j.1365-2249.1996.d01-746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ding L, Linsley PS, Huang LY, Germain RN, Shevach EM. IL-10 inhibits macrophage costimulatory activity by selectively inhibiting the up regulation of B-7 expression. J Immunol. 1993;151:1224–31. [PubMed] [Google Scholar]
- 17.de Waal Malefyt R, Haanen J, Spits H, et al. Interleukin 10 (IL-10) and viral IL-10 strongly reduce antigen-specific human T cell proliferation by diminishing the antigen-presenting capacity of monocytes via down regulation of class II major histocompatibility complex expression. J Exp Med. 1991;174:915–24. doi: 10.1084/jem.174.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Howard M, O'Garra A, Ishida H, de Waal Malefyt R, de Vries J. Biological properties of interleukin 10. J Clin Immunol. 1992;12:239–47. doi: 10.1007/BF00918147. [DOI] [PubMed] [Google Scholar]
- 19.Del Prete G, De Carli M, Almerigogna F, Giudizi MG, Biagiotti R, Romagnani S. Human IL-10 is produced by both type 1 helper (Th1) and type 2 helper (Th2) T cell clones and inhibits their antigen-specific proliferation and cytokine production. J Immunol. 1993;150:353–60. [PubMed] [Google Scholar]
- 20.Groux H, Bigler M, de Vries JE, Roncarolo M-G. Interleukin-10 induces a long-term antigen-specific anergic state in human CD4+ T cells. J Exp Med. 1994;184:19–29. doi: 10.1084/jem.184.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bacchetta R, Bigler M, Touraine J-L, Parkman R, Tovo P-A, Abrams J, de Waal Malefyt R, Roncarolo M-G. High levels of interleukin 10 production in vivo are associated with tolerance in SCID patients transplanted with HLA mismatched hematopoietic stem cells. J Exp Med. 1994;179:493–502. doi: 10.1084/jem.179.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saville MW, Taga K, Foli A, Broder S, Tosato G, Yarchoan R. Interleukin-10 suppresses human immunodeficiency virus-1 replication in vitro in cells of the monocyte/macrophages lineage. Blood. 1994;83:3591–9. [PubMed] [Google Scholar]
- 23.Akridge RE, Oyafuso LK, Reed SG. IL-10 is induced during HIV-1 infection and is capable of decreasing viral replication in human macrophages. J Immunol. 1994;153:5782–9. [PubMed] [Google Scholar]
- 24.Clerici M, Wynn TA, Berzofsky JA, et al. Role of interleukin-10 in T helper cell dysfunction in asymptomatic individuals infected with the immunodeficiency virus. J Clin Invest. 1994;93:768–75. doi: 10.1172/JCI117031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Diaz-Mitoma F, Kumar A, Karimi S, Kryworuchko M, Daftarian MP, Creery WD, Filion L, Cameron W. Expression of IL-10, IL-4 and interferon-gamma in unstimulated and mitogen-stimulated peripheral blood lymphocytes from HIV-seropositive patients. Clin Exp Immunol. 1995;102:31–39. doi: 10.1111/j.1365-2249.1995.tb06632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Daftarian MP, Diaz-Mitoma F, Creery WD, Cameron W, Kumar A. Dysregulated production of interleukin-10 (IL-10) and IL-12 by peripheral blood lymphocytes from human immunodeficiency virus-infected individuals is associated with altered proliferative responses to recall antigens. Clin Diagn Lab Immunol. 1995;2:712–8. doi: 10.1128/cdli.2.6.712-718.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meyaard L, Hovenkamp E, Keet IPM, Hooibrink B, de Jong IH, Otto SA, Miedema F. Single-cell analysis of IL-4 and IFN-γ production by T cells from HIV-infected individuals. J Immunol. 1996;157:2712–8. [PubMed] [Google Scholar]
- 28.Emilie D, Fior R, Llorente L, et al. Cytokines from lymphoid organs of HIV infected patients: production and role in the immune disequilibrium of the disease and in the development of B lymphomas. Immunol Rev. 1994;140:5–34. doi: 10.1111/j.1600-065x.1994.tb00863.x. [DOI] [PubMed] [Google Scholar]
- 29.Graziosi C, Pantalea G, Gantt KR, Fortin JP, Demarest J-F, Cohen OJ, Sekaly R-P, Fauci AS. Lack of evidence for the dichotomy of Th1 and Th2 predominance in HIV-infected individuals. Science. 1994;265:248–52. doi: 10.1126/science.8023143. [DOI] [PubMed] [Google Scholar]
- 30.Maggi E, Mazzetti M, Ravina A, et al. Ability of HIV to promote a Th1 to Th0 shift and to replicate preferentially in Th2 and Th0 cells. Science. 1994;265:244–8. doi: 10.1126/science.8023142. [DOI] [PubMed] [Google Scholar]
- 31.Chehimi J, Starr SE, Frank I, D'Andrea A, Ma X, MacGregor R, Sennelier J, Trinchieri G. Impaired interleukin-12 production in human immunodeficiency virus-infected patients. J Exp Med. 1994;179:1351–66. doi: 10.1084/jem.179.4.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Romagnani S, Del Prete G, Manetti R, et al. Role of TH1/TH2 cytokines in HIV infection. Immunol Rev. 1994;140:73–92. doi: 10.1111/j.1600-065x.1994.tb00865.x. [DOI] [PubMed] [Google Scholar]
- 33.Zhang M, Gong J, Iyer DV, Jones BE, Modlin RL, Barnes PF. T cell cytokine responses in persons with tuberculosis and human immunodeficiency virus infection. J Clin Invest. 1994;94:2435–42. doi: 10.1172/JCI117611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Estaquier J, Idziorek T, Zou W, Emilie D, Farber CM, Bourez J-M, Ameisen JC. T helper type-1/T helper type-2 cytokines and T cell death. Preventive effect of interleukin 12 on activation induced and CD95 (FAS/APO-1)-mediated apoptosis of CD4+ T cells from human immunodeficiency virus infected persons. J Exp Med. 1995;182:1759–67. doi: 10.1084/jem.182.6.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Coligan JE, Konisbee AM, Margulies DH, Shevach EM, Strober W. New York: John Wiley & Sons; 1992. Current protocols in immunology. [Google Scholar]
- 36.Daftarian MP, Kumar A, Kryworuchko M, Diaz-Mitoma F. IL-10 production is enhanced in human T cells by IL-12 and IL-6 and in monocytes by tumor necrosis factor-α. J Immunol. 1996;157:12–20. [PubMed] [Google Scholar]
- 37.Thiele DL, Lipsky PE. The immunosuppressive activity of L-leucyl-L-leucine methyl ester: selective ablation of cytotoxic lymphocytes and monocytes. J Immunol. 1996;136:1038–48. [PubMed] [Google Scholar]
- 38.Schwartz RH. Costimulation of T lymphocytes: the role of CD28, CTLA-4, and B7/BB1 in interleukin-2 production and immunotherapy. Cell. 1992;71:1065–8. doi: 10.1016/s0092-8674(05)80055-8. [DOI] [PubMed] [Google Scholar]
- 39.Brodie SJ, Sasseville VG, Reimann KA, Simon MA, Sehgal PK, Ringler DJ. Macrophage function in simian AIDS. Killing defects in vivo are independent of macrophage infection, associated with alterations in Th phenotype, and reversible with IFN-γ. J Immunol. 1994;153:5790–801. [PubMed] [Google Scholar]
- 40.Yoo J, Chen H, Kraus T, Hirsch D, Polyak S, George I, Sperber K. Altered cytokine production and accessory cell function after HIV-1 infection. J Immunol. 1996;157:1313–20. [PubMed] [Google Scholar]
- 41.Kootstra NA, van'T Wout AB, Huisman HG, Miedema F, Schuitemaker H. Interference of interleukin-10 with human immunodeficiency virus type 1 replication in primary monocyte-derived macrophages. J Virol. 1994;68:6967–75. doi: 10.1128/jvi.68.11.6967-6975.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gutheil WG, Subramanyam M, Flentke GR, Sanford DG, Munoz E, Huber BT, Bachovchin WW. Human immunodeficiency virus type 1 tat binds to dipeptidyl aminopeptidase 10 (CD26): a possible mechanism for tat's immunosuppressive activity. Proc Natl Acad Sci USA. 1994;91:6594–8. doi: 10.1073/pnas.91.14.6594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Niedermann TM, Garcia JV, Hastings WR, Luria S, Ratner L. Human immunodeficiency virus type 1 nef protein inhibits NF-kappa B induction in human T cells. J Virol. 1992;66:6213–9. doi: 10.1128/jvi.66.10.6213-6219.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Luria S, Chambers I, Berg P. Expression of the type I human immunodeficiency virus nef protein in T cells prevent antigen receptor mediated induction of interleukin-2 mRNA. Proc Natl Acad Sci USA. 1991;88:5326–30. doi: 10.1073/pnas.88.12.5326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gerard C, Bruyns C, Marchant A, et al. Interleukin 10 reduces the release of tumor necrosis factor and prevents lethality in experimental endotoxaemia. J Exp Med. 1993;177:547–50. doi: 10.1084/jem.177.2.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Silva JSP, Morrissey PJ, Grabstein KH, Mohler KM, Anderson D, Reed SG. Interleukin-10 and interferon gamma regulation of experimental Trypanosoma cruzi infection. J Exp Med. 1992;175:169–74. doi: 10.1084/jem.175.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.De Waal Malefyt R, Yssel H, De Vries JE. Direct effects of IL-10 on subsets of human CD4+ T cell clones and resting T cells. Specific inhibition of IL-2 production and proliferation. J Immunol. 1993;150:4754–65. [PubMed] [Google Scholar]
- 48.Creery D, Diaz-Mitoma F, Filion L, Kumar A. Differential modulation of B7-1 and B7-2 isoform expression on human monocytes by cytokines which influence the development of T helper cell phenotype. Eur J Immunol. 1996;26:1273–7. doi: 10.1002/eji.1830260614. [DOI] [PubMed] [Google Scholar]
- 49.Kubin M, Kamoun M, Trinchieri G. Interleukin-12 synergizes with B7/CD28 interaction in inducing efficient proliferation and cytokine production of human T cells. J Exp Med. 1994;180:211–22. doi: 10.1084/jem.180.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cayotta A, Vuillier F, Siciliano J, Dighiero G. Defective protein tyrosine phosphorylation and altered levels of p59fyn and p56lck in CD4 T cells from HIV-1 infected patients. Int Immunol. 1994;6:611–21. doi: 10.1093/intimm/6.4.611. [DOI] [PubMed] [Google Scholar]
- 51.Meyaard L, Kuiper H, Otto SA, Wolthers KC, van Lier RA, Miedema F. Evidence for intact costimulation via CD28 and CD27 molecules in hyporesponsive T cells from human immunodeficiency virus-infected individuals. Eur J Immunol. 1995;25:232–7. doi: 10.1002/eji.1830250138. [DOI] [PubMed] [Google Scholar]
- 52.Noel PJ, Boise LH, Green JM, Thompson CB. CD28 costimulation prevents cell death during primary T cell activation. J Immunol. 1996;157:636–42. [PubMed] [Google Scholar]
- 53.Corry DB, Reiner SL, Linsley PS, Locksley RM. Differential effects of blockade of CD28-B7 on development of Th1 or Th2 effector cells in experimental Leishmaniasis. J Immunol. 1994;153:4142–8. [PubMed] [Google Scholar]
- 54.Sayegh MH, Akalin E, Hancock WW, Russel ME, Carpenter CB, Linsley PS, Turka LA. CD28-B7 blockade after alloantigenic challenge in vivo inhibits Th1 cytokines but spares Th2. J Exp Med. 1995;181:1869–74. doi: 10.1084/jem.181.5.1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.King CL, Stupi RJ, Craighead N, June CH, Thyphronitis GH. CD28 activation promotes Th2 subset differentiation by human CD4+ cells. Eur J Immunol. 1995;25:587–95. doi: 10.1002/eji.1830250242. [DOI] [PubMed] [Google Scholar]
- 56.Levine BL, Mosca JD, Riley JL, et al. Antiviral effect and ex vivo CD4+ T cell proliferation in HIV-positive patients as a result of CD28 costimulation. Science. 1996;272:1939–43. doi: 10.1126/science.272.5270.1939. [DOI] [PubMed] [Google Scholar]
- 57.Asjo B, Cefai D, Debre P, Dudoit Y, Autran B. A novel mode of human immunodeficiency virus type 1 (HIV-1) activation: ligation of CD28 alone induces HIV-1 replication in naturally infected lymphocytes. J Virol. 1993;67:4395–8. doi: 10.1128/jvi.67.7.4395-4398.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Thompson CB. Distinct roles for the costimulatory ligands B7-1 and B7-2 in T helper cell differentiation? Cell. 1995;81:979–82. doi: 10.1016/s0092-8674(05)80001-7. [DOI] [PubMed] [Google Scholar]
- 59.Angel JB, Kumar A, Parato K, et al. Rapid and sustained improvement in cell mediated immune responses during potent anti-HIV therapy with ritonavir and saquinavir. J Infect Dis. 1998;177:898–904. doi: 10.1086/515244. [DOI] [PubMed] [Google Scholar]