Abstract
Paracoccidioidomycosis (PCM) is a systemic mycosis endemic in Latin America, with a high prevalence in Brazil, Argentina, Colombia and Venezuela. The aetiologic agent of disease is a thermal dimorphic fungus, Paracoccidioides brasiliensis. A glycoprotein of 43 000 D (gp43) is the major antigen of P. brasiliensis. Antibodies directed to this antigen are detected in the sera of all patients with PCM. Gp43 binds to laminin, thus participating in adhesion, invasion and pathogenesis of the fungus. As the role of antibodies in PCM is not fully understood, we decided to investigate the outcome of mice immunization with three distinct anti-gp43 MoAbs (17c, 8a and 24a) coupled with keyhole limpet haemocyanin (KLH). Results show not only the expected presence of anti-Id (AB2) antibodies in the sera of these animals but also a spontaneous and increasing amount of anti-anti-Id (AB3) antibodies after the third course of immunization. Hybridomas producing both AB2 and AB3 MoAbs were obtained using spleen cells from mice immunized with MoAb 17c. AB3 MoAbs were also obtained with spleen cells of mice immunized with MoAbs 8a and 24a. It was also shown that human PCM patients' sera with high titres of anti-gp43 antibodies generate anti-Id antibodies. These data suggest that the immune response to P. brasiliensis can be spontaneously modulated by the idiotypic network.
Keywords: Paracoccidioides brasiliensis, gp43, idiotypic network, monoclonal antibodies, human infection
INTRODUCTION
Paracoccidioidomycosis (PCM) is a systemic mycosis restricted to Latin America, with large endemic areas in Brazil, Colombia, Venezuela and Argentina [1]. The disease is caused by the thermal dimorphic fungus Paracoccidioides brasiliensis, which grows as a characteristic multiple budding yeast in the host or at 37°C, or as mycelium at 25°C [1]. Although the natural habitat of P. brasiliensis is not known, it is assumed that humans become infected by inhalation of fungal propagules which differentiate into infective yeast forms in the lungs. Yeast multiplies in the parenchyma, inducing a host response characterized by a primary complex followed by granuloma formation [1–3]. Pulmonary or lymph node granulomas may remain dormant for years or progress to overt disease, depending on the host's immune defence. Different courses of the disease are described, ranging from chronic to acute forms of PCM. In both cases, dissemination to other organs can occur [1–3].
The main antigenic component of P. brasiliensis is a 43-kD surface glycoprotein (gp43). Gp43 is biochemically characterized as a high-mannose concanavalin A (Con A) binding glycoprotein, reviewed by Travassos [4]. Since gp43 is recognized by all patients' sera, the molecule is used in serological assays for diagnostic purposes [5,6]. Other biological functions have been proposed for this glycoprotein. Our group has characterized gp43 as a laminin-binding protein implicated in fungal pathogenesis in vivo [7]. Others have shown that gp43 express immunodominant epitopes eliciting T cell-dependent delayed hypersensitivity reaction inducing a T CD4+ lymphocyte proliferation response in humans and experimental animals [8].
MoAbs anti-gp43 were obtained and fully characterized by our group [9]. Our panel of 12 anti-gp43 MoAbs is directed against peptidic portions of the target molecule and recognize at least three distinct epitopes. All anti-gp43 MoAbs were able to recognize all gp43 isoforms, with pIs ranging from 5.8 to 8.5 [9,10]. It was also shown that some MoAbs such as 17c and 24a were able to partially inhibit the laminin-dependent fungal adhesion to epithelial cells, and one of them, MoAb 24a, significantly reduced P. brasiliensis infection in vivo. On the other hand, one anti-gp43 MoAb (8a) increased both laminin-dependent fungal adhesion and pathogenesis [9].
The role played by anti-gp43 antibodies in the evolution and maintenance of PCM infection is not fully understood. As antibodies against this glycoprotein can be detected during all stages of the disease, some authors consider these antibodies non-protective and unrelated to fungal pathogenesis.
According to the network hypothesis proposed by Jerne [11], anti-idiotypic antibodies (anti-Id) are part of the normal immune response resulting in a web of interacting idiotypic (Id) antibodies. Idiotypes (Id) are the sum of idiotopes or serologically determined antigenic determinants, unique to an antibody or group of antibodies [11,12]. The potential immune regulatory role of Id–anti-Id interactions has been intensively investigated [13,14]. Anti-Id antibodies recognizing antigenic determinants that overlap a portion of the combining site that is in contact with the original antigen are said to carry its ‘internal image’. Although those anti-Id antibodies, also known as AB2β, are able to mimic the antigen, they represent a small fraction of all anti-Id antibodies produced [12]. By definition, AB2β compete with the external antigen in the binding to antibodies (AB1) raised against it [15]. This mimicry points out the relevance of these antibodies from both immunological and biological points of view. Recently, the therapeutic potential of anti-Id antibodies has been exploited in different systems [12,16,17].
Considering that anti-Id antibodies are components of the immune response, we decided to investigate the possible generation of anti-anti-Id (AB3) in mice immunized with MoAbs directed to gp43. The presence of anti-Id (AB2) in the sera of PCM-infected patients was also studied. Our results show spontaneous modulation of the idiotypic cascade in the P. brasiliensis gp43 system in both mice and humans.
MATERIALS AND METHODS
Animals
Six-week-old female BALB/c mice (H-2d) were obtained from the Ludwig Institute for Cancer Research, São Paulo branch. Mice were 6–8 weeks old at the beginning of the study.
MoAbs
All anti-gp43 MoAbs 17c, 24a and 8a (all IgG2b, κ light chain) [9], MoAb anti-carcinoembryonic antigen (CEA) 5.D11 (IgG2a, κ light chain) [18], anti-Id MoAb 6.C4 (IgG2b) [19], anti-anti-Id anti-CEA MoAb 1.H2 (IgG1, κ light chain) [20] and anti-Schistosoma mansoni (IgM, κ light chain) (a generous gift from Dr C. R. W. Carneiro, UNIFESP) were used for immunization purposes or as irrelevant antibodies. All MoAbs used in the study were affinity-purified in Sepharose Protein A (Pharmacia, Uppsala, Sweden) or Sepharose Protein G (Pharmacia), according to their isotypes and to the manufacturer's instructions.
Immunization
Groups of five BALB/c mice were immunized subcutaneously or intraperitoneally with 100 μg of AB1 MoAbs coupled to keyhole limpet haemocyanin (KLH), obtained as described before [19]. For the first and second immunizations, mice were always injected subcutaneously in four sites (axillary and inguinal) with MoAbs coupled to KLH emulsified in Freund's complete adjuvant (FCA; 1:2, v/v) to a final volume of 100 μl/site (first immunization). All subsequent immunizations, i.p. or s.c., were performed as described above with the immunogen emulsified in Freund's incomplete adjuvant (FIA), each at 3-week intervals. For hybridoma production, 2 days before cell fusion, a final booster of 100 μg of KLH-coupled anti-gp43 Moabs was administered intravenously. Before each immunization mice were bled through the ocular plexus, serum was separated by centrifugation and stored at −20°C.
Human serum specimens
All PCM patients' sera or those from healthy donors were obtained from Hospital São Paulo, Federal University of São Paulo. Individual serum specimens from 13 patients with PCM with chronic multifocal form of the disease were selected based on clinical diagnosis of PCM and confirmed by positive direct examination of characteristic multiple budding yeast forms either in histopathology or in other biological fluids. All patients' sera were positive for anti-gp43 antibodies by both immunodiffusion test [21] and capture enzyme immunoassay (EIA) [6].
Gp43 purification, enzymatic deglycosylation and iodination
Paracoccidioides brasiliensis strain B-339 was grown in TOM medium and exoantigen was prepared as described elsewhere [9]. For gp43 purification, exoantigen was fractionated by affinity chromatography in Sepharose coupled with anti-gp43 MoAb 17c [9]. The resulting affinity-purified gp43 was always checked in 10% polyacrylamide gels (SDS–PAGE) and protein content was estimated by the Bradford method [22]. Deglycosylation of affinity-purified gp43 was performed with recombinant PGNase (New England Biolabs, Beverly, MA) as described elsewhere [9]. For radioimmunoassays (RIAs), affinity-purified gp43 was labelled with 125I by the IodoGen method [23] to a specific activity of 3–5 μCi/μg.
Immunoassays
Detection of anti-Id (AB2) antibodies in immunized mice and AB2 MoAb inhibition assays
Mice sera anti-Id titres were determined by competitive inhibition RIA, as previously described [19], with some modifications. Briefly, 20 μg/ml of anti-gp43 MoAbs (17c, 24a or 8a) in PBS were added to 96-well polystyrene microtitre plate (Costar Corp., Cambridge, MA) (100 μl/well) for 1 h at 37°C. Free sites were blocked with PBS containing 1% bovine serum albumin (PBS–BSA 1%) for 1 h at room temperature. Wells were incubated overnight with 50 μl of 125I-gp43 in the presence or absence of 50 μl of serially diluted mice sera. Wells were then exhaustively washed with PBS containing 0.5% gelatin (Difco Labs, Detroit, MI) and 0.05% Tween 20 (Sigma Chemical Co., St Louis, MO) (PBS–T–G) and radioactivity was counted in a γ counter (Packard Instrument Co., Grove, IL). The same competitive inhibition assay was used to characterize anti-Id MoAbs (AB2 MoAbs). Briefly, wells were coated with 20 μg/ml anti-gp43 MoAbs (17c, 40.d7, 10d, 27a, 3e or 8a), blocked with PBS–BSA 1% and incubated with 125I-gp43 in the absence or presence of an excess of anti-Id MoAb (AB2). Detection of captured 125I-gp43 was estimated as described above.
Detection and characterization of anti-anti-Id (AB3) in immunized mice sera
Detection of anti-anti-Id (AB3) antibodies in both gp43 and CEA systems was performed by EIA. Briefly, coated polyvinyl microplates (Costar) with affinity-purified gp43 or CEA (100 ng/well), diluted in PBS, were adsorbed for 1 h at 37°C. After blocking free sites with PBS–BSA 1%, 50 μl of PBS-diluted mice sera were incubated for 1 h at 37°C. Microplates were then washed with PBS–T–G and treated with goat anti-mouse IgG labelled with peroxidase at 37°C for 1 h. Wells were washed with PBS–T–G, reactions developed with o-phenylenediamine (OPD) in 0.1 m acetate-phosphate buffer pH 5.8, interrupted with 4 n H2SO4 and read in a Titertek (Finland) Multiscan EIA reader at 492 nm. The same EIA was employed to characterize the affinity-purified AB3 MoAbs for their ability to recognize the target antigen (gp43).
Detection of anti-gp43 antibodies (AB1 and AB3) in human PCM sera
PCM patients' sera were diluted 1:2500, 1:25 000 and 1:250 000 (v/v) in PBS and assayed as described before [6,24]. Briefly, polyvinyl microplates (Costar) were coated with 20 μg/ml of anti-gp43 MoAb 17c in PBS (100 μl/well), during 1 h at 37°C. After blocking free sites with PBS–BSA 1%, wells were treated with 100 μl of affinity-purified gp43 (10 μg/ml) for 2 h, 37°C. After gp43 capture, wells were exhaustively washed with PBS–T–G and incubated with human PCM sera diluted 1:2500, 1:25 000 and 1:250 000 in PBS (v/v) for 1 h, 37°C. After incubation, wells were washed three times with PBS–T–G and treated for 1 h, 37°C, with 100 μl of goat anti-human IgG coupled with peroxidase (Sigma). After that, wells were treated as described above.
Detection of anti-gp43 antibodies in human PCM sera using anti-Id MoAb as antigen surrogate
Anti-Id MoAb 7.B12 (20 μg/ml, 100 μl/well) was used to coat polyvinyl microplates (Costar) and free sites were blocked as described above. Wells were treated with PBS-diluted human PCM sera for 1 h at 37°C. After washing with PBS–T–G, microplates were treated with peroxidase-labelled goat anti-human IgG (Sigma), washed exhaustively with PBS–T–G. Reactions and readings were performed as described above.
Detection of anti-Id (AB2) in human PCM sera
All human PCM sera or from healthy volunteers were diluted 1:2000 (v/v) in PBS and the presence of AB2 was determined by a modification of the assay described for the detection of human anti-gp43 antibodies as described above. Briefly, microplate (Costar) wells were coated with 100 μl of 20 μg/ml of a mixture of murine anti-gp43 MoAbs 8a and 17c (10 μg/ml each) for 1 h, 37°C, and free sites were blocked with PBS–BSA 1%. Then wells were incubated with PCM patients' sera for 2 h at 37°C. Afterwards, plates were treated using the same conditions already described.
Hybridoma production
Anti-Id (AB2) and anti-anti-Id (AB3) MoAbs for the gp43 system were produced according to Lopes & Alves [25]. Double screening of colonies was made by both competitive inhibition RIA assay (for AB2 detection of hybridoma cells) and EIA (for AB3 antibodies), as described above. After cloning by limiting dilution and expanding positive clones, large amounts of antibodies were obtained by production of ascites in BALB/c mice previously primed with Pristane (Sigma). MoAbs were purified from ascites fluids by affinity chromatography in a Protein G column. Immunoglobulin isotyping was performed with Mouse Antibody Isotyping kit (Gibco, BRL Life Technologies, Grand Island, NY), following the manufacturer's instructions.
SDS–PAGE and immunoblot analysis
SDS–PAGE was performed on vertical slab gels of 10% acrylamide, always under reducing conditions [26]. Immunoblots were performed as described elsewhere [27].
Statistical analysis
Data were analysed by Student's t-test or by anova.
RESULTS
Induction of anti-Id antibodies (AB2) in BALB/c mice
The outcome of BALB/c mice i.p. or s.c. immunization with KLH-coupled anti-gp43 MoAb 17c (AB1) was based on the fact that anti-Id antibodies (AB2), especially those mimicking the target antigen (AB2β), can inhibit the binding of 125I-gp43 to MoAb 17c, used as immunogen. After the second immunization high AB2 titres were observed in all immunized mice, despite some individual variations (data not shown). However, further immunizations produced a marked reduction in the binding inhibition of 125I-gp43 to MoAb 17c in all mice sera. Figure 1a shows AB2 titres from a representative mouse.
Fig. 1.
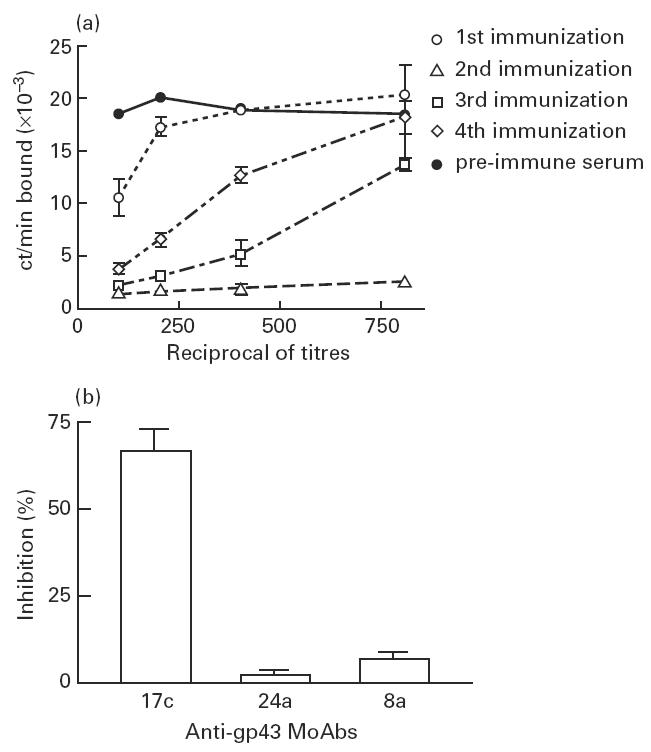
Mice AB2 titres after immunization with anti-gp43 MoAb 17c and idiotope specificity of sera. (a) Binding of 125I-gp43 to anti-gp43 MoAb 17c-coated plates performed in the presence of diluted sera from a representative immunized mouse with anti-gp43 MoAb 17c, as indicated in Materials and Methods. (b) Binding of 125I-gp43 to anti-gp43 MoAb 17c-coated plates performed in the presence of diluted sera from immunized mice with anti-gp43 MoAb 24a and 8a. No relevant inhibition of binding was observed when mice sera immunized with the latter MoAbs were added. Assays were made in triplicates.
Since those findings could reflect a particularity of the Id expressed by MoAb 17c, we investigated AB2 induction by two other anti-gp43 MoAbs, 8a and 24a. In relation to anti-gp43 MoAb 17c, these MoAbs recognize another distinct epitope of the target antigen, gp43. Figure 2 shows the results obtained with both anti-gp43 MoAbs 8a (Fig. 2a) and 24a (Fig. 2b) from representative immunized mice. Similarly to MoAb anti-gp43 17c, higher AB2 titres were observed after the second immunization, and reduction of titres in subsequent immunizations.
Fig. 2.
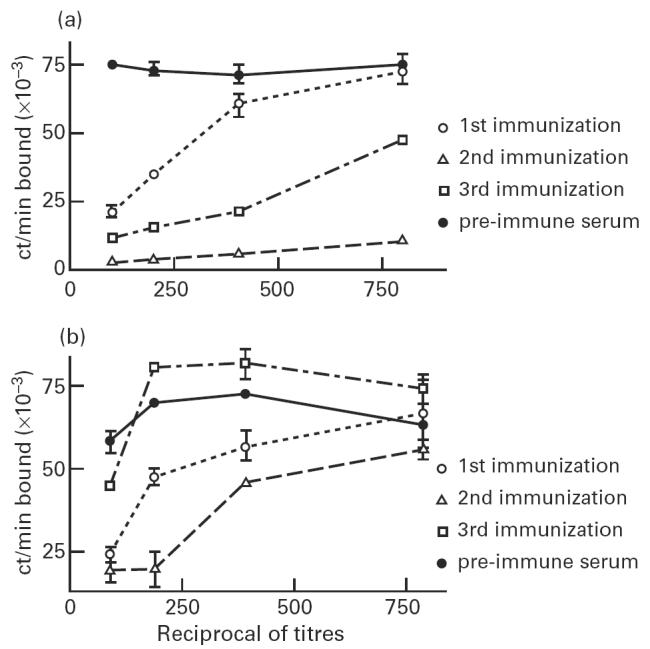
Mice AB2 titre after immunization with anti-gp43 MoAbs 8a and 24a. Binding of 125I-gp43 to anti-gp43 MoAb 8a (a) or 24a-coated plates (b) was performed in the presence of diluted sera from representative immunized mice with anti-gp43 MoAb 8a or 24a, respectively, as indicated in Materials and Methods. All assays were made in triplicates.
The same binding inhibition assay was performed to determine the specificity of idiotope reactivity from sera of mice immunized with anti-gp43 MoAbs. As shown in Fig. 1b, diluted sera from the second immunization of immunized mice with either anti-gp43 MoAb 24a or 8a did not inhibit the binding of 125I-gp43 to MoAb 17c. Contrariwise, an average of 72% inhibition was observed when sera of mice immunized with MoAb 17c were used.
Detection of anti-anti-Id antibodies (AB3) in BALB/c mice immunized with anti-gp43 MoAbs
Since all sera from mice immunized with anti-gp43 MoAbs showed lower AB2 titres after the second immunization, we investigated whether this reduction was associated with the appearance of AB3 antibodies, directed against the induced AB2. If so, mice sera should recognize gp43. The results obtained in all mice sera showed a significant enhancement of antibodies recognizing gp43 after the third immunization, correlating with reduced AB2 titres. Figure 3 shows AB3 titres of a representative mouse immunized with anti-gp43 MoAb 17c. Similar results were obtained with mice immunized with other anti-gp43 MoAbs (not shown).
Fig. 3.
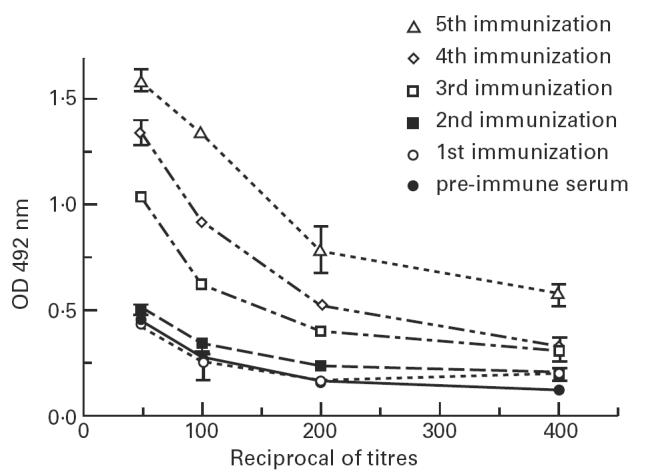
Mice AB3 titres after immunization with anti-gp43 MoAb 17c. Recognition of gp43 from Paracoccidioides brasiliensis by diluted mice sera immunized with anti-gp43 MoAb 17c by EIA, as described in Materials and Methods. All assays were made in triplicates.
Comparison between mice immunized with anti-gp43 and anti-CEA MoAbs
Figure 4 shows AB3 induction of antibodies in immunized mice in two different systems. For this purpose groups of mice were immunized with two MoAbs, one anti-gp43 (MoAb 17c) and the other directed against CEA (MoAb 5.D11). After the 5th immunization it can be seen that anti-CEA MoAb 5.D11 did not induce significant AB3 titres, compared with mice immunized with anti-gp43 MoAb 17c (P < 0.001).
Fig. 4.
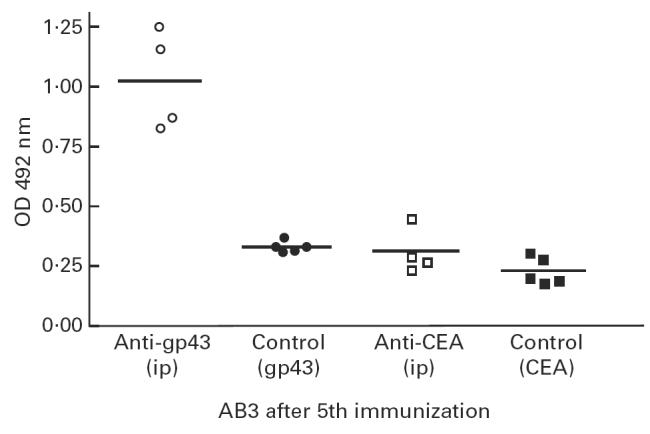
Comparison of AB3 induction in mice immunized intraperitoneally (i.p.) with anti-gp43 MoAb 17c and anti-carcinoembryonic antigen (CEA) MoAb 5.D11. After the fifth immunization, diluted mice sera in triplicates (1:50 v/v in PBS) were assayed for recognition of coated gp43 from Paracoccidioides brasiliensis or CEA, as indicated in Materials and Methods. Induction of AB3 production in mice immunized with anti-gp43 MoAb was statistically significant (anova, P < 0.001).
Hybridoma production
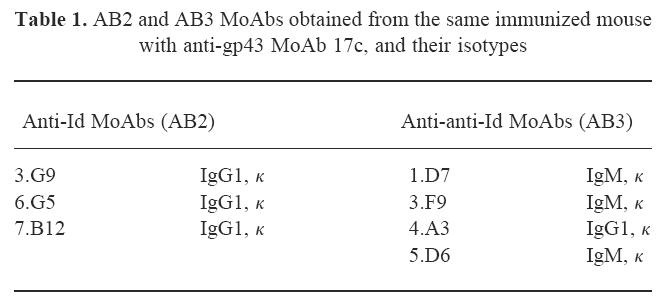
To characterize further the idiotypic cascade modulation in mice immunized with anti-gp43 MoAb 17c, hybridomas simultaneously producing MoAbs AB2 and AB3 were obtained from fused spleen cells of the same animal. Table 1 shows the MoAbs AB2 and AB3, with their isotypes. MoAbs AB3 were also obtained from immunized mice with anti-gp43 MoAbs 8a (MoAb 3.D6, IgM, κ) and 24a (MoAb 2.D5, IgM, κ).
Table 1.
AB2 and AB3 MoAbs obtained from the same immunized mouse with anti-gp43 MoAb 17c, and their isotypes
Characterization of anti-Id MoAbs (MoAbs AB2)
All affinity-purified AB2 MoAbs were able to inhibit (> 90%) the binding of 125I-gp43 to MoAb 17c (data not shown). Further studies indicated that AB2 MoAb 7.B12 was able inhibit, on different levels, the binding of 125I-gp43 to other anti-gp43 MoAbs, which were seen to compete with MoAb 17c in gp43 recognition. As can be seen in Fig. 5, anti-Id MoAb 7.B12 was able to inhibit completely (> 98%) AB1 MoAbs 10d and 3e, whereas no inhibition could be detected in others (anti-gp43 MoAbs 40.d7 and 27a). No inhibition was observed with MoAb 8a, which clearly binds to another portion of the antigen.
Fig. 5.
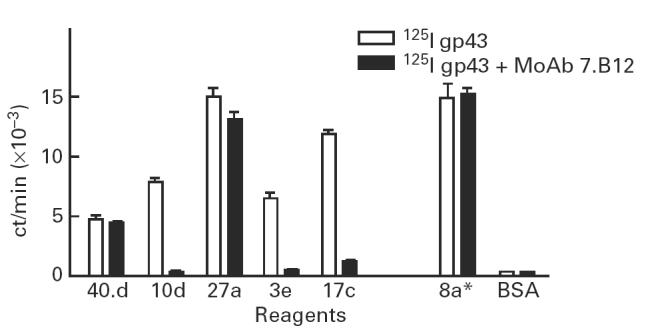
Binding inhibition of 125I-gp43 to anti-gp43 MoAbs by anti-Id MoAb 7.B12. Binding of 125I-gp43 to anti-gp43 MoAbs 40.d7, 10d, 27a, 3e and 17c in the presence or absence of 100 μg/ml of anti-Id MoAb 7.B12 (AB2). Binding of 125I-gp43 to anti-gp43 MoAb 8a, which recognized another peptidic epitope of the target antigen, was used as control. Bovine serum albumin (BSA) was used as an irrelevant protein. Assays were made in triplicates.
One AB2 MoAb, 7.B12, was able to detect the presence of anti-gp43 antibodies in human PCM sera. Figure 6 shows the results obtained from three representative PCM patients, showing the potential use of AB2 in serological assays.
Fig. 6.
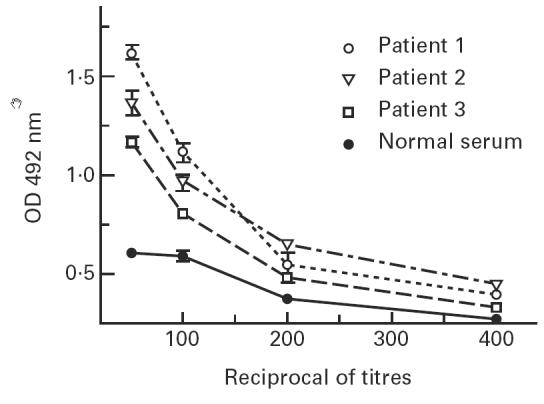
Detection of anti-gp43 antibodies in paracoccidioidomycosis patients’ sera using anti-Id MoAb 7.B12 as gp43 surrogate. Coated wells with anti-Id MoAb 7.B12, which carries the internal image of gp43, were used as antigen substitute for human sera anti-gp43 antibody determination, as described in Materials and Methods.
Characterization of anti-anti-Id MoAbs (MoAbs AB3)
Affinity-purified anti-anti-Id MoAbs (AB3) were shown to recognize specifically gp43 from P. brasiliensis in both EIA and immunoblots. Figure 7 shows an EIA assay with one of those AB3, the MoAb 1.D7.
Fig. 7.
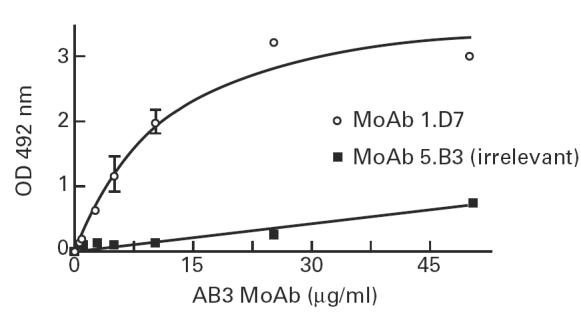
Binding of anti-anti-Id MoAb 1.D7 (AB3) to gp43 in EIA assay. Gp43-coated plates were recognized by purified anti-anti-Id MoAb 1.D7, in concentrations ranging from 1 to 50 μg/ml. MoAb 5.B9 was used as irrelevant IgM, as indicated in Materials and Methods.
Through immunoblotting, all AB3 MoAbs were seen to detect specifically gp43 from exoantigens from yeast cultures of P. brasiliensis. Figure 8, lane C shows the gp43 recognition by MoAb 4.A3. It was also observed that AB3 MoAbs recognized the deglycosylated form of gp43 (p38), thus binding through peptidic epitopes on the gp43 (Fig. 8, lane D). No reactivity with either gp43 or p38 was observed with anti-anti-Id 1.H2 (CEA AB3) used as irrelevant antibody (data not shown).
Fig. 8.
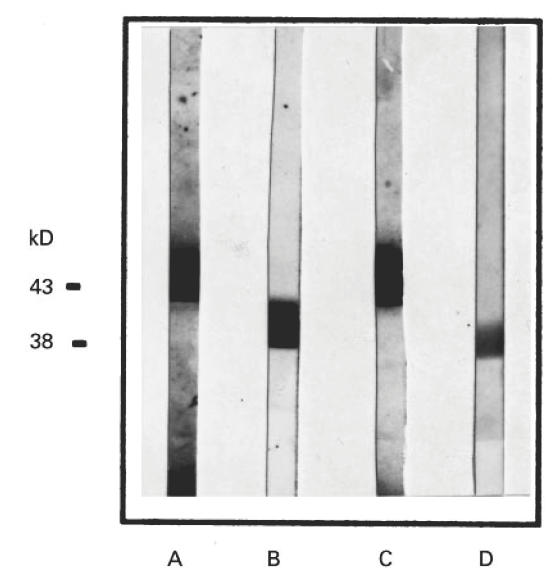
Reactivity of anti-anti-Id MoAb 4.A3 to gp43 and its deglycosylated form, p38, in immunoblots. Exoantigens and deglycosylated gp43 (p38) were transferred to nitrocellulose membranes as indicated in Materials and Methods. Lanes A and C show the specific recognition of gp43 in crude exoantigens by anti-gp43 MoAb 17c (control) and anti-anti-Id MoAb 4.A3 (AB3), respectively. Lanes B and D show the recognition of the gp43 deglycosylated form by AB1 MoAbs 17c and AB3 MoAb 4.A3, respectively.
Detection of human anti-Id antibodies in PCM sera
After characterization of idiotypic modulation in the gp43 system in mice, we examined whether the idiotypic cascade could also occur in human PCM infection. For this purpose, we selected PCM sera with high titres of anti-gp43 antibodies. Using a modification of the EIA capture assay, human anti-Id antibodies (AB2) were detected. Figure 9 shows significant detection of AB2 in 1:2000 diluted PCM sera (P < 0.0001) compared with sera from healthy volunteers.
Fig. 9.
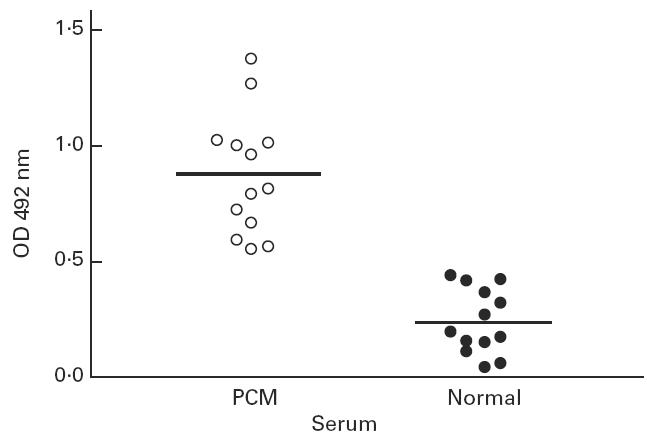
Detection of anti-Id antibodies (AB2) in paracoccidioidomycosis (PCM) patients. Human PCM patients' sera with high titres of anti-gp43 antibodies were assayed as described in Materials and Methods. The lines indicate the mean values for both PCM sera and healthy donors (Student's t-test, P < 0.0001).
DISCUSSION
Lymphocyte antigen receptors (i.e. immunoglobulin on B cells or T cell receptor on T cells) express antigenic determinants that can be recognized and elicit a humoral or cellular immune response in xenogeneic, allogeneic, syngeneic or even in autologous animals [11–13,15,16]. Thus, foreign antibody molecules given to an individual or new antibodies arising in serum have the potential to induce immune responses, mainly directed to variable region determinants known collectively as idiotypes (Ids). Jerne's network theory predicts that anti-Id antibodies bearing the internal image of the corresponding antigen are able to induce immunity to the original antigen in hosts not previously exposed to it [11,15–17]. It has been suggested that the immune response can be regulated at the level of recognition of idiotype determinants via the idiotype–anti-idiotype network [13,14,28]. While there is substantial evidence indicating that anti-idiotypic antibodies are normal components of the immune response, their significance in its regulation remains unclear [29,30]. Although it is generally assumed that anti-Id antibodies which arise in an intact network will reduce immune responses associated with the particular idiotype, anti-idiotypic networks may also have the capacity to increase immune responses [31,32].
All patients with PCM mount both humoral and cellular immune responses against gp43 from P. brasiliensis [5,8]. The role of antibodies against gp43 in the evolution and maintenance of P. brasiliensis infection remains unclear. Gp43 has also been characterized as a potential virulence factor [9,10]. To understand further the importance of anti-gp43 antibodies in the fungal infection, we describe in both mice and humans that Id antibodies against gp43 spontaneously induce an idiotypic cascade.
All immunized BALB/c mice with anti-gp43 MoAbs (AB1), which recognize different peptidic epitopes on the molecule, produced a humoral response characterized by inhibiting antibodies of the binding of labelled gp43 to the anti-gp43 MoAbs (AB1) used as immunogen. The idiotope specificity of sera from anti-gp43 MoAb (AB1)-immunized mice was also verified. No significant cross-reactivity was detected when mice sera from the second immunization with either anti-gp43 MoAb 8a or 24a were added in the binding assay of 125I-gp43 to anti-gp43 MoAb 17c (Fig. 1b). These results show that immunization with anti-gp43 MoAbs produced a specific response against the idiotope displayed by the AB1 used as immunogen. Moreover, these results suggest that those induced anti-Id antibodies (AB2) recognize the same epitopes reactive with the original antibody (AB1). By this criterion, such anti-Id antibodies are referred to as internal images or AB2β [15,16]. Antibodies with these properties have been induced in several animal species and with different antigenic systems involving virus, bacteria, fungi, parasites, toxins, hormones and tumoural antigens [12,16]. Such AB2β antibodies can be used as antigen surrogates in several circumstances, especially to overcome immunological tolerance as seen in tumour-associated antigens (TAA), opening perspectives in cancer immunotherapy, as recently reviewed by Herlyn et al. [17].
Mice immunized with anti-gp43 MoAbs showed higher AB2β titres after the second challenge. Surprisingly, however, subsequent injections resulted in a significant reduction of AB2β levels. These results could be due either to a tolerized response to subsequent immunizations with the same MoAbs or, more probably, to stimulation of other B cell clones producing antibodies directed against AB2β, or anti-anti-Id antibodies (AB3). In the latter scenario, depending on affinities involved, AB3 could form immune complexes with AB2β, thus resulting in a lower inhibition by mice sera of the binding of labelled gp43 to anti-gp43 MoAbs. If AB2β antibodies were inducing an AB3 humoral response, mice sera should then contain antibodies recognizing gp43, even though the animals were never exposed to it. Thus, when sera from AB1-immunized mice were assayed for direct recognition of gp43, results showed that significant levels of AB3 could be observed after the third immunization, in parallel with the diminution of AB2β detection. Subsequent immunizations with AB1 resulted in further enhancement of AB3 antibody production, probably reflecting a higher production of AB2β antibodies, although in this phase of the immunization procedures they could not be detected by our assay.
While evidence to support the immunomodulatory role of anti-idiotypes in an intact system is not extensive, there are considerable data demonstrating the connectivity of network reactions, such as those that follow injections of antigen giving rise to AB2 [16,17] as well as injections of AB1 inducing antigen-specific AB3 [31–40], in several antigenic systems such as systemic lupus erythematosus (SLE) [31–33], cancer patients treated with anti-TAA MoAbs [34–36], viruses [37] or parasites such as S. mansoni [38].
To investigate whether the generation of AB3 by AB1 is a common feature, we compared serological recognition of nominal antigen in two distinct systems. Groups of mice were injected with anti-gp43 MoAb 17c or with MoAb 5.D11, an antibody against CEA [18,19]. High densities of this molecule are expressed on the cell surface of a majority of human colorectal and also on several other adenocarcinomas [39–41]. Mice sera recognition of gp43 in the group treated with anti-gp43 MoAb 17c produced, after the 5th injection, high titres of AB3, as expected. However, sera of anti-CEA MoAb 5.D11-immunized mice did not significantly recognize CEA, indicating a lower, if any, induction of AB3 antibodies, despite previous reports that this MoAb is able to induce high titres of AB2β antibodies [19]. Moreover, in the CEA system, our group demonstrated that high-affinity AB3 could be induced in mice immunized with AB2β [19,20]. Those different results can depend on specific characteristics of Ids expressed by anti-gp43 AB1s, which have the capacity to modulate the idiotypic cascade. Recently, Comacchio et al. [42] observed marked differences between an AB1-induced antigen-specific AB3 response in both rabbits and mice, depending on the antigen system used, human albumin or trinitrophenyl (TNP) hapten.
Because of the complexity and heterogeneity of antibody populations in conventional antisera, it is not possible to analyse the fine specificity of anti-Id (AB2) and anti-anti-Id (AB3) elicited by anti-gp43 MoAbs. Therefore, to dissect the humoral immune response induced by anti-gp43 MoAbs, the production of a panel of AB2 and AB3 MoAbs could be of help. If our assumptions are correct, i.e. that anti-gp43 MoAbs were spontaneously modulating the idiotypic cascade, we should then be able to obtain hybridomas producing both MoAbs AB2β and AB3 from fused spleen cells of the same immunized animal. This was achieved by means of double screening the hybridoma supernatants. Supernatants that competed in the binding of anti-gp43 MoAb 17c to labelled gp43 were from AB2β-secreting hybridomas, whereas those secreting antibodies recognizing gp43 contained AB3 antibodies. Thus, three AB2β stable hybridomas (all IgG1, κ light chain) and four AB3 MoAbs (three IgM and one IgG1, all κ light chain) were obtained. It is interesting to note that at least one AB3 MoAb elicited by MoAb 17c belongs to the IgG1 subclass, suggesting that the immune response involved in the generation of AB3 is T cell-dependent. Similar results were obtained in other systems which describe AB3 MoAbs [20,43].
All AB2 MoAbs inhibited (> 90%) the binding of gp43 to MoAb 17c, suggesting that those anti-Id MoAbs bind to the idiotope, thus fulfilling the internal image criteria proposed by Nisonoff & Lamoyi [15] and confirming our previous findings with mice sera. We then investigated whether the AB2 MoAbs could inhibit the binding of gp43 to other anti-gp43 MoAbs which are known to compete with MoAb 17c in gp43 peptidic recognition [9]. Our results show that AB2 MoAb 7.B12 was able to inhibit (> 95%) the binding of gp43 to anti-gp43 MoAbs 17c, 10d and 3e. As expected, anti-Id MoAb 7.B12 was not able to inhibit gp43 binding to MoAb 8a, since this anti-gp43 MoAb recognizes the antigen through a distinct epitope. However, MoAb 7.B12 was not able to inhibit the binding of labelled antigen by MoAbs 40.d7 and 27a, which compete with MoAb 17c in gp43 binding assays [9]. The use of anti-Id MoAbs in the study of fine epitope recognition of AB1 MoAbs to their antigen was also performed in other systems [19]. Recently, Fields et al. [44] studied the interaction of an idiotope–anti-idiotope through crystallography, showing that MoAb AB1 contacts the antigen and the anti-Id MoAb through essentially the same combining-site residues. Furthermore, it was shown that contacts between anti-Id MoAb and AB1 MoAb were similar to those between the antigen and AB1. The authors suggest that the mimicking of the antigen epitope by anti-Id MoAb is functional, involving similar binding interactions between atoms of amino acid residues in the binding site rather than exact topological replicas [44]. It can thus be speculated that anti-Id MoAb 7.B12 contacts efficiently anti-gp43 MoAbs 17c, 10d and 3e, but not the other anti-gp43 MoAbs (40.d7 and 27a), since the presence of Ab2β MoAb 7.B12 caused no interference in their binding to gp43.
Taking the investigation further, we asked whether Ab2β MoAb 7.B12 could act as antigen surrogate in serological assays. By testing three PCM patients' sera, it was found that anti-Id MoAb 7.B12 could substitute gp43. The fact that the patients' sera reacted with relatively low titres could be due to intrinsic affinities of both anti-Id MoAb 7.B12 and human anti-gp43 antibodies, or it could reflect the small population of human anti-gp43 antibodies which can bind to the Ab2β MoAb idiotope.
By immunochemical criteria it is difficult to discriminate between AB2β and AB2γ. To sort out both types of AB2 a functional demonstration should be observed. Since AB2β express an idiotype that mimics the antigen, it might have the capacity to elicit antibodies specific for the antigen in the same or different species [16]. The production and characterization of AB3 MoAbs confirms our findings that immunization with anti-gp43 MoAbs to modulate the idiotypic cascade by eliciting AB2β antibodies that, in turn, have the capacity to stimulate specific B cell clones to secrete AB3 antibodies. All AB3 MoAbs recognized specifically gp43 in EIA assays, showing a typical saturation pattern. Moreover, one AB3 MoAb, 4.A3, recognized both gp43 as well as its deglycosylated form by immunoblots, meaning that this AB3 MoAb binds the antigen through peptidic portions of the molecule, as AB1 MoAb 17c does.
According to Goldbaum et al. [45], there are two major possibilities for the induction of anti-anti-Id (AB3) that resemble AB1. One is that a suitably selected anti-Id will mimic the antigen so closely as to produce the equivalent of an antibody response to the original antigen. A second possibility is that the anti-Id is specific for the Id and will thus stimulate lymphocytes displaying anti-anti-Id immunoglobulins that should be identical or essentially identical to the AB1. In their study, results seem to favour the second hypothesis, since AB3 showed greater binding equilibrium constants for the anti-Id than for the nominal antigen and because of sequence similarities between AB1 and AB3 MoAbs. In this light, it is conceivable to hypothesize that our anti-gp43 AB3 MoAbs were generated through a similar mechanism.
To corroborate those findings, we investigated whether the idiotypic cascade is also triggered in human PCM. In order to identify AB2 antibodies in human PCM, we selected sera with high anti-gp43 titres, more prone to contain AB2 antibodies. Our results showed significant levels of AB2 antibodies reacting with the MoAb 17c idiotype, suggesting a modulation of the idiotypic cascade during the course of the disease.
Although no autoimmune phenomena have been associated with PCM, it should be mentioned that the spontaneous modulation of the idiotypic cascade in anti-gp43 MoAbs (ABs1)-immunized mice resulted in great mortality, especially when those animals were further immunized (6th immunization) (data not shown). Although histopathological studies could not detect any pathology caused by immune complexes in spleen, lungs, liver or kidneys, sera analysis revealed an important increase of gammaglobulin levels, suggesting a polyclonal activation (data not shown). In several systems it has been shown that immunization of various strains of mice with monoclonal or polyclonal human or mouse anti-DNA antibodies, carrying a pathogenic idiotype, ended in the production of a set of SLE-related autoantibodies by the animals [46]. Recently, Shoenfeld [47] proposed that an infection with any microorganism or parasite leads to the generation of antibodies against the invading organism and that some of these antibodies carry ‘pathogenic idiotypes’, which exist as part of the normal immune response repertoire. These Ids recognized by T helper cells generate an anti-Id reaction which in the healthy subjects leads, after eradication of the invading organism, to the decrease in the idiotype titre to an almost undetectable level [47]. In subjects prone to autoimmunity, the idiotypic cascade will proceed from AB2 to the generation of AB3, in a process that may last weeks, months or years [47]. It should be noted that titre of anti-gp43 antibodies in human patients does not correlate with the clinical criteria of cure. Some patients can have high titres of anti-gp43 antibodies even though no overt clinical manifestation of the disease is observed (Z. P. Camargo and J. R. Alves, personal communication). In this case, the stimulation of the idiotypic cascade could be responsible for the observed high anti-gp43 antibodies in some patients.
The results presented here show for the first time the modulation of the idiotypic network in P. brasiliensis infection and open the possibility of a better understanding of the importance of anti-gp43 antibodies in paracoccidioidomycosis.
Acknowledgments
We are indebted to Drs Rosana Puccia and Luiz R. Travassos for the generous gift of anti-gp43 MoAbs, and to Dr Célia R. W. Carneiro for providing the irrelevant monoclonal antibody used and for helpful discussions. We are also indebted to Dr Euripides Ferreira for helpful suggestions. We thank Creuza Rosa de Oliveira and Laura Dias Batista for technical assistance. This work was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) and Financiadora Nacional de Projetos (FINEP). A.R.S. and C.R.B.C. are recipients of grants from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and J.S. from PIBIC-CNPq.
REFERENCES
- 1.Brummer E, Castañeda E, Restrepo A. Paracoccidioidomycosis: an update. Clin Microbiol Rev. 1993;6:89–117. doi: 10.1128/cmr.6.2.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Montenegro MR, Franco M. Pathology. In: Franco M, Lacaz CS, Restrepo-Moreno A, Del Negro G, editors. Paracoccidioidomycosis. Boca Raton: CRC Press Inc; 1994. pp. 131–50. [Google Scholar]
- 3.Negroni R. Paracoccidioidomycosis (South American Blastomycosis, Lutz's mycosis) Intl J Dermatol. 1993;32:847–59. doi: 10.1111/j.1365-4362.1993.tb01396.x. [DOI] [PubMed] [Google Scholar]
- 4.Travassos LR. Immunochemistry of Paracoccidioides brasiliensis antigens. In: Franco M, Lacaz CS, Restrepo-Moreno A, Del Negro G, editors. Paracoccidioidomycosis. Boca Raton: CRC Press Inc; 1994. pp. 67–86. [Google Scholar]
- 5.Camargo ZP, Cano LER. Humoral immunity. In: Franco M, Lacaz CS, Restrepo-Moreno A, Del Negro G, editors. Paracoccidioidomycosis. Boca Raton: CRC Press Inc; 1994. pp. 187–201. [Google Scholar]
- 6.Camargo ZP, Gesztesi JL, Saraiva ECO, et al. Monoclonal antibody capture enzyme immunoassay for detection of Paracoccidioides brasiliensis antibodies in paracoccidioidomycosis. J Clin Microbiol. 1994;32:2377–81. doi: 10.1128/jcm.32.10.2377-2381.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vicentini AP, Gesztesi JL, Franco MM, et al. Binding of Paracoccidioides brasiliensis to laminin through surface glycoprotein gp43 leads to enhancement of fungal pathogenesis. Infect Immun. 1994;62:1465–9. doi: 10.1128/iai.62.4.1465-1469.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Travassos LR, Puccia R, Cisalpino P, et al. Biochemistry and molecular biology of the main antigen of Paracoccidioides brasiliensis. Arch Med Res. 1995;26:297–304. [PubMed] [Google Scholar]
- 9.Gesztesi J-L, Puccia R, Travassos LR, et al. Monoclonal antibodies against the 43,000 Da glycoprotein from Paracoccidioides brasiliensis modulate laminin-mediated fungal adhesion to epithelial cells and pathogenesis. Hybridoma. 1996;15:415–21. doi: 10.1089/hyb.1996.15.415. [DOI] [PubMed] [Google Scholar]
- 10.Moura Campos MCR, Gesztesi J-L, Vicentini AP, et al. Expression and isoforms of gp43 in different strains of Paracoccidioides brasiliensis. J Med Vet Mycol. 1995;33:223–7. doi: 10.1080/02681219580000461. [DOI] [PubMed] [Google Scholar]
- 11.Jerne NK. Towards a network theory of the immune response. Ann Immunol Paris. 1974;125C:373–89. [PubMed] [Google Scholar]
- 12.Pan Y, Yuhasz SC, Amzel LM. Anti-idiotypic antibodies: biological function and structural studies. FASEB J. 1995;9:43–49. doi: 10.1096/fasebj.9.1.7821758. [DOI] [PubMed] [Google Scholar]
- 13.Jerne NK, Roland J, Cazenave PA. Recurrent idiotopes and internal images. EMBO J. 1982;1:243–7. doi: 10.1002/j.1460-2075.1982.tb01154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bona CA, Köhler H. Anti-idiotypic antibodies and internal images. Recept Biochem Methodol. 1984;14:141–9. [Google Scholar]
- 15.Nisonoff A, Lamoyi E. Implications of the presence of internal images of the antigen in anti-idiotypic antibodies: possible applications to vaccine production. Clin Immunol Immunopathol. 1981;21:397–406. doi: 10.1016/0090-1229(81)90228-2. [DOI] [PubMed] [Google Scholar]
- 16.Bona CA. Internal image concept revisited. Proc Soc Exp Biol Med. 1996;213:32–42. doi: 10.3181/00379727-213-44033. [DOI] [PubMed] [Google Scholar]
- 17.Herlyn D, Somasundaram R, Li W, et al. Anti-idiotype cancer vaccines: past and future. Cancer Immunol Immunother. 1996;43:65–76. doi: 10.1007/s002620050305. [DOI] [PubMed] [Google Scholar]
- 18.Carneiro CRW, Lopes JD, Brentani RR. Carcinoembryonic antigen (CEA): production of immunoprecipitating monoclonal antibodies and development of an enzyme immunoassay. Hybridoma. 1987;6:689–92. doi: 10.1089/hyb.1987.6.689. [DOI] [PubMed] [Google Scholar]
- 19.Moraes JZ, Carneiro CRW, Buchegger F, et al. Induction of an immune response through the idiotypic network with monoclonal anti-idiotype in the carcinoembryonic antigen system. J Cell Biochem. 1992;50:324–35. doi: 10.1002/jcb.240500313. [DOI] [PubMed] [Google Scholar]
- 20.Moraes JZ, Gesztesi J-L, Westermann P, et al. Anti-idiotypic monoclonal antibody AB3, reacting with the primary antigen (CEA), can localize human colon-carcinoma xenografts as efficiently as AB1. Int J Cancer. 1994;57:586–91. doi: 10.1002/ijc.2910570424. [DOI] [PubMed] [Google Scholar]
- 21.Camargo ZP, Unterkircher C, Campoy S, et al. Production of Paracoccidioides brasiliensis exoantigens for immunodiffusion tests. J Clin Microbiol. 1988;26:2147–51. doi: 10.1128/jcm.26.10.2147-2151.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bradford MM. A rapid and sensitive method for the quantification of micrograms quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 23.Fraker JP, Speck J C. Protein and cell membrane iodinations with a sparingly soluble chloramine 1,3,4,6-tetra-chloro-3a,6a-diphenylglycoluryl. Biochem Biophys Res Commun. 1978;80:849–57. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- 24.Souza MC, Gesztesi J-L, Souza AR, et al. Differences in reactivity of paracoccidioidomycosis sera with gp43 isoforms. J Med Vet Mycol. 1997;35:13–18. doi: 10.1080/02681219780000811. [DOI] [PubMed] [Google Scholar]
- 25.Lopes JD, Alves MJM. Production of monoclonal antibodies by somatic cell hybridization. In: Morel CM, editor. Genes and parasites: a laboratory manual. Rio de Janeiro: Fundação Oswaldo Cruz; 1983. pp. 386–98. [Google Scholar]
- 26.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 1970;224:680–5. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 27.Townbin H, Staehelin T, Gordon J. Electrophoretic transfer of the proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang YF, Thanavala Y. A comparison of the antibody and T cell response elicited by internal image and noninternal image anti-idiotypes. Clin Immunol Immunopathol. 1995;75:154–8. doi: 10.1006/clin.1995.1065. [DOI] [PubMed] [Google Scholar]
- 29.Rodey GE. Anti-idiotypic antibodies and regulation of immune responses. Transfusion. 1992;32:361–72. doi: 10.1046/j.1537-2995.1992.32492263453.x. [DOI] [PubMed] [Google Scholar]
- 30.Nisonoff A. Idiotypes: concepts and applications. J Immunol. 1991;147:2429–38. [PubMed] [Google Scholar]
- 31.Dang H, Ogawa N, Takei M, et al. Induction of lupus-associated autoantibodies by immunization with native and recombinant Ig polypeptides expressing a cross-reactive idiotype 4b4. J Immunol. 1993;151:7260–7. [PubMed] [Google Scholar]
- 32.Faberger J, Frodin J-E, Wigzell H, et al. Induction of an immune network cascade in cancer patients treated with monoclonal antibodies (ab1): I. May induction of ab1-reactive T cells and anti-anti-idiotypic antibodies (ab3) lead to tumor regression after mAb therapy? Cancer Immunol Immunother. 1993;37:264–70. doi: 10.1007/BF01518521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mendlovic S, Brocke S, Shoenfeld Y, et al. Induction of a systemic lupus erythematosus-like disease in mice by a common human anti-DNA idiotype. Proc Natl Acad Sci USA. 1988;85:2260–4. doi: 10.1073/pnas.85.7.2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wettendorff M, Iliopoulos D, Tempero M, et al. Idiotypic cascades in cancer patients treated with monoclonal antibody CO17-1A. Proc Natl Acad Sci USA. 1989;86:3787–91. doi: 10.1073/pnas.86.10.3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Viale G, Flamini G, Grassi F, et al. Idiotypic replica of an anti-human tumor-associated antigen monoclonal antibody. Analysis of monoclonal Ab1 and Ab3 fine specificity. J Immunol. 1989;143:4338–44. [PubMed] [Google Scholar]
- 36.Fagerberg J, Ragnhammar P, Liljefors M, et al. Humoral anti-idiotypic and anti-anti-idiotypic immune response in cancer patients treated with monoclonal antibody 17-1A. Cancer Immunol Immunother. 1996;42:81–87. doi: 10.1007/s002620050255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McCullough KC, Butcher RN. Function of idiotypic networks in vivo: immunisation with idiotype-bearing (Id1) antibody induces further production of Id1 antibody. Immunol Letters. 1991;27:9–12. doi: 10.1016/0165-2478(91)90236-4. [DOI] [PubMed] [Google Scholar]
- 38.Percy ASJ, Harn TM, Vakil M, et al. Monoclonal anti-idiotypic and anti-anti-idiotypic antibodies from mice immunized with a protective monoclonal antibody against Schistosoma mansoni. J Immunol. 1988;140:2760–2. [PubMed] [Google Scholar]
- 39.Muraro R, Wunderlich D, Thor A. Definition by monoclonal antibodies of repertoire of epitopes on carcinoembryonic antigen differentially expressed in human colon carcinoma versus normal adult tissues. Cancer Res. 1985;45:5769–80. [PubMed] [Google Scholar]
- 40.Steward AM, Nixon D, Zamcheck N, et al. Carcinoembryonic antigen in breast cancer patients: serum levels and disease progress. Cancer. 1974;33:1246–52. doi: 10.1002/1097-0142(197405)33:5<1246::aid-cncr2820330509>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 41.Vincent RG, Chu TM. Carcinoembryonic antigen in patients with carcinoma of the lung. J Throrac Cardiovasc Surg. 1978;45:5769–80. [PubMed] [Google Scholar]
- 42.Comacchio RM, Bradley J, Hohmann AW. A comparison of the anti-idiotypic responses generated by antibodies to a protein and a hapten: a common interspecies idiotype on antibodies against human albumin induces an idiotypic network in rabbits. Immunol Cell Biol. 1996;74:72–80. doi: 10.1038/icb.1996.10. [DOI] [PubMed] [Google Scholar]
- 43.Yang H, Chen ZJ, Kageshita T, et al. Idiotypic cascade in the human high molecular weight melanoma-associated antigen system: fine specificity and idiotypic profile of anti-anti-idiotypic monoclonal antibodies. Eur J Immunol. 1993;23:1671–7. doi: 10.1002/eji.1830230741. [DOI] [PubMed] [Google Scholar]
- 44.Fields BA, Goldbaum FA, Ysern X, et al. Molecular basis of antigen mimicry by an anti-idiotope. Nature (London) 1995;374:739–42. doi: 10.1038/374739a0. [DOI] [PubMed] [Google Scholar]
- 45.Goldbaum FA, Velikovsky CA, Dall'Acqua W, et al. Characterization of anti-anti-idiotypic antibodies that bind antigen and an anti-idiotype. Proc Natl Acad Sci USA. 1997;94:8697–701. doi: 10.1073/pnas.94.16.8697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Blank M, Krup M, Mendlovic S, et al. The importance of the pathogenic 16/6 idiotype in the induction of SLE in naive mice. Scand J Immunol. 1990;13:291–302. doi: 10.1111/j.1365-3083.1990.tb02741.x. [DOI] [PubMed] [Google Scholar]
- 47.Shoenfeld Y. Common infection, idiotypic dysregulation, autoantibody spread and induction of autoimmune diseases. J Autoimmun. 1996;9:235–9. doi: 10.1006/jaut.1996.0029. [DOI] [PubMed] [Google Scholar]


