Abstract
Neutrophils are the predominant inflammatory cell in the lung tissues and airways in RSV infection, and can augment the epithelial cell damage induced by RSV. Neutrophil apoptosis has been suggested to be a mechanism to reduce the potential for tissue injury. The apoptosis of neutrophils from nasopharyngeal aspirates (NPA) (n = 19) and peripheral blood (PB) of infants with RSV bronchiolitis (n = 11) and PB from healthy controls (n = 9) was investigated. Monoclonal antibody against CD95 (Fas) and a binding protein Annexin V were used to determine the apoptosis of neutrophils. The expression of CD11b and CD18 on neutrophils was also detected with flow cytometry. The mean fluorescence intensity (MFI) of CD95 on neutrophils from RSV+ NPA was increased compared with cells from control PB (73.6 ± 7.6 versus 31.5 ± 4.3); the MFI of Annexin V, CD11b and CD18 on neutrophils from RSV+ NPA was up-regulated compared with cells from both control PB (105.3 ± 18.1 versus 11.8 ± 1.5; 1683 ± 153.3 versus 841.1 ± 72.3; 517 ± 50.5 versus 147 ± 8.7, respectively) and RSV+ PB (105.3 ± 18.1 versus 35.8 ± 4.1; 1683 ± 153.3 versus 818 ± 141.2; 517 ± 50.5 versus 260 ± 25.8, respectively). Furthermore, the percentage of neutrophils expressing Annexin V and the MFI of CD18 on neutrophils from RSV+ PB were increased compared with neutrophils from control PB. In addition, both CD11b (MFI) and CD18 (MFI) correlated with Annexin V (MFI) on neutrophils. We conclude that neutrophil apoptosis in RSV bronchiolitis is accelerated; and CD11b/CD18 may play an important role in RSV infection by influencing neutrophil apoptosis.
Keywords: neutrophil, apoptosis, regulation, respiratory syncytial virus, bronchiolitis
INTRODUCTION
Polymorphonuclear neutrophils (PMN) play an important role in inflammation. A mechanism determining if inflammation resolves or progresses to a chronic inflammatory state, characteristic of some inflammatory diseases, is the process by which recruited neutrophils and their potentially injurious contents are removed from inflamed tissues [1]. Cell death can be thought of as occurring by either necrosis or apoptosis. Neutrophil death by necrosis or disintegration has the potential to induce cellular damage by release of granular contents [2]. Apoptotic neutrophils are recognized and phagocytosed by macrophages via either the vitronectin receptor or the phosphatidylserine (PS) receptor [3]. This apoptotic process has been suggested to represent a mechanism in vivo to limit the release of neutrophil contents from disintegrating cells that could have the potential to cause tissue injury and amplify inflammation [4].
RSV is the most frequent cause of bronchiolitis and pneumonia in infants requiring hospitalization [5], and is associated with significant morbidity and mortality in seriously affected children [6]. This virus causes epithelial necrosis and peribronchial infiltration with leucocytes, especially with a high percentage of neutrophils in the lung tissues [7] and airway lumen [8]. Neutrophils and their products are likely to have an important role in the various clinical and pathological changes occurring in RSV infection of the airways. It has been reported that RSV infection of respiratory epithelial monolayers can promote neutrophil adherence which could, in turn, potentially contribute to the airway injury and obstruction that accompanies bronchiolitis [9]. Our recent studies show that neutrophils augment the damage to respiratory epithelial cells induced by RSV infection in vitro [10]. The modulation of neutrophil apoptosis in RSV infection in vivo may regulate the epithelial damage induced by neutrophils. To date, however, there are no published studies of neutrophil apoptosis in RSV infection, although a recent study shows that HIV infection accelerates neutrophil apoptosis [11].
We hypothesized that RSV infection may accelerate neutrophil apoptosis in vivo, which could subsequently modulate the neutrophil-induced damage to epithelial cells. In the following study we investigate the apoptosis of neutrophils from peripheral blood (PB) and nasopharyngeal aspirates (NPA) of infants with RSV bronchiolitis and from PB of control infants, by detecting the expression of Fas (CD95) and PS receptor on neutrophils with MoAbs against CD95 and a binding protein Annexin V, respectively [12,13]. As neutrophil apoptosis has been shown to be associated with the expression of CD11b/CD18 on neutrophils [14], the relationship between neutrophil apoptosis and the expression of β2-integrin CD11b/CD18 on neutrophils in RSV bronchiolitis is also investigated.
MATERIALS AND METHODS
Reagents
MoAbs against CD95 (Fas, Dx22; Pharmingen, San Diego, CA) and CD18 (7E4; Coulter, Hialeah, FL) were used in this study. MoAb against CD11b (2LPM19c; Dako, Carpinteria, CA) was directly conjugated to PE. Silenus anti-mouse Fab2 fragment (second antibody) was directly conjugated to FITC (AMRAD, Boronia, Australia). Fluorescein-conjugated Annexin V (Annexin V–FITC) and propidium iodide (PI; 50 μg/ml) were supplied by Pharmingen. A binding buffer (10 mm HEPES/NaOH, pH 7.4, 140 mm NaCl, 2.5 mm CaCl2) was used to wash cells after staining.
Selection of patients
Nineteen RSV+ infants between 1 and 13 months old (median 4.9 months), admitted for acute bronchiolitis to Flinders Medical Centre (Adelaide, South Australia), were enrolled. The RSV antigens were detected from an aliquot of NPA by ELISA. The infants were healthy until the time of RSV infection, and all had a severe infection with coughing, wheezing, chest overinflation and tachypnoea.
A healthy control group of nine infants between 1 and 12 months of age (median 5.2 months) who required blood tests prior to minor surgery was selected.
Informed consent for this study was given by the parents of RSV-infected and control infants. The study was approved by the Clinical Investigations (Ethics) Committee at Flinders Medical Centre.
Collection and separation of NPA
For the nasal wash, children were placed in the supine position, and one or two drops of sterile normal saline were placed in each nostril. The nasal airway was suctioned with a fine catheter. Secretions and lavage fluid were collected into a trap which was connected to a suction tube About 0.5 ml of NPA was collected from both nostrils and diluted with saline to a volume of 4 ml. A 2-ml aliquot of the diluted NPA was used for detecting viruses (RSV, influenza virus A and B, adenovirus, and parainfluenza virus 1, 2, 3). The other 2-ml aliquot of diluted NPA was put on ice immediately for isolation of neutrophils.
To dissolve the mucus further, 50 μl of N-acetylcysteine (2 g in 10 ml) (David Bull Labs, Adelaide, Australia) were added to the 2 ml NPA. The NPA was mixed gently with a 5-ml syringe or a pipette. Total cell counts were obtained by haemocytometer, and an aliquot was smeared onto slides, then dried, fixed and stained for morphologic assessment of cells in the fluid. The suspended NPA was spun at 514 g for 5 min at 4°C. The supernatant was collected and stored at −80°C for later cytokine analysis. The cell pellet was resuspended in 5 ml of Dulbecco's modified Eagle's medium (DMEM; Gibco BRL, Life Technologies, Inc., Grand Island, NY) with high glucose, l-glutamine, and 5% fetal calf serum (FCS) for isolating neutrophils. The process used for the isolation of neutrophils from NPA is similar to the isolation of neutrophils from PB.
Isolation of neutrophils
Immediately before each experiment, NPA neutrophils (n = 19) and PB neutrophils (n = 11) from infants infected with RSV and from healthy control infants (n = 9) were isolated by Lymphoprep (Hycomed Pharma AS, Oslo, Norway) and 3% dextran (T500; Pharmacia Biotech, Uppsala, Sweden) sedimentation techniques [15]. Residual erythrocytes in the granulocyte-rich fraction were eliminated by hypotonic lysis in 0.2% sodium chloride twice for 20 s each time. This resulted in a cell fraction containing > 97% neutrophils with > 97% viability as determined by trypan blue exclusion. Neutrophils were suspended in DMEM at a concentration of 1 × 107/ml and were put on ice for flow cytometric analysis.
Duplicate controls were undertaken on the effect of N-acetylcysteine, which was used in the process of isolating neutrophils from NPA. No effect was observed on the apoptosis of neutrophils from PB of healthy controls.
Neutrophils were attempted to be obtained from NPA of four healthy age-matched control infants. Insufficient neutrophils were obtained for analysis by NPA from these infants.
Flow cytometric analysis
Neutrophils (5 × 105 in 50 μl) were incubated for 15 min at room temperature in the dark with 5 μl of Annexin V, or 10 μl of PI (50 μg/ml), with one tube as blank control. Binding buffer (400 μl) was added to each tube. Cells were analysed by flow cytometry within 1 h.
Neutrophils (5 × 105 in 50 μl) were incubated on ice for 30 min with an excess concentration of MoAbs (CD95, CD18, with X63 used as a negative control). The cells were washed twice with PBS plus sodium azide and labelled with Silenus anti-mouse Fab2 fragment conjugated to FITC diluted 1:100. The cells were incubated on ice for another 30 min. After two washes with PBS, the cells were incubated for 10 min on ice with 5 μl of mouse IgG to block non-specific binding. Then 5 μl of MoAb against CD11b conjugated with PE were added to the cells and incubated on ice for another 30 min in order to discriminate neutrophils from other cells. After washing in PBS, the cells were analysed by FACScan flow cytometry (CellQuest; Becton Dickinson, Mountain View, CA) with standard settings. Ten thousand cells were analysed from each sample. Mean fluorescence intensity (MFI) was measured by flow cytometry as a marker of cell surface expression of adhesion molecules.
Statistical analysis
Data are given as mean ± s.e.m. Data were analysed using a one-way analysis of variance (anova). A Bonferroni test was used to identify differences between individual groups except where indicated. P < 0.05 was considered significant.
RESULTS
The expression of CD95 (Fas) on neutrophils in RSV bronchiolitis
The MFI of CD95 on neutrophils from RSV+ NPA (73.6 ± 7.6) was markedly increased compared with cells from control PB (31.5 ± 4.3). No significance was seen between RSV+ PB group and control PB group, although independent t-test showed a significant difference (P < 0.01) (Fig. 1).
Fig. 1.
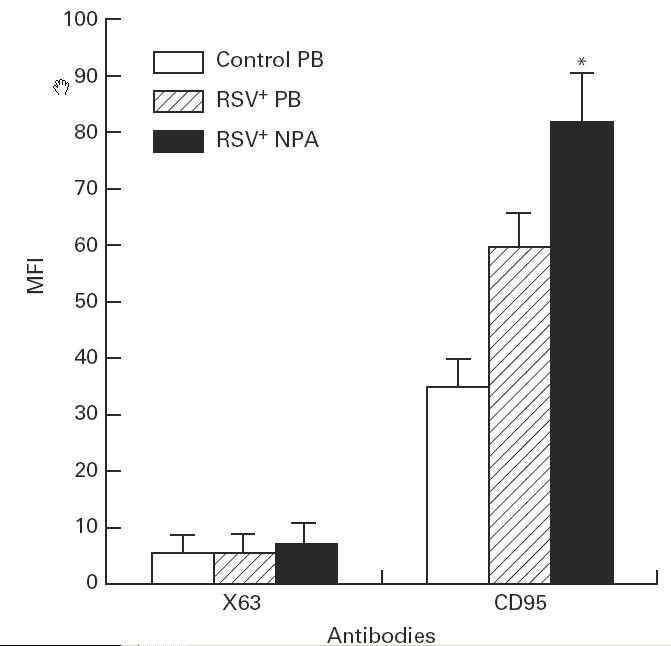
The mean fluorescence intensity (MFI) of CD95 expressed on neutrophils in RSV bronchiolitis. The MFI of CD95 on neutrophils obtained from control peripheral blood (PB) (n = 9), RSV+ PB (n = 11), and from RSV+ nasopharyngeal aspirates (NPA) (n = 19) was detected by cytometric analysis. The values are expressed as mean ± s.e.m. *P < 0.05 compared with control PB group.
The expression of PI on neutrophils in RSV bronchiolitis
The positive percentage expression of PI on neutrophils from both RSV+ PB (22.1 ± 3.8) and RSV+ NPA (24.6 ± 3.4) was increased compared with cells from control PB (7.5 ± 0.5). There was no difference between RSV+ PB group and RSV+ NPA groups (Fig. 2). The MFI of PI on neutrophils from RSV+ NPA (54.8 ± 14.6) was increased compared with cells from control PB (15.4 ± 0.8) (Fig. 3). No statistical difference was seen between the RSV+ PB group and the control group.
Fig. 2.
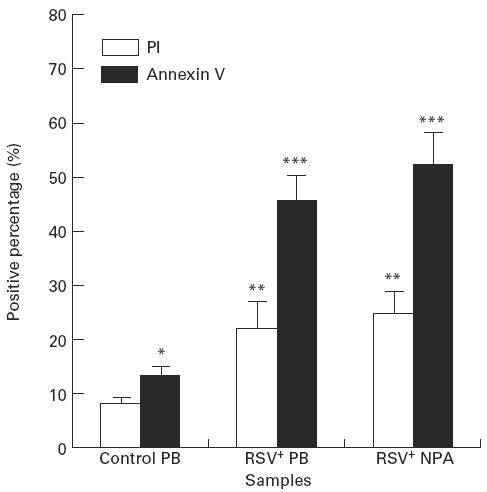
The comparison of positive percentage expression of Annexin V with propidium iodide (PI) on neutrophils in RSV bronchiolitis. The positive percentage of PI and Annexin V on neutrophils obtained from control peripheral blood (PB) (n = 9), RSV+ PB (n = 8), and from RSV+ nasopharyngeal aspirates (NPA) (n = 13) was determined by flow cytometric analysis. The values are expressed as mean ± s.e.m. *P < 0.05 compared with PI group (paired t-test); **P ≤ 0.05 compared with control PB group; ***P ≤ 0.05 compared with both control PB group and PI group.
Fig. 3.
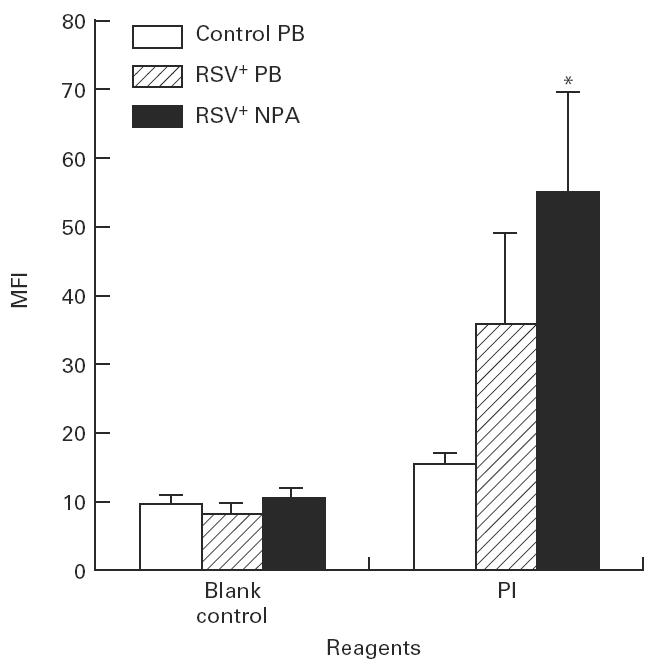
The mean fluorescence intensity (MFI) of propidium iodide (PI) expressed on neutrophils in RSV bronchiolitis. The MFI of PI on neutrophils obtained from control peripheral blood (PB) (n = 9), RSV+ PB (n = 8), and from RSV+ nasopharyngeal aspirates (NPA) (n = 13) was detected by flow cytometric analysis. The values are expressed as mean ± s.e.m. *P < 0.05 compared with control PB group.
The expression of Annexin V on neutrophils in RSV bronchiolitis
The positive percentage expression of Annexin V on neutrophils from both RSV+ PB (45.8 ± 4.8) and RSV+ NPA (52.5 ± 4.4) was increased compared with cells from control PB (13.5 ± 1.2) (Fig. 2). There was no significant difference between the RSV+ PB group and the RSV+ NPA group. The MFI of Annexin V on neutrophils from RSV+ NPA (105.3 ± 18.1) was increased compared with cells from both control PB (11.8 ± 1.5) and RSV+ PB (35.8 ± 4.1) (Fig. 4). No statistical significance was seen between the RSV+ PB group and the control PB group using Bonferroni test, although independent t-test showed that the MFI of Annexin V on neutrophils from RSV+ PB was increased compared with cells from control PB (P < 0.001).
Fig. 4.
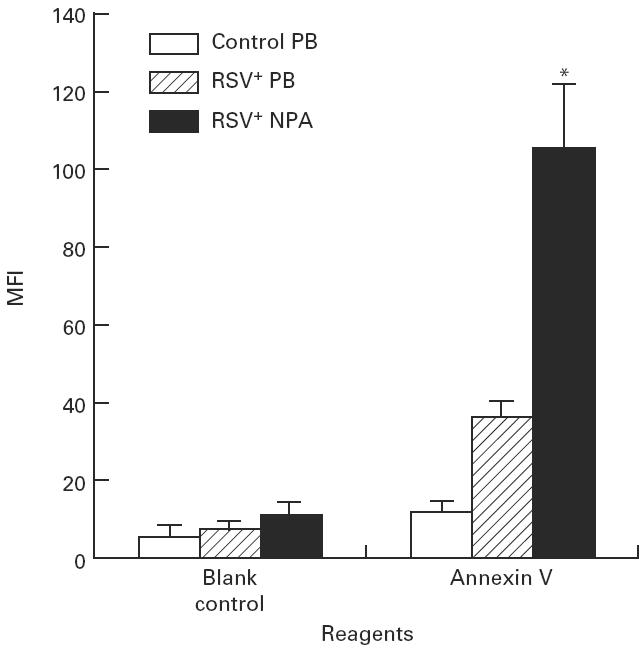
The mean fluorescence intensity (MFI) of Annexin V expressed on neutrophils in RSV bronchiolitis. The MFI of Annexin V on neutrophils obtained from control peripheral blood (PB) (n = 9), RSV+ PB (n = 8), and from RSV+ nasopharyngeal aspirates (NPA) (n = 13) was detected with flow cytometric analysis. The values are expressed as mean ± s.e.m. *P < 0.05 compared with control PB group.
The positive percentage expression of Annexin V was significantly higher compared with the positive percentage of PI on neutrophils from control PB, RSV+ PB, and RSV+ NPA, respectively, using independent t-test (Fig. 2).
Regression analysis showed that Annexin V (MFI) and Annexin V (%) correlated well with CD95 (MFI) (r = 0.71, P = 0.0007; r = 0.66, P = 0.0028, respectively).
The expression of CD11b and CD18 on neutrophils in RSV bronchiolitis
The MFI of CD11b on neutrophils from RSV+ NPA (1683 ± 153.3) was increased compared with cells from both control PB (841.1 ± 72.3) and RSV+ PB (818 ± 141.2). The MFI of CD18 on neutrophils from RSV+ NPA (517 ± 50.5) was up-regulated compared with cells from both control PB (147 ± 8.7) and RSV+ PB (260 ± 25.8). The MFI of CD18 on neutrophils from RSV+ PB was also up-regulated compared with cells from control PB (Fig. 5).
Fig. 5.
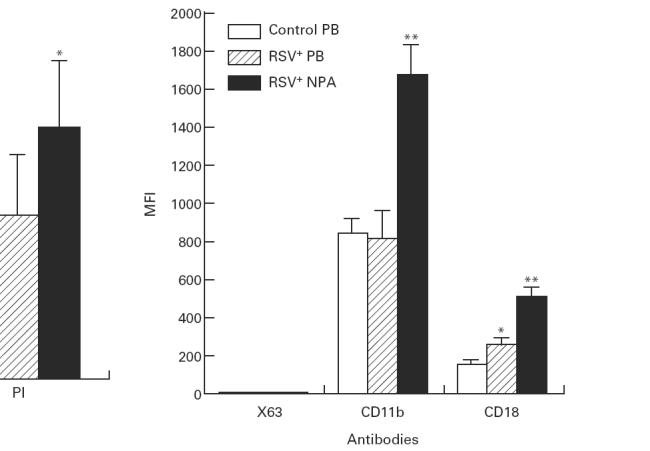
The mean fluorescence intensity (MFI) of CD11b and CD18 expressed on neutrophils in RSV bronchiolitis. The MFI of CD11b and CD18 on neutrophils obtained from control peripheral blood (PB) (n = 9), RSV+ PB (n = 11), and from RSV+ nasopharyngeal aspirates (NPA) (n = 19) was detected with flow cytometric analysis. The values are expressed as mean ± s.e.m. *P < 0.05 compared with control PB group; **P < 0.05 compared with both control PB group and RSV+ PB group.
Furthermore, the up-regulation of CD11b and CD18 on neutrophils was accompanied by the up-regulation of Annexin V on neutrophils. Regression analysis showed that expression of CD11b (MFI) and CD18 (MFI) on neutrophils (n = 19) correlated very well with expression of Annexin V (MFI) (r = 0.87, P < 0.0001; r = 0.94, P < 0.0001, respectively).
DISCUSSION
CD95 (APO-1; Fas) has emerged as an important cellular pathway regulating the induction of apoptosis in a wide variety of tissues [16]. Fas is a widely expressed 45-kD type I membrane protein member of the tumour necrosis factor (TNF)/nerve growth factor family of cell surface molecules [17]. Fas is activated to mediate apoptosis in susceptible tissues following interaction with agonistic anti-Fas IgM MoAb or its natural ligand, FasL, a 37-kD type II protein [17,18]. The up-regulation of Fas on neutrophils suggests that the apoptosis of neutrophils from RSV+ NPA is increased.
Changes in the plasma membrane of the cell surface are one of the earliest features of cells undergoing apoptosis. In apoptotic neutrophils, the membrane phospholipid PS is translocated from the inner to the outer leaflet of the plasma membrane, thereby exposing PS to the external cellular environment [13,19], which is recognized by a specific receptor on macrophages [2]. Macrophages phagocytose apoptotic neutrophils. Annexin V is a member of a family of proteins that are structurally related and exhibit Ca2+-dependent phospholipid-binding properties [20]. Annexin V shows highest affinity for PS and binds to cells with exposed PS [21]. The increased Annexin V binding and positive percentage indicate that the apoptosis of neutrophils from both RSV+ PB and RSV+ NPA is up-regulated, and the intensity of neutrophil apoptosis from RSV+ NPA is stronger than cells from both control PB and RSV+ PB. The accelerated neutrophil apoptosis in RSV+ PB indicates that there must be a systemic factor in RSV bronchiolitis which transfers signal to neutrophils in PB and accelerates neutrophil apoptosis. Neutrophil apoptosis could be part of neutrophil activation in RSV bronchiolitis.
Since translocation of PS to the external cell surface also occurs during necrosis, Annexin V–FITC is typically used in conjunction with the non-vital dye PI. Viable cells with intact membranes exclude PI, whereas the membranes of dead and damaged cells are permeable to PI. Therefore, this test discriminates between intact cells (FITC−/PI−), apoptotic cells (FITC+/PI−) and cells either in the end stage of apoptosis, undergoing necrosis, or already dead (FITC+/PI+) [22]. Our results show that although the positive percentage of PI on neutrophils from both RSV+ PB and RSV+ NPA is increased compared with cells from control PB, the positive percentage of Annexin V is higher than the positive percentage of PI on neutrophils from control PB, RSV+ PB, and RSV+ NPA, respectively. This indicates that more cells are undergoing early apoptosis in addition to some necrotic cells, which are the result of late apoptosis or non-apoptotic cell death.
By comparing the expression of CD95 with Annexin V on neutrophils, our data show that CD95 (MFI) on neutrophils from RSV+ NPA is increased only compared with cells from control PB; but Annexin V (MFI) on neutrophils from RSV+ NPA is increased compared with cells from both control PB and RSV+ PB; and furthermore, the positive percentage of Annexin V on neutrophils from both RSV+ NPA and RSV+ PB is increased compared with cells from control PB. These data indicate that Annexin V is very sensitive in detecting neutrophil apoptosis. Recent studies also show that Annexin V is a sensitive probe for identifying cells that are undergoing apoptosis, because PS exposure occurs during the early stage of apoptosis [23,24].
The regulation of neutrophil apoptosis in inflammation is not well defined. The mechanisms by which neutrophil apoptosis is accelerated as a consequence of RSV infection are not known. There are several possible explanations. Fas (CD95) is an important cellular pathway inducing apoptosis [16]. The up-regulation of Fas on neutrophils from RSV+ NPA suggests that the accelerated neutrophil apoptosis may be mediated through a Fas pathway. TNF-α is one of the cytokines known to accelerate neutrophil apoptosis [25–27]. RSV-infected macrophages have been reported to produce TNF-α [28] and RSV infection of epithelial cells could release TNF receptor type I (TNF-RI) [29]. Furthermore, a recent clinical study shows that TNF-α is markedly elevated in nasal lavage fluid during viral acute respiratory infection of childhood [30]. Neutrophil activation will accelerate the process of apoptosis, and our data on CD11b/CD18 expression on neutrophils indicate that neutrophils from RSV-infected infants undergo in vivo activation.
Our data indicate that the up-regulation of CD11b/CD18 on neutrophils accompanies the increased neutrophil apoptosis. It has been reported that neutrophil apoptosis is associated with the expression of adhesion molecules on neutrophils [14,31]. Spontaneous apoptotic neutrophils during in vitro culture showed increased expression of CD11b/CD18 integrin [31]. Of further relevance, phagocytosis of opsonized particles by human neutrophils can rapidly induce apoptosis that can be blocked with CD11b/CD18 antibodies in vitro [14]. These results suggest that CD11b/CD18 plays a novel and unsuspected homeostatic role in inflammation by accelerating neutrophil apoptosis [14].
It should be emphasized that the apoptosis of neutrophils from RSV+ PB is up-regulated, compared with cells from control PB. Similarly, the expression of CD18 on neutrophils from RSV+ PB is also up-regulated, compared with cells from control PB. However, the expression of Fas on neutrophils from RSV+ PB is not significantly increased, compared with cells from control PB. These data indicate that PB neutrophils from infants with severe RSV bronchiolitis have been activated systemically, and in turn, PB neutrophil apoptosis may be accelerated through a systemic factor other than Fas.
The accelerated neutrophil apoptotic process in vivo may limit the release of neutrophil contents from disintegrating cells in the airways in RSV bronchiolitis [8] and prevent further injuries to the epithelial cells induced by neutrophils in RSV infection [4,10]. On the other hand, because apoptotic neutrophils are functionally impaired [32], accelerated neutrophil apoptosis in vivo may contribute to the risk of secondary infection [11], which has been associated with RSV infection [33,34]. In addition, although in this study neutrophil apoptosis due to RSV infection was investigated, it is likely that other respiratory viral infections could have a similar effect.
In conclusion, the results confirm our hypothesis that neutrophil apoptosis in RSV bronchiolitis is accelerated, and CD11b/CD18 may play an important role in RSV infection by influencing neutrophil apoptosis.
Acknowledgments
The authors thank Ms L. Wheatland and Dr P. MacArdle for assistance in analysing cells with flow cytometry. The authors also thank all the staff in the Departments of Paediatrics, Paediatric Surgery, and Anaesthesia for assistance in collecting NPA and PB. S.-Z.W. was supported by an Australian Overseas Postgraduate Research Scholarship and a Flinders University Research Scholarship. This research was in part funded by The Channel Seven Children's Research Foundation of South Australia Inc. (Grant No. 21, 94/95).
REFERENCES
- 1.Haslett C, Savill JS, Meagher L. The neutrophil. Curr Opin Immunol. 1989;2:10–18. doi: 10.1016/0952-7915(89)90091-5. [DOI] [PubMed] [Google Scholar]
- 2.Henson PM, Johnston RB. Tissue injury in inflammation. Oxidants, proteinases and cationic proteins. J Clin Invest. 1987;79:669–74. doi: 10.1172/JCI112869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fadok VA, Savill JS, Haslett C, et al. Different populations of macrophages use either the vitronectin receptor or the phosphatidylserine receptor to recognize and remove apoptotic cells. J Immunol. 1992;149:4029–35. [PubMed] [Google Scholar]
- 4.Savill JS, Henson PM, Haslett C. Phagocytosis of aged neutrophils by macrophages is mediated by a novel ‘charge-sensitive’ recognition mechanism. J Clin Invest. 1989;84:1518–27. doi: 10.1172/JCI114328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holberg CJ, Wright AL, Martinez FD. Risk factors for respiratory syncytial virus-associated lower airway illness in the first year of life. Am J Epidemiol. 1991;133:1135–51. doi: 10.1093/oxfordjournals.aje.a115826. [DOI] [PubMed] [Google Scholar]
- 6.Anderson LJ, Parker RA, Strukes RL. Association between respiratory syncytial virus outbreaks and lower respiratory tract deaths of infants and young children. J Infect Dis. 1990;161:640–6. doi: 10.1093/infdis/161.4.640. [DOI] [PubMed] [Google Scholar]
- 7.Dakhama A, Vitalis TZ, Hegele RG. Persistence of respiratory syncytial virus (RSV) infection and development of RSV-specific IgG1 response in a guinea-pig model of acute bronchiolitis. Eur Respir J. 1997;10:20–26. doi: 10.1183/09031936.97.10010020. [DOI] [PubMed] [Google Scholar]
- 8.Everard ML, Awarbrick A, Wrightham M, et al. Analysis of cells obtained by bronchial lavage of infants with respiratory syncytial virus infection. Arch Dis Child. 1994;71:428–32. doi: 10.1136/adc.71.5.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stark JM, Godding V, Sedgwick JB, Busse WW. Respiratory syncytial virus infection enhances neutrophil and eosinophil adhesion to cultured respiratory epithelial cells: role of CD18 and intercellular adhesion molecule-1. J Immunol. 1996;156:4774–82. [PubMed] [Google Scholar]
- 10.Wang S-Z, Xu H, Wraith A, Bowden JJ, Alpers JH, Forsyth KD. Neutrophils induce damage to respiratory epithelial cells infected with respiratory syncytial virus. Eur Respir J. doi: 10.1183/09031936.98.12030612. in press. [DOI] [PubMed] [Google Scholar]
- 11.Pitrak DJ, Tsai HC, Mullane KM, Sutton SH, Stevens P. Accelerated neutrophil apoptosis in the acquired immunodeficiency syndrome. J Clin Invest. 1996;98:2714–9. doi: 10.1172/JCI119096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liles WC, Kiener PA, Ledbetter JA, Aruffo A, Klebanoff SJ. Differential expression of Fas (CD95) and Fas ligand on normal human phagocytes: implications for the regulation of apoptosis in neutrophils. J Exp Med. 1996;184:429–40. doi: 10.1084/jem.184.2.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Homburg CHE, de Haas M, von dem Borne AEGKr, Verhoeven AJ, Reutelingsperger CPM, Roos D. Human neutrophils lose their surface FcγRIII and acquire Annexin V binding sites during apoptosis in vitro. Blood. 1995;85:532–40. [PubMed] [Google Scholar]
- 14.Coxon A, Rieu P, Barkalow FIJ, et al. A novel role for the beta 2 integrin CD11b/CD18 in neutrophil apoptosis: a homeostatic mechanism in inflammation. Immunity. 1996;5:653–66. doi: 10.1016/s1074-7613(00)80278-2. [DOI] [PubMed] [Google Scholar]
- 15.Metcalf JA, Gallin JI, Nauseef WM, Root RK. New York: Raven Press; 1986. Laboratory manual of neutrophil function. [Google Scholar]
- 16.Cohen JJ. Programmed cell death in the immune system. Adv Immunol. 1991;50:55–85. doi: 10.1016/s0065-2776(08)60822-6. [DOI] [PubMed] [Google Scholar]
- 17.Nagata S, Golstein P. The Fas death factor. Science (Wash DC) 1995;267:1449–56. doi: 10.1126/science.7533326. [DOI] [PubMed] [Google Scholar]
- 18.Suda T, Nagata S. Purification and characterization of the Fas-ligand that induces apoptosis. J Exp Med. 1994;179:873–9. doi: 10.1084/jem.179.3.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martin SJ, Reutelingsperger CPM, McGahon AJ, et al. Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: inhibition by overexpression of Bcl-2 and Abl. J Exp Med. 1995;182:1545–56. doi: 10.1084/jem.182.5.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moss SE, Edwards HC, Crumpton MJ. Diversity in the annexin family. In: Heizmann CW, editor. Novel calcium binding proteins. Berlin: Springer Verlag; 1991. p. 535. [Google Scholar]
- 21.Andree HAM, Reutelingsperger CPM, Hauptmann R, Hemker HC. Hermens WT, Willems GM. Binding of vascular anticoagulant alpha to planar phospholipid bilayers. J Biol Chem. 1990;265:4923–8. [PubMed] [Google Scholar]
- 22.Vermes I, Haanen C, Steffens-Nakken H, Reutelingsperger C. A novel assay for apoptosis: flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J Immunol Methods. 1995;184:39–51. doi: 10.1016/0022-1759(95)00072-i. [DOI] [PubMed] [Google Scholar]
- 23.Emoto K, Toyama-Sorimachi N, Karasuyama H, Inoue K, Umeda M. Exposure of phosphatidylethanolamine on the surface of apoptotic cells. Exp Cell Res. 1997;232:430–4. doi: 10.1006/excr.1997.3521. [DOI] [PubMed] [Google Scholar]
- 24.Hertveldt K, Philippe J, Thierens H, Cornelissen M, Vral A, De-Ridder L. Flow cytometry as a quantitative and sensitive method to evaluate low dose radiation induced apoptosis in vitro in human peripheral blood lymphocytes. Int J Radiat Biol. 1997;71:429–33. doi: 10.1080/095530097144049. [DOI] [PubMed] [Google Scholar]
- 25.Takeda Y, Watanabe H, Yonehara S, Saito S, Sendo F. Rapid acceleration of neutrophil apoptosis by TNF-α. Int Immunol. 1993;5:691–4. doi: 10.1093/intimm/5.6.691. [DOI] [PubMed] [Google Scholar]
- 26.Gon S, Gatanaga T, Sendo F. Involvement of two types of TNF receptor in TNF-alpha induced neutrophil apoptosis. Microbiol Immunol. 1996;40:463–5. doi: 10.1111/j.1348-0421.1996.tb01095.x. [DOI] [PubMed] [Google Scholar]
- 27.Sullivan GW, Gelrud AK, Carper HT, Mandell GL. Interaction of tumour necrosis factor-alpha and granulocyte colony-stimulating factor on neutrophil apoptosis, receptor expression, and bactericidal function. Proc Assoc Am Physicians. 1996;108:455–66. [PubMed] [Google Scholar]
- 28.Matsuda K, Tsutsumi H, Sone S, et al. Characteristics of IL-6 and TNF-alpha production by respiratory syncytial virus-infected macrophages in the neonate. J Med Virol. 1996;48:199–203. doi: 10.1002/(SICI)1096-9071(199602)48:2<199::AID-JMV13>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 29.Arnold R, Humbert B, Werchao H, Gallati H, Konig W. Interleukin -8, interleukin-6 and soluble tumour necrosis factor receptor type I release from a human pulmonary epithelial cell line (A549) exposed to respiratory syncytial virus. Immunology. 1994;82:126–33. [PMC free article] [PubMed] [Google Scholar]
- 30.Noah TL, Henderson FW, Wortman IV, Devlin RB, Handy J, Koren HS, Becker S. Nasal cytokine production in viral acute upper respiratory infection of childhood. J Infect Dis. 1995;171:584–92. doi: 10.1093/infdis/171.3.584. [DOI] [PubMed] [Google Scholar]
- 31.Dransfield I, Stocks SC, Haslett C. Regulation of cell adhesion molecule expression and function associated with neutrophil apoptosis. Blood. 1995;85:3264–73. [PubMed] [Google Scholar]
- 32.Whyte MKB, Meaggher LC, MacDermot J, Haslett C. Impairment of function in aging neutrophils is associated with apoptosis. J Immunol. 1993;150:5124–34. [PubMed] [Google Scholar]
- 33.Korppi M, Leinonen M, Koskela M, Makela PH, Launiala K. Bacterial coinfection in children hospitalized with respiratory syncytial virus infections. Pediatr Infect Dis J. 1989;8:687–92. doi: 10.1097/00006454-198910000-00005. [DOI] [PubMed] [Google Scholar]
- 34.Patel J, Faden H, Sharma S, Ogra PL. Effect of respiratory syncytial virus on adherence, colonization and immunity of non-typable Haemophilus influenzae: implications for otitis media. Internat J Pediatr Otor. 1992;23:15–23. doi: 10.1016/0165-5876(92)90075-z. [DOI] [PubMed] [Google Scholar]


