Abstract
We examined the secretion and expression by peripheral blood mononuclear cells (PBMC) of TNF-α and TNF-related molecules with regard to Th1/Th2-type cytokine production. In 76 HIV+ patients at different disease stages and in 25 controls we measured cytokine (TNF-α/β, interferon-gamma (IFN-γ), IL-2, IL-4, IL-10), and activation marker secretion (sCD4, sCD8, sCD30) in phytohaemagglutinin (PHA)-stimulated and unstimulated PBMC cultures by ELISA, and membrane-bound TNF-α and CD30 expression by flow cytometry. We found an expansion of the TNF system in HIV+ individuals, that positively correlated with TNF-α, IFN-γ and sCD8, probably representing activation of the cytotoxic compartment. In advanced disease these correlations disappeared, and TNF-α and TNF-related molecules positively correlated with IL-10. Our results are in line with the hypothesis that an expanded TNF system is immunopathological in conjunction with Th2-type immunity in the advanced stage of disease and with the inexorable progression to disease seen when both IL-10 and TNF-α are elevated.
Keywords: HIV-1 infection, TNF-α, mbTNF-α, soluble CD30, CD30 antigen, IL-10, cellular factors/cytokines, disease progression
INTRODUCTION
The role of cytokines in HIV disease progression has recently been extensively investigated. It has been postulated that a dominant Th1-type immune response is prevalent and protective during early asymptomatic HIV infection, whereas the onset of Th2-type immune responses is associated with progressive disease [1–4]. In addition, recent studies have focused on the association between markers of immune activation and inflammatory cytokines with disease progression. Elevated levels of TNF-α, soluble TNF receptors (sTNFR) and sCD8, as well as sCD30, a member of the TNF/nerve growth factor superfamily, have been shown to be increased in the majority of patients in early HIV-1 infection and were prognostic for poor clinical outcome [5–13]. Indeed, elevated levels of TNF-α and sCD30 were found to predict accurately a bad clinical outcome in patients with primary HIV-1 infection [14]. Altogether, these findings suggest that there is a close association between disease progression and early stage activation markers, as well as with loss of Th1-type cytokine production. The possible relationship between immune activation markers and Th1-type and Th2-type cytokines is supported by recent observations that CD30 defines a selective differentiation and/or activation pathway associated with T cells producing Th2-type cytokines [12,15]. TNF-α, however, is not defined in terms of Th1/Th2-type cytokine production, although it would appear to be an important factor in the interaction between cytokines and other immune factors during the immune response. We therefore examined the secretion and expression by peripheral blood mononuclear cells (PBMC) of TNF and TNF-related molecules with regard to the production of different cytokines.
PATIENTS AND METHODS
Patients
We included 76 HIV-1+ subjects at different stages of disease, classified according to the Centers for Disease Control and Prevention criteria (CDC [16]) on the basis of the CD4+ cell counts: ≥ 500/μl (category 1, n = 16), 200–499/μl (category 2, n = 29) and < 200/μl (category 3, n = 31). Sixty-two were males and 14 females, and the mean (± s.e.m.) age was 38.4 ± 0.9 years (range 24–45 years). The distribution of risk categories (21 ex-intravenous drug users, 37 homosexuals and 18 heterosexuals) was comparable in the three CDC groups. Fifty-four of the 76 included in the study were taking zidovudine at a daily dosage between 500 and 750 mg. As control group, we included 25 healthy HIV-1− blood donors (20 males and five females, age 36 ± 2 years (range 22–44 years)).
Lymphocyte culture conditions and laboratory tests
PBMC, separated from heparinized blood by Ficoll–Isopaque density gradient centrifugation, were resuspended in RPMI 1640 culture medium supplemented with 2 mm l-glutamine, 100 U/ml penicillin, 0.1 mg/ml streptomycin (Sigma, Poole, UK), and 10% heat-inactivated fetal calf serum (FCS; HyClone Labs, Logan, UT). Twenty-four-hour cultures were set up with 1 × 106/ml PBMC in 1 ml final volume in 12 × 75 mm round-bottomed tubes in the presence of 1 μg/ml phytohaemagglutinin (PHA; Gibco, Grand Island, NY) or medium alone. All patients were positive in both anti-HIV-1 + 2 antibody recombinant enzyme immunoassay (EIA; HIV-1 + 2, Ortho Diagnostics Systems, Raritan, NJ) and immunoblot analysis (Gene Labs, Singapore Science Park, Singapore). p24 antigen release (Retro-Tek, Cellular Products Inc., Buffalo, NY) was determined in the culture supernatants by ELISA.
The following cytokine and soluble receptors were measured in the culture supernatants with ELISA kits, according to the manufacturers' specifications: TNF-α, TNF-β, interferon-gamma (IFN-γ), IL-2, IL-4, IL-10 (R&D Systems, Minneapolis, MN), sCD30 (Ki-1 antigen; Dako, Glostrup, Denmark), sCD4 and sCD8 (T-Cell Diagnostics, Cambridge, MA). The limits of detection of the assays performed were: 15.6–1000 pg/ml for TNF-α and IFN-γ, 31.2–2000 pg/ml for TNF-β, IL-2 and IL-4, 7.8–500 pg/ml for IL-10, 2–191 U/ml for sCD4, 50–2280 U/ml for sCD8, and 6–230 U/ml for sCD30; when required, samples were diluted before testing.
Flow cytometric analysis
PBMC (1 × 106) were harvested for each test and pelleted by centrifugation at 400 g for 5 min. Cell pellets were resuspended in 100 μl PBS containing 2% bovine serum albumin (BSA; Sigma) plus 0.01% sodium azide to which 10 μl of FITC-conjugated anti-CD30 MoAb (Dako) were added. Cells were incubated for 30 min at 4°C, then washed three times, and finally fixed in 300 μl 1% paraformaldehyde in PBS before analysis by flow cytometry in an Ortho FACScan system (Raritan). For membrane-bound (mb)TNF-α, cells were first Fc-blocked by treatment with 1 μg of human IgG/105 cells (Sigma) for 15 min at room temperature, and then 10 μl of FITC-conjugated anti-mbTNF-α MoAb (R&D Systems) were added and incubated for 60 min at 4°C. Cells were then washed and fixed as described. As negative controls we used subclass-matched FITC-conjugated mouse anti-human IgG1 (Dako). Results are given as percentage of positive cells.
Statistical analysis
The Statistical Package of Social Science (SPSS release 4.0; SPSS Inc., Chicago, IL) was used to analyse data. Comparisons were performed by Mann–Whitney test, and correlation coefficients were calculated by Spearman's test. P (two-tailed) < 0.05 was considered significant.
RESULTS
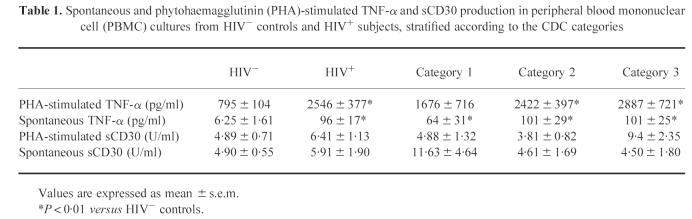
Spontaneous and PHA-stimulated TNF-α production was significantly increased in HIV+ subjects versus HIV− controls, and showed an increasing trend with disease progression (Table 1). Levels of unstimulated and PHA-stimulated sCD30 release in cultures of HIV+ and HIV− subjects did not differ. However, as far as CDC categories are concerned, spontaneous sCD30 secretion decreased, whereas mitogen-stimulated production increased, being significantly greater in category 3 than in category 2 (P = 0.032). Furthermore, in 25 of the 76 HIV+ individuals enrolled, we evaluated the number of fresh PBMC expressing either mbTNF-α or CD30 molecule. HIV+ subjects showed a higher percentage of mbTNF-α-positive PBMC than HIV− controls (43.97 ± 6.91% versus 20.25 ± 2.24%, P = 0.0027). Likewise, the percentage of PBMC expressing CD30 surface antigen was increased in HIV+ subjects, although not significantly (8.79 ± 3.57% versus 4.89 ± 0.32%). Finally, in HIV+ subjects, levels of stimulated TNF-α production in cultures were positively correlated with the percentage of cells expressing mbTNF-α (r = 0.57, P < 0.019) and CD30 (r = 0.92, P < 0.001), and the number of CD30+ cells was positively correlated with that of cells expressing mbTNF-α (r = 0.92, P < 0.001) (Fig. 1a).
Table 1.
Spontaneous and phytohaemagglutinin (PHA)-stimulated TNF-α and sCD30 production in peripheral blood mononuclear cell (PBMC) cultures from HIV− controls and HIV+ subjects, stratified according to the CDC categories
Fig. 1.
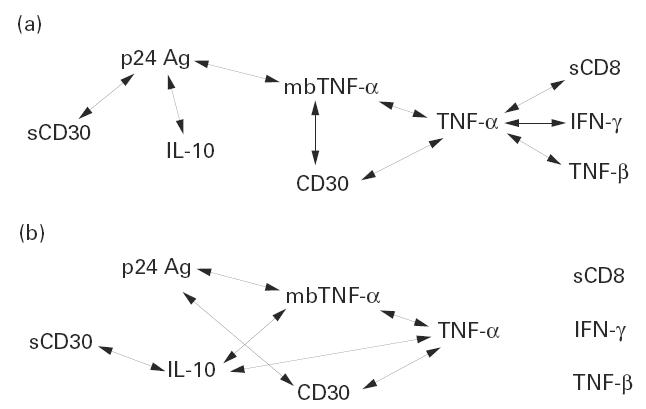
Patterns of statistically significant positive correlations among cytokine (TNF-α, TNF-β, IFN-γ and IL-10) and soluble receptor (sCD8 and sCD30) levels in phytohaemagglutinin (PHA)-stimulated cultures and percentages of CD30 and membrane-bound (mb)TNF-α-positive peripheral blood mononuclear cells (PBMC) (see text) (a) in all HIV+ subjects, and (b) in patients belonging to CDC category 3. The double-headed arrows connecting two parameters indicate a positive correlation between these paramenters.
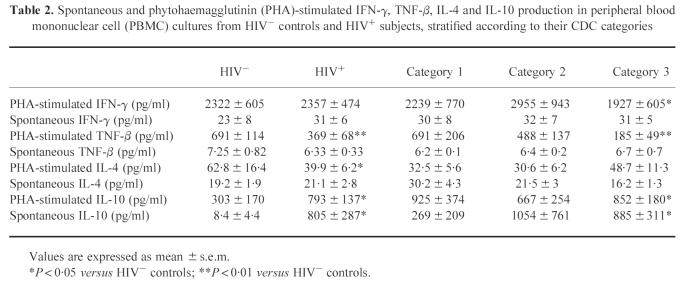
As far as Th1-type and Th2-type cytokine secretion is concerned (Table 2), IFN-γ release in mitogen-induced PBMC cultures was comparable in HIV+ individuals and HIV− subjects. Mitogen-induced TNF-β production was significantly decreased in HIV+ subjects versus HIV− controls (P = 0.007), and showed a decreasing trend with disease progression. A similar pattern of cytokine secretion was observed for IL-2 (not shown). Spontaneous IFN-γ, TNF-β and IL-2 release was not different between HIV+ and HIV− individuals. PHA-induced IL-4 production was significantly decreased in HIV+ subjects versus controls (P = 0.015), and levels of spontaneous and mitogen-induced IL-10 production were significantly increased in HIV+ subjects (P = 0.01 and P = 0.031, respectively), particularly in CDC category 3 (P = 0.017 and P = 0.032 versus controls, respectively).
Table 2.
Spontaneous and phytohaemagglutinin (PHA)-stimulated IFN-γ, TNF-β, IL-4 and IL-10 production in peripheral blood mononuclear cell (PBMC) cultures from HIV− controls and HIV+ subjects, stratified according to their CDC categories
We then investigated the release in cultures of sCD4 and sCD8, as indirect markers of T cell subset activation. In HIV+ individuals compared with HIV− controls, levels of sCD8 release were significantly increased both in spontaneous (161 ± 45 U/ml versus 0.1 ± 0.02 U/ml, respectively; P = 0.0027) and in PHA-induced cultures (414 ± 31 U/ml versus 200 ± 70 U/ml, respectively; P = 0.0012). As far as disease progression is concerned, sCD8 production did not significantly change in the three categories (not shown). The reduction in PHA-induced sCD4 release was significantly greater in HIV+ individuals than in HIV− controls (25.5 ± 0.9 versus 39.1 ± 3, respectively; P < 0.001), whereas no difference was observed in values of spontaneous secretion (22.2 ± 0.5 versus 23.6 ± 0.9, respectively). As expected, sCD4 release significantly decreased with disease progression (not shown).
Finally, in the same cultures spontaneous and PHA-stimulated p24 antigen release significantly increased during disease progression, being higher in categories 2 and 3 than in category 1 (not shown).
We then evaluated the patterns of correlation among the cytokines and markers studied in PHA-stimulated cultures from HIV+ subjects (Fig. 1). As mentioned above, levels of TNF-α production were positively correlated with the percentage of cells expressing mbTNF-α and CD30. Furthermore, TNF-α levels were positively correlated with those of TNF-β (r = 0.521, P < 0.01), IFN-γ (r = 0.298, P < 0.05) and sCD8 (r = 0.434, P < 0.01). Moreover, values of sCD8 secretion were positively correlated with those of IFN-γ (0.314, P < 0.01) and TNF-β (r = 0.41, P < 0.05). As far as p24 antigen release is concerned, its values were positively correlated with those of sCD30 (r = 0.46, P < 0.001), IL-10 (r = 0.43, P = 0.018), and with the percentage of cells expressing mbTNF-α (r = 0.752, P < 0.05) (Fig. 1a). Finally, an overall positive correlation was observed both among Th1-type (IFN-γ, IL-2 and TNF-β) and between Th2-type (IL-4 and IL-10) cytokines (not shown).
Considering patients at advanced disease stage, the patterns of correlation changed (Fig. 1b). First, correlations among TNF-α, TNF-β, IFN-γ and sCD8 were no longer present. Furthermore, levels of IL-10 were positively correlated with those of TNF-α (r = 0.474, P < 0.05), sCD30 (r = 0.338, P = 0.046) and with the percentage of cells expressing mbTNF-α (r = 0.94, P = 0.026). Finally, the percentage of cells expressing CD30 was positively correlated with values of p24 antigen (r = 0.99, P < 0.001).
DISCUSSION
In this cohort of 76 HIV+ subjects at different disease stages, levels of TNF-α were significantly elevated compared with controls, and increased with disease progression. Similarly, levels of sCD30 were raised in advanced disease, although they were not different comparing all HIV+ with HIV− subjects. HIV+ individuals had a higher proportion of cells expressing CD30 and mbTNF-α, which correlated with each other and also with levels of secreted TNF-α. Furthermore, mbTNF-α expression and sCD30 release were positively correlated with p24 antigen levels. Consistently, it has been demonstrated that cross-linking of CD30 and activation via cell surface TNF-α induce HIV expression in chronically infected cells [17,18]. Both these membrane-mediated pathways may be important in the activation of latent HIV, especially in lymphoid organs where cell–cell contact is conducive to receptor–ligand interactions. Collectively, our results suggest the existence of an overall positive correlation between TNF-α secretion and TNF-related molecule expression, and also an expansion of the TNF system in HIV+ individuals, which is associated with enhanced HIV expression and with a more rapid progression of the disease [5,14,19].
The relationship between TNF-α/TNF-related molecules and Th1/Th2-type cytokines in HIV infection is not clear. It is known that TNF-α can synergize with IFN-γ in activating the cellular immune responses to intracellular microorganisms [20]. In this study, we have found that, in the whole HIV+ population, levels of TNF-α were positively correlated with those of IFN-γ and TNF-β and that all three were correlated with those of sCD8, the latter probably representing activation of the cytotoxic compartment. In the complex interplay between host and virus, it is likely that many factors determine cytokine effector function and that these may change with disease progression. This has been demonstrated in a murine mycobacterial model, where TNF-α appears to be beneficial in the context of a dominant Th1-type immune response but detrimental in the context of a Th2-type immune response [21]. Interestingly, considering all HIV+ individuals, we failed to find a direct correlation between the levels of TNF-α and those of IL-4 and IL-10. However, these patterns of correlation changed with the progression of the disease. In CDC category 3, the correlations among TNF-α, IFN-γ, TNF-β and sCD8 were no longer present, whereas levels of sCD30, mbTNF-α and secreted TNF-α were positively correlated with those of IL-10. The interplay between TNF-α and IL-10, both in modulating HIV expression and affecting the immune responses, appears to change according to the different stages of infection. IL-10 has been shown to suppress HIV replication in in vitro acutely infected monocyte-derived macrophages [22–24]. Furthermore, it has been recently reported that in vivo IL-10 administration to HIV− volunteers induces a subsequent resistance to in vitro acute PBMC infection with HIV [25]. Conversely, in a promonocytic cell line model of latent HIV infection, it has been shown that IL-10 can synergize with multiple cytokines, including TNF-α, in enhancing HIV production [26], and also activate HIV expression through the induction of mbTNF-α and TNFR-I [27]. Altogether, these data suggest that IL-10 can exert differential effects on HIV, in relation to the presence of an acute or a latent infection and varying cytokine expression, thus emphasizing the importance of these factors in determining disease progression. In this regard, the correlation between IL-10 and TNF-α, mbTNF-α and sCD30, observed in the advanced stage of disease only, is consistent with a putative detrimental role for IL-10 in the presence of elevated levels of TNF-α and TNF-related molecules. Although no correlation between levels of IL-4 and TNF-α was observed, our results are in line with the hypothesis that an expanded TNF system is immunopathological in conjunction with Th2-type immunity in the advanced stage of disease and with the inexorable progression to disease seen when both IL-10 and TNF-α are elevated. Whether TNF-α is beneficial in association with a Th1-type immune response is unclear, although the pattern of correlation observed in CDC categories 1 and 2 may mirror beneficial cytotoxic responses. However, other results indicate that a persistent immune activation over time is detrimental, since it may lead to the availability of activated uninfected cells and subsequently enhanced HIV-1 infection and replication [14,28]. More understanding of the multi-component shifts in cytokine profiles would derive from studies with specific cell subpopulations, although this approach would be farther away from the in vivo situation.
Altogether, our results provide support for the crucial role of TNF-α and TNF-related molecules in AIDS pathogenesis, and may warrant the design of future therapeutic strategies that down-modulate the TNF system during the course of HIV infection.
Acknowledgments
This work was supported by ISS/9403-11 and MRC.
REFERENCES
- 1.Clerici M, Shearer GM. A TH1→TH2 switch is a critical step in the etiology of HIV infection. Immunol Today. 1993;14:107–11. doi: 10.1016/0167-5699(93)90208-3. [DOI] [PubMed] [Google Scholar]
- 2.Clerici M, Shearer GM. The Th1-Th2 hypothesis of HIV infection: new insights. Immunol Today. 1994;15:575–81. doi: 10.1016/0167-5699(94)90220-8. [DOI] [PubMed] [Google Scholar]
- 3.Romagnani S, Maggi E, Del Prete G. Tenth anniversary perspectives on AIDS. An alternative view of the Th1/Th2 switch hypothesis in HIV infection. AIDS Res Hum Retrovir. 1994;10:3–9. doi: 10.1089/aid.1994.10.iii. [DOI] [PubMed] [Google Scholar]
- 4.Barcellini W, Rizzardi GP, Borghi MO, Fain C, Lazzarin A, Meroni PL. TH1 and TH2 cytokine production by peripheral blood mononuclear cells from HIV-infected patients. AIDS. 1994;8:757–62. doi: 10.1097/00002030-199406000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Barcellini W, Rizzardi GP, Poli G, et al. Cytokines and soluble receptor changes in the transition from primary to early chronic HIV type 1 infection. AIDS Res Hum Retrovir. 1996;12:325–30. doi: 10.1089/aid.1996.12.325. [DOI] [PubMed] [Google Scholar]
- 6.Aukrust P, Liabakk N, Müller F, Lien E, Espevik T, Frøland SS. Serum levels of tumor necrosis factor-α (TNF-α) and soluble TNF receptors in human immunodeficiency virus type 1 infection—correlations to clinical, immunologic, and virologic parameters. J Infect Dis. 1994;169:420–4. doi: 10.1093/infdis/169.2.420. [DOI] [PubMed] [Google Scholar]
- 7.Godfried ME, van der Poll T, Jansen J, et al. Soluble receptors for tumour necrosis factor: a putative marker of disease progression in HIV infection. AIDS. 1993;7:33–36. [PubMed] [Google Scholar]
- 8.Godfried MH, van der Poll T, Weverling GJ, et al. Soluble receptors for tumor necrosis factor as predictors of progression to AIDS in asymptomatic human immunodeficiency virus type 1 infection. J Infect Dis. 1994;169:739–45. doi: 10.1093/infdis/169.4.739. [DOI] [PubMed] [Google Scholar]
- 9.Aukrust P, Lien E, Kristoffersen AK, et al. Persistent activation of the tumor necrosis factor system in a subgroup of patients with common variable immunodeficiency—possible immunologic and clinical consequences. Blood. 1996;87:674–81. [PubMed] [Google Scholar]
- 10.Zangerle R, Gallati H, Sarcletti M, Weiss G, Denz H, Wachter H, Fuchs D. Increased serum concentrations of soluble tumor necrosis factor receptors in HIV-infected individuals are associated with immune activation. J AIDS. 1994;7:79–85. [PubMed] [Google Scholar]
- 11.Nishanian P, Hofmann B, Wang Y, Jackson AL, Detels R, Fahey M. Serum soluble CD8 molecule is a marker of CD8 T cell activation in HIV-1 disease. AIDS. 1991;5:805–12. doi: 10.1097/00002030-199107000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Del Prete G, Maggi E, Pizzolo G, Romagnani S. CD30, Th2 cytokines and HIV infection: a complex and fascinating link. Immunol Today. 1995;16:76–80. doi: 10.1016/0167-5699(95)80092-1. [DOI] [PubMed] [Google Scholar]
- 13.Pizzolo G, Vinante F, Morosato L, et al. High serum level of the soluble form of CD30 molecule in the early phase of HIV-1 infection as an independent predictor of progression to AIDS. AIDS. 1994;8:741–5. doi: 10.1097/00002030-199406000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Rizzardi GP, Barcellini W, Tambussi G, Lillo F, Malnati M, Perrin L, Lazzarin A. Plasma levels of soluble CD30, tumour necrosis factor (TNF)-α and TNF receptors during primary HIV-1 infection. Correlation with HIV-1 RNA and the clinical outcome. AIDS. 1996;10:F45–F50. doi: 10.1097/00002030-199611000-00001. [DOI] [PubMed] [Google Scholar]
- 15.Del Prete G, De Carli M, Almerigogna F, et al. Preferential expression of CD30 by human CD4+ T cells producing Th2-type cytokines. Faseb J. 1995;9:81–86. [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention. 1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR. 1992;41:1–19. [PubMed] [Google Scholar]
- 17.Biswas P, Smith CA, Goletti D, Hardy EC, Jackson RW, Fauci AS. Cross-linking of CD30 induces HIV expression in chronically infected T cells. Immunity. 1995;2:587–96. doi: 10.1016/1074-7613(95)90003-9. [DOI] [PubMed] [Google Scholar]
- 18.Tadmori W, Mondal D, Tadmori I, Prakash O. Transactivation of human immunodeficiency virus type 1 long terminal repeats by cell surface tumor necrosis factor α. J Virol. 1991;65:6425–9. doi: 10.1128/jvi.65.12.6425-6429.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bilello JA, Stellrecht K, Drusano GL, Stein DS. Soluble tumor necrosis factor-α receptor type II (sTNF-RII) correlates with human immunodeficiency virus (HIV) RNA copy number in HIV-infected patients. J Infect Dis. 1996;173:464–7. doi: 10.1093/infdis/173.2.464. [DOI] [PubMed] [Google Scholar]
- 20.Blanchard DK, Friedman H, Klein TW, Djeu JY. Induction of interferon-γ and tumor necrosis factor by Legionella pneumophila: augmentation of human neutrophil bactericidal activity. J Leuk Biol. 1989;45:538–45. doi: 10.1002/jlb.45.6.538. [DOI] [PubMed] [Google Scholar]
- 21.Hernandez-Pando R, Rook GA. The role of TNF-alpha in T-cell-mediated inflammation depends on the Th1/Th2 cytokine balance. Immunology. 1994;82:591–5. [PMC free article] [PubMed] [Google Scholar]
- 22.Akridge RE, Oyafuso LK, Reed SG. IL-10 is induced during HIV-1 infection and is capable of decreasing viral replication in human macrophages. J Immunol. 1994;153:5782–9. [PubMed] [Google Scholar]
- 23.Kootstra NA, van't Wout AB, Huisman HG, Miedema F, Schuitemaker H. Interference of interleukin-10 with human immunodeficiency virus type 1 replication in primary monocyte-derived macrophages. J Virol. 1994;68:6967–75. doi: 10.1128/jvi.68.11.6967-6975.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saville MW, Kazayuki T, Foli A, Broder S, Tosato G, Yarchoan R. Interleukin-10 suppresses human immunodeficiency virus-1 replication in vitro in cells of the monocyte/macrophage lineage. Blood. 1994;83:3591–9. [PubMed] [Google Scholar]
- 25.Fauci AS. XI International Conference on AIDS. Vancouver, Canada: 1996. Host factors in the pathogenesis of HIV disease. [Google Scholar]
- 26.Weissman D, Poli G, Fauci AS. IL-10 synergizes with multiple cytokines in enhancing HIV production in cells of monocytic lineage. J AIDS. 1995;9:442–9. [PubMed] [Google Scholar]
- 27.Barcellini W, Rizzardi GP, Marriott JB, Fain C, Shattock RJ, Meroni PL, Poli G, DaIgleish AG. Interleukin-10-induced HIV-1 expression is mediated by induction of both membrane bound tumour necrosis factor (TNF)-α and TNF receptor type 1 in U1 cell line. AIDS. 1996;10:835–42. doi: 10.1097/00002030-199607000-00006. [DOI] [PubMed] [Google Scholar]
- 28.Fauci AS. Multifactorial nature of human immunodeficiency virus disease: implications for therapy. Science. 1993;262:1011–8. doi: 10.1126/science.8235617. [DOI] [PubMed] [Google Scholar]