Abstract
Chronic meningococcaemia is a relatively benign manifestation of meningococcal disease. Whether bacterial virulence factors are responsible for this benign course has not been studied. We compared the in vitro endotoxin-liberating ability and cytokine-inducing potential of 31 Neisseria meningitidis isolates obtained from children with acute septic shock with that of nine isolates obtained from patients with chronic meningococcaemia and 12 isolates obtained from carriers with respiratory symptoms. The median endotoxin level released in vitro after 3 h of incubation was significantly higher for isolates causing septic shock compared with isolates from the other two groups (P = 0.01 and 0.02, Mann–Whitney test). This was not explained by differences in bacterial growth rate in vitro. The median IL-6 levels in whole blood ex vivo after 4 h of incubation were also significantly lower for isolates causing chronic meningococcaemia (P = 0.04, Mann–Whitney test). The endotoxin and cytokine levels measured on admission in the 31 children with acute meningococcal septic shock showed a 1000-fold variation. No relationship was established between the amount of endotoxin released by the causative microorganisms in vitro and the endotoxin or cytokine levels in the corresponding 31 children. These results suggest a diminished bacterial virulence for isolates causing chronic meningococcaemia. However, other factors than the endotoxin-releasing potential of the microorganism involved are responsible for the wide variation in endotoxin and therefore cytokine levels in patients with acute meningococcal septic shock.
Keywords: meningococci, chronic meningococcaemia, sepsis, endotoxin, cytokines
INTRODUCTION
Neisseria meningitidis is carried in the nasopharynx of humans. It is suggested that disruption of mucosal integrity, for instance due to a coincident viral infection, promotes the transition from nasopharyngeal carriage to invasive disease [1,2]. The clinical spectrum of invasive infections with N. meningitidis ranges from chronic meningococcaemia to acute sepsis, with or without meningitis. Host factors as well as characteristics of the microorganism may both determine the clinical manifestation of meningococcal infection.
Chronic meningococcaemia is an unusual, relatively benign manifestation of Neisserial disease. It is characterized by prolonged and often relapsing fever and the presence of meningococci in the bloodstream. Other clinical characteristics are rash, arthralgia, and headache [3,4]. In cases of chronic meningococcaemia host factors rather than determinants of bacterial virulence are held responsible for the relatively benign clinical course [3], but the importance of bacterial virulence factors has not been studied in detail.
Acute meningococcal septic shock has a high fatality rate, and bacterial endotoxin is considered to be the most important bacterial factor in the pathogenesis of infection [2,5,6]. Tumour necrosis factor-alpha (TNF-α) and other cytokines are released in response to endotoxin, but a marked variation in cytokine levels is found in patients suffering from severe meningococcal septic shock [7–9]. As in several studies the levels of endotoxin and cytokine levels were strongly correlated [7,8], variations in endotoxin level probably explain most of the variation in cytokine levels. The circulating endotoxin level is influenced by bacterial numbers and by the endotoxin-releasing potential of the causative microorganism, but the relative contribution of each of these factors to the variation in endotoxin and cytokine levels is not known.
Therefore, we compared the in vitro endotoxin-liberating ability and cytokine-inducing potential of N. meningitidis isolates obtained from patients with chronic meningococcaemia with that of meningococcal isolates obtained from patients with severe meningococcal septic shock or from carriers with respiratory symptoms. Second, we determined whether the endotoxin-liberating ability in vitro of the causative microorganisms contributed to the variation in endotoxin and cytokine levels found in the serum of 31 children admitted with severe meningococcal septic shock.
MATERIALS AND METHODS
Clinical isolates
All bacterial isolates were retrieved from the National Reference Laboratory for Bacterial Meningitis (University of Amsterdam, and RIVM, Bilthoven, The Netherlands). Isolates from three different patient categories were studied.
Patients with severe meningococcal septic shock
Thirty-one isolates were obtained from the blood of children (median age 3 years, range 3 months to 16 years) with a fulminant meningococcal septic shock, who were prospectively enrolled in a clinical trial between 1991 and 1995. Fulminant meningococcal septic shock was defined as the presence of purpura (with or without petechiae) and persisting hypotension despite volume loading or three or more signs of poor end-organ perfusion. Hypotension was defined according to age [10]. Metabolic acidosis, elevated lactate levels, arterial hypoxia, oliguria, and elevated difference in temperature between Tcore and Tperipheral (> 3°C) were considered as signs of poor end-organ perfusion [9,10].
Blood samples were drawn within 90 min of admittance. For endotoxin levels, blood was collected in pyrogen-free tubes (Falcon 2063; Becton Dickinson, Lincoln Park, NJ) and immediately immersed in melting ice. Anticoagulation was obtained by pyrogen-free heparin (final concentration 50 IE/ml blood). After centrifugation at 190 g at 4°C for 10 min, platelet-rich plasma was collected, immediately frozen and stored at −20°C. For TNF-α and IL-6, serum was collected, immediately frozen and stored at − 20°C.
Patients with chronic meningococcaemia
Nine isolates were obtained from the blood of patients with chronic meningococcaemia. This was defined as a case of meningococcal sepsis with a febrile episode of at least 1 week, without meningeal symptoms [4]. In addition, the patients had other clinical characteristics of chronic meningococcaemia, such as rash and/or arthralgia [3].
Carriers with respiratory tract symptoms
Twelve isolates were obtained from the sputum of patients with signs of infection of the respiratory tract.
Identification of the isolates was performed using standard methods [11]. The isolates were serogrouped by means of Ouchterlony tests with use of rabbit antisera (produced at the Reference Laboratory) to the capsular polysaccharides.
The isolates were stored at −70°C. Prior to the experiments, the isolates were subcultured on chocolate agar at 37°C in 5% CO2. One colony was suspended in 5 ml of RPMI 1640 (Bio Whittaker, Verviers, Belgium) and incubated overnight at 37°C in 5% CO2. This suspension was diluted 10-fold the next morning in fresh RPMI, incubated at 37°C in 5% CO2, and after 2 h further diluted 100-fold in prewarmed RPMI 1640, immediately before the start of the experiments. For counting of viable colony-forming units (CFU), 10-fold dilutions of this bacterial suspension were made in PBS and 10-μl aliquots were plated on chocolate plates. The plates were incubated at 37°C in 5% CO2 overnight and counting was performed. All countings were performed in triplicate.
Endotoxin release in vitro
Bacterial suspension (0.5 ml; containing 1.0–3.0 × 104 CFU) was added to pyrogen-free tubes (Falcon 2059) containing 9.5 ml of prewarmed RPMI 1640. The tubes were subsequently incubated at 37°C in 5% CO2. After 3 h, the tubes were centrifuged at 3300 g for 30 min at 4°C, supernatant was collected, immediately frozen and stored at −70°C. We previously demonstrated that in such supernatants bacterial numbers are reduced to ≤ 0.1% of the numbers before centrifugation [12], so the endotoxin in the supernatant is non-cell-bound or ‘free’ endotoxin. All experiments were performed in triplicate.
Whole blood stimulations
Blood was collected from a single donor by venepuncture by using a pyrogen-free collecting system. The donor was known to have no bactericidal antibodies against group B or C meningococci (assay modified after [13]). Anticoagulation was obtained with pyrogen-free heparin (Organon Teknika BV, Boxtel, The Netherlands; final concentration 50 IE/ml blood). Bacterial suspension (50 μl; containing 1.0–3.0 × 103 CFU) was added to pyrogen-free tubes (Falcon 2063) containing 950 μl of heparinized whole blood. The tubes were incubated at 37°C in 5% CO2 for 4 h. Incubations were terminated by centrifugation (1500 g for 20 min at 4°C). The plasma was collected, immediately frozen and stored at −70°C until assessment of TNF-α and IL-6. All experiments were performed in triplicate.
Bacterial growth in vitro
To establish whether differences in endotoxin release in vitro were related to differences in bacterial growth rate, the in vitro growth rate was established for nine isolates causing acute septic shock and nine isolates causing chronic meningococcaemia. An overnight culture suspension was prepared in RPMI 1640 or in trypticase soy broth (TSB), as described above. The next morning this suspension was diluted 10-fold, incubated for 2 h and subsequently diluted 1000-fold in prewarmed RPMI 1640 or TSB, and incubated for 3 h at 37°C in 5% CO2. Counts were performed at the beginning and at the end of this incubation, as described above.
Assays
The endotoxin content was determined by a chromogenic Limulus amebocyte lysate assay (Coatest Endotoxin; Chromogenix AB, Mölndal, Sweden). The method has a detection limit in blood of 0.036 endotoxin units (EU)/ml, the intra-assay variation is < 10%. Each sample was assayed in duplicate, and the results were expressed as the mean. In the in vitro experiments, the obtained values were corrected for the inoculum added at t = 0 h, and expressed as EU/ml, per 104 CFU/ml added.
TNF-α levels in the serum of the children with meningococcal septic shock were determined by TNF-IRMA (Medgenix, Fleurus, Belgium; detection limit 5 pg/ml, intra-assay variation < 5%), and the IL-6 levels by the B9 bioassay (detection limit 1 pg/ml). TNF-α levels obtained in the ex vivo whole blood stimulations were measured using an ELISA for human TNF-α, according to the instructions of the manufacturer (Central Laboratory of the Netherlands Red Cross Blood Transfusion Service, Amsterdam, The Netherlands; intra-assay variation < 10%). IL-6 was assessed by ELISA using anti-human IL-6 MoAb (Pharmingen, San Diego, CA; clone MQ2-13A5 and MQ2-39C3). Both assays had a detection limit of 2 pg/ml.
Statistical analysis
The values of the endotoxin and cytokine levels obtained in the in vitro experiments were expressed as means of three experiments. For comparison of groups the Mann–Whitney test was applied. Pearson's r or Spearman's ρ were used to determine correlation coefficients. α was set at 0.05.
RESULTS
Bacteria
Of the 31 isolates obtained from children with acute meningococcal septic shock, 27 belonged to serogroup B, three to serogroup C and one to serogroup A. Of the isolates from patients with chronic meningococcaemia or from carriers with respiratory symptoms, four and 10 belonged to serogroup B, and five and two belonged to serogroup C, respectively.
Endotoxin and cytokine levels in vitro
The endotoxin concentration in RPMI before addition of bacterial suspension was below the detection limit. Mean TNF-α and IL-6 levels in whole blood ex vivo after 4 h of incubation at 37°C without addition of bacteria were also below the detection limit.
The free endotoxin levels in the supernatant after 3 h of incubation and the TNF-α and IL-6 levels in plasma after 4 h of ex vivo stimulation with the three groups of meningococci are shown in Fig. 1. The median free endotoxin level was significantly higher for isolates causing septic shock compared with isolates causing chronic meningococcaemia (P = 0.01, Mann–Whitney test) or isolates from carriers with respiratory symptoms (P = 0.02, Mann–Whitney test). The median TNF-α and IL-6 levels were lowest for the isolates causing chronic meningococcaemia, although this difference reached statistical significance only for IL-6 levels.
Fig. 1.
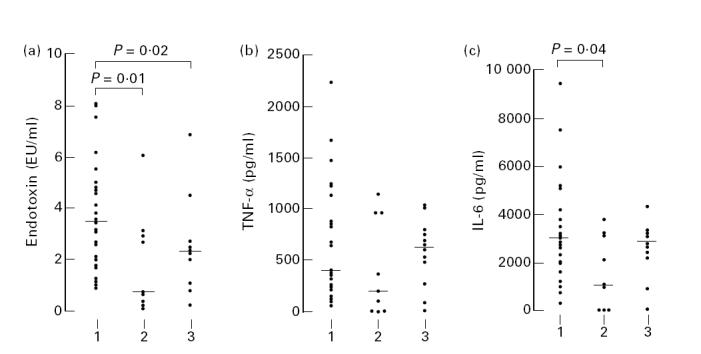
Free endotoxin levels in RPMI 1640 after 3 h of incubation and tumour necrosis factor-alpha (TNF-α) and IL-6 levels in plasma after 4 h of ex vivo stimulation with meningococci obtained from (1) children with severe meningococcal septic shock (n = 31), (2) patients with chronic meningococcaemia (n = 9) and (3) carriers with respiratory symptoms (n = 12). The horizontal bar represents the median value. For statistical comparison of groups the Mann–Whitney test was used.
In all three groups endotoxin levels were not significantly different for group B or C meningococci (P > 0.05, Mann–Whitney test). There was variation in inoculum added at t = 0 h (1.0–3.0 × 103 CFU), but TNF-α and IL-6 levels showed only a weak correlation with the inoculum size: r = 0.33, P = 0.02, and r = 0.29, P = 0.03, respectively. Hence, as r2 was only 0.09 and 0.07, respectively, this inoculum effect explained only a minor part of the variation in cytokine levels in the in vitro stimulations. The free endotoxin levels in RPMI and the TNF-α or IL-6 levels in whole blood ex vivo strongly correlated (ρ = 0.63, P < 0.001) (Fig. 2), as did TNF-α and IL-6 levels (ρ = 0.95, P < 0.001).
Fig. 2.
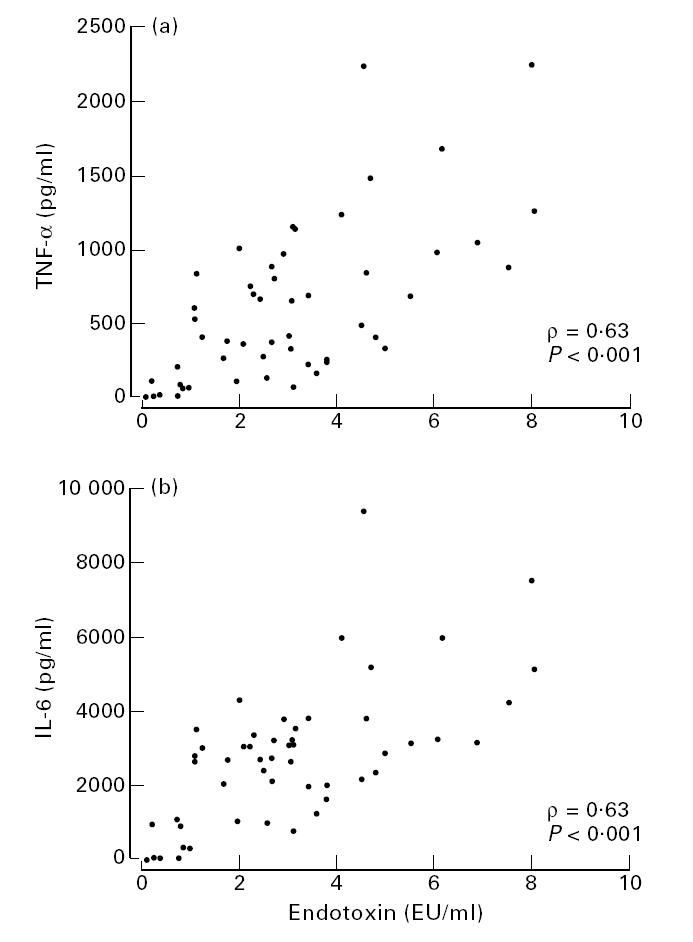
Correlation between free endotoxin levels in RPMI 1640 after 3 h incubation and tumour necrosis factor-alpha (TNF-α) (a) and IL-6 levels (b) in plasma after 4 h of ex vivo stimulation with meningococci (n = 52), obtained from children with severe meningococcal septic shock (n = 31), patients with chronic meningococcaemia (n = 9) and carriers with respiratory symptoms (n = 12).
Bacterial growth in vitro
Nine isolates causing acute septic shock (five isolates releasing the highest and four isolates releasing the lowest endotoxin levels), and nine isolates causing chronic meningococcaemia were studied. In RPMI, no significant growth occurred during 3 h incubation. In TSB, maximal 24-fold growth occurred during 3 h incubation. No differences could be established between the growth rate of isolates causing acute or chronic meningococcaemia.
In vitro versus in vivo endotoxin and cytokine levels
The median endotoxin level on admission in the 31 children with septic shock was 5.6 EU/ml (range 0.036–40.2 EU/ml), the median TNF-α level was 226 pg/ml (range 14–727 pg/ml), and the median IL-6 level was 11 545 pg/ml (range 141–167.139 pg/ml). Endotoxin and TNF-α levels strongly correlated (ρ = 0.84, P < 0.01), as did endotoxin and IL-6 levels (ρ = 0.79, P < 0.01), and TNF-α and IL-6 levels (ρ = 0.91, P < 0.01). No relationship was established between the amount of endotoxin released by the causative microorganisms in vitro and the corresponding endotoxin level obtained in the children with septic shock: ρ = 0.02 (P > 0.2) (Fig. 3); or between in vitro endotoxin levels and TNF-α or IL-6 levels in these children: ρ = − 0.29 (P > 0.1), and ρ = − 0.27 (P > 0.1), respectively. Finally, there was no correlation between in vitro and in vivo TNF-α or IL-6 levels (P > 0.1 in both cases).
Fig. 3.
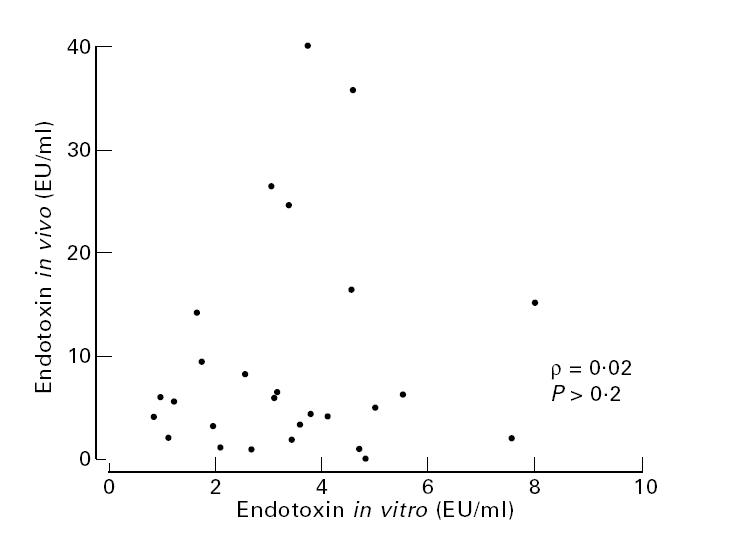
Correlation between endotoxin levels in children with severe meningococcal septic shock, and those levels released by the causative microorganisms in vitro.
DISCUSSION
The clinical spectrum of infections with meningococci ranges from asymptomatic carriership to acute, fulminant sepsis with a rapidly fatal outcome. Characteristics of the microorganism and host factors may both determine the clinical presentation. This study focused on the effect of microbial factors on the human host response.
Isolates obtained from patients with chronic meningococcaemia released significantly less endotoxin and induced significantly less IL-6 in vitro than invasive strains causing acute sepsis or carrier strains. This could not be explained by differences in growth rate in vitro. Hence, the two mechanisms which might explain the lower endotoxin release of these isolates are differences in shedding of membrane particles during growth, differences in biological activity of released endotoxin, or differences in lipopolysaccharide (LPS) immunotype.
An important question is whether the behaviour of the bacteria in this in vitro model reflects the situation in vivo. To address this question, we not only measured free endotoxin release in vitro, but we also measured cytokine production in whole blood ex vivo, which is a well established method to study the human host response. Bacteria were grown overnight in RPMI 1640, which is an endotoxin-free culture medium. Other media like TSB are heavily contaminated with endotoxin, and this is undesirable when performing whole blood stimulations. The levels of (free) endotoxin released in RPMI 1640 and the cytokine levels in whole blood ex vivo strongly correlated, suggesting that the free endotoxin level was a major determinant of cytokine production in this ex vivo model. Therefore, endotoxin release in RPMI 1640 is a meaningful parameter to study, as it clearly predicts the human host response.
Host factors rather than determinants of bacterial virulence are usually held responsible for the relatively benign clinical course of chronic meningococcaemia [3]. Indeed, a number of these patients were proven to have a late complement component deficiency [14]. However, in a series of 15 patients with chronic meningococcaemia, total plasma IgG levels were somewhat lower, but the vast majority of patients had a normal humoral immune system [15]. To our knowledge, strains causing chronic meningococcaemia have never been compared with carrier strains or with meningococcal strains causing fulminant septicaemia. In the only study available, a series describing 14 cases of chronic meningococcaemia, it was concluded that 13 of the 14 isolates belonged to serogroups A, B or C [16]. This is comparable to what is usually found in patients with fulminant septicaemia. Our data suggest that these strains are less virulent than carrier strains or strains isolated from patients with septic shock. A lower endotoxin-releasing potential of the causative microorganism in vitro associated with diminished virulence has been demonstrated also in a mouse model of acute meningococcal septicaemia [17]. In conclusion, our observations suggest that a diminished bacterial virulence contributes significantly to the relatively benign clinical course of chronic meningococcaemia [3].
We also demonstrated that isolates obtained from patients with fulminant septic shock have a significantly higher endotoxin-releasing potential in vitro compared with isolates obtained from carriers with respiratory symptoms. This is in accordance with earlier studies [18–20], again supporting the methodology used. It is known that isolates from patients with septic shock have different lipooligosaccharide (LOS) immunotypes than isolates obtained from carriers [21]. In contrast, the cytokine-inducing potential of carrier isolates and of isolates causing fulminant septic shock was similar. A possible explanation for this high cytokine-inducing potential of the carrier isolates might be the fact that endotoxin concentration and TNF-α release in vitro are usually correlated in a semilogarithmic fashion [22]. As a result, the relatively small difference between isolates causing septic shock and carrier isolates in free endotoxin levels released (median values 3.42 EU/ml versus 2.26 EU/ml) will not result in significant differences in cytokine production.
Endotoxin and cytokine levels in patients with meningococcal septic shock were strongly correlated, supporting the notion that endotoxin plays a key role in the pathogenesis of meningococcal septic shock [2,5,6]. No correlation was observed between the level of endotoxin release by the causative microorganism in vitro, and the corresponding endotoxin and cytokine levels in the patients with septic shock. Therefore, the endotoxin-releasing potential of the causative microorganism does not explain the well known variation in endotoxin [5] and cytokine [7–9] levels in patients with severe meningococcal septic shock. As total endotoxin levels have been demonstrated to correlate with outcome [5], it is clear that this variation in endotoxin levels is clinically important. Other factors which might contribute to the variation in total endotoxin levels are the bacterial numbers in the blood, and the clearance of endotoxin from the circulation.
In conclusion, we demonstrated a diminished bacterial virulence of meningococci causing chronic meningococcaemia. However, other factors than the endotoxin-releasing potential of the microorganism involved are responsible for the wide variation in endotoxin and therefore cytokine levels in patients with acute meningococcal septic shock.
Acknowledgments
We thank Dr A. J. W. van Alphen for his pivotal contribution to the study.
REFERENCES
- 1.Apicella MA. Principles and practice of infectious diseases. In: Mandell GL, Bennett JE, Dolin R, editors. Neisseria meningitidis. 4. New York: Churchill Livingstone; 1995. pp. 1896–909. [Google Scholar]
- 2.Verheul AFM, Snippe H, Poolman JT. Meningococcal lipopolysaccharides: virulence factor and potential vaccine component. Microbiol Rev. 1993;57:34–49. doi: 10.1128/mr.57.1.34-49.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benoit FL. Chronic meningococcemia. Case report and review of the literature. Am J Med. 1963;35:103–12. doi: 10.1016/0002-9343(63)90167-0. [DOI] [PubMed] [Google Scholar]
- 4.Dock W. Intermittent fever of seven months' duration due to meningococcemia (with an analysis of sixty-eight reported cases of meningococcemia) JAMA. 1924;83:31–33. [Google Scholar]
- 5.Brandtzaeg P, Kierulf P, Gaustad P, Skulberg A, Bruun JN, Halvorsen S, Sørensen E. Plasma endotoxin as a predictor of multiple organ failure and death in systemic meningococcal disease. J Infect Dis. 1989;159:195–204. doi: 10.1093/infdis/159.2.195. [DOI] [PubMed] [Google Scholar]
- 6.Dunn KLR, Virji M, Moxon ER. Investigations into the molecular basis of meningococcal toxicity for human endothelial and epithelial cells: the synergistic effect of LPS and pili. Microb Pathogen. 1995;18:81–96. doi: 10.1016/s0882-4010(95)90085-3. [DOI] [PubMed] [Google Scholar]
- 7.Waage A, Brandtzaeg P, Halstensen A, Kierulf P, Espevik T. The complex pattern of cytokines in serum from patients with meningococcal septic shock. J Exp Med. 1989;169:333–8. doi: 10.1084/jem.169.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Deuren M, van der Ven-Jongekrijg J, Bartelink AKM, van Dalen R, Sauerwein RW, van der Meer JWM. Correlation between proinflammatory cytokines and antiinflammatory mediators and the severity of disease in meningococcal infections. J Infect Dis. 1995;172:433–9. doi: 10.1093/infdis/172.2.433. [DOI] [PubMed] [Google Scholar]
- 9.Derkx B, Marchant A, Goldman M, Bijlmer R, van Deventer S. High levels of interleukin-10 during the initial phase of fulminant meningococcal septic shock. J Infect Dis. 1995;171:229–32. doi: 10.1093/infdis/171.1.229. [DOI] [PubMed] [Google Scholar]
- 10.Derkx HHF, van den Hoek J, Redekop WK, Bijlmer Rpgm, van Deventer SJH, Bossuyt PMM. Meningococcal disease: a comparison of eight severity scores in 125 children. Intens Care Med. 1996;22:1433–41. doi: 10.1007/BF01709565. [DOI] [PubMed] [Google Scholar]
- 11.Balows A, Hausler WJ JR, Herrmann KL, Isenberg HD, Shadomy HJ, editors. 5. Washington: American Society for Microbiology; 1991. Manual of clinical microbiology. [Google Scholar]
- 12.Prins JM, Speelman P, Kuijper EJ, Dankert J, van Deventer SJH. No increase in endotoxin release during antibiotic killing of meningococci. J Antimicrob Chemother. 1997;39:13–18. doi: 10.1093/jac/39.1.13. [DOI] [PubMed] [Google Scholar]
- 13.Zangwill KM, Stout RW, Carlone GM, et al. Duration of antibody response after meningococcal polysaccharide vaccination in US Air Force personnel. J Infect Dis. 1994;169:847–52. doi: 10.1093/infdis/169.4.847. [DOI] [PubMed] [Google Scholar]
- 14.Fasano MB, Sullivan KE, Ibsen L, Winkelstein JA. Chronic meningococcemia in a child with a deficiency of the sixth component of complement. Pediatr Allergy Immunol. 1993;4:214–6. doi: 10.1111/j.1399-3038.1993.tb00094.x. [DOI] [PubMed] [Google Scholar]
- 15.Nielsen HE, Koch C, Mansa B, Magnussen P, Bergmann OJ. Complement and immunoglobulin studies in 15 cases of chronic meningococcemia: properdin deficiency and hypoimmunoglobulinemia. Scand J Infect Dis. 1990;22:31–36. doi: 10.3109/00365549009023116. [DOI] [PubMed] [Google Scholar]
- 16.Spanjaard L, Bol P, Zanen HC. Chronische meningokokkensepsis, een vergeten ziekte. Ned Tijdschr Geneesk. 1985;129:352–5. [PubMed] [Google Scholar]
- 17.Andersen BM, Solberg O. Effect of benzylpenicillin in mice infected with endotoxin-liberating or non-liberating variant strains of Neisseria meningitidis. Scand J Infect Dis. 1984;16:257–66. doi: 10.3109/00365548409070398. [DOI] [PubMed] [Google Scholar]
- 18.Andersen BM, Solberg O. Endotoxin liberation and invasivity of Neisseria meningitidis. Scand J Infect Dis. 1984;16:247–54. doi: 10.3109/00365548409070397. [DOI] [PubMed] [Google Scholar]
- 19.Andersen BM, Solberg O, Bryn K, Frøholm LO, Gaustad P, Høiby EA, Kristiansen BE, Bøvre K. Endotoxin liberation from Neisseria meningitidis isolated from carriers and clinical cases. Scand J Infect Dis. 1987;19:409–19. doi: 10.3109/00365548709021673. [DOI] [PubMed] [Google Scholar]
- 20.Mellado MC, Rodríguez-Contreras R, Fernandez-Crehuet M, Lopez-Gigosos R, Delgado Rodríguez M, Galvez-Vargas R. Endotoxin liberation by strains of N. meningitidis isolated from patients and healthy carriers. Epidemiol Infect. 1991;106:289–95. doi: 10.1017/s0950268800048433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones DM, Borrow R, Fox AJ, Gray S, Cartwright KA, Poolman JT. The lipooligosaccharide immunotype as a virulence determinant in Neisseria meningitidis. Microb Pathogen. 1992;13:219–24. doi: 10.1016/0882-4010(92)90022-g. [DOI] [PubMed] [Google Scholar]
- 22.Bruin KF, von der Möhlen MAM, Derkx BHF, Jansen J, ten Cate JW, van Deventer SJH. Characterization of the endotoxin-induced TNF release in whole blood and peripheral blood mononuclear cells. In: Bruin KF, editor. In: Endotoxin responsiveness in humans. University of Amsterdam; 1994. pp. 31–42. PhD Thesis. [Google Scholar]


