Abstract
Perivascular infiltrates of inflammatory cells are a hallmark of lesional skin in scleroderma. We have explored the potential for scleroderma fibroblasts to modulate mononuclear leucocyte migration across endothelial cell monolayers in tissue culture, and to regulate expression of endothelial cell adhesion molecules. Fibroblasts were grown from skin biopsies of eight patients with active diffuse cutaneous scleroderma and from four healthy controls. Co-culture and conditioned medium transfer experiments examined the effect of soluble fibroblast products on mononuclear leucocyte (U937) cell migration across endothelial cell (1E-7) monolayers grown on tissue culture inserts. Co-culture of scleroderma, but not control fibroblasts, promoted transendothelial migration of U937 cells. Scleroderma fibroblast-conditioned medium had qualitatively similar effects and equivalent results were obtained using Jurkat-6 (T lymphocyte) cells, and with peripheral blood mononuclear cells from a patient with diffuse cutaneous scleroderma. Promotion of leucocyte migration does not appear to result from increased endothelial adhesion molecule expression, since fibroblast-conditioned medium did not up-regulate endothelial cell expression of intercellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1) or E-selectin. Moreover, leucocyte migration across cytokine-activated endothelial cell layers in co-cuture with fibroblasts was less than across resting cells, although the selective effect of scleroderma fibroblast co-culture persisted. Recombinant monocyte chemoattractant protein-1 (MCP-1) or IL-8 increased passage of mononuclear leucocytes across endothelial cell monolayers, whilst anti-MCP-1, but not anti-IL-8 antibodies, significantly reduced the effect of fibroblast conditioned medium. These data suggest that systemic sclerosis (SSc) fibroblasts promote leucocyte migration across endothelial cell monolayers in tissue culture via an MCP-1-dependent mechanism. These findings may be relevant to the perivascular mononuclear leucocyte infiltrates characteristic of early SSc lesions.
Keywords: scleroderma, systemic sclerosis, endothelium, fibroblast, co-culture
INTRODUCTION
Scleroderma (systemic sclerosis) is a fibrosing connective tissue disease of unknown aetiology and uncertain pathogenesis [1]. A growing body of evidence suggests that cellular components of the immune system [2] and vasculature [3] are important in modulating fibroblast properties within lesional tissues. Perhaps as a consequence of cytokine stimulation, an activated population of fibrogenic fibroblasts deposit excessive extracellular matrix, which is the pathological hallmark of the established disease [4]. Studies have confirmed the potential for increased direct interactions between lymphocytes and fibroblasts in scleroderma [5], and other reports suggest that soluble products of mononuclear leucocytes isolated from scleroderma patients can activate fibroblasts in vitro [6].
Our own work has shown that endothelial cells (EC) modulate fibroblast properties [7] and that scleroderma fibroblasts show increased responsiveness to some EC-derived factors, including IL-1 and basic fibroblast growth factor (bFGF) [8]. Since perivascular fibroblasts show increased collagen gene expression in lesional tissues [9], one mechanism which may operate in this disease is that activated, or damaged, EC release factors which stimulate fibroblast matrix synthesis and increase expression of fibroblast surface adhesion molecules, including intercellular adhesion molecule-1 (ICAM-1), to facilitate interactions between extravasated inflammatory leucocytes and fibroblasts. However, scleroderma fibroblasts are also reported to release a range of soluble factors which can influence other cell types. These products include a number of cytokines and growth factors which can modulate EC phenotype, including IL-1, IL-6, tumour necrosis factor-alpha (TNF-α) and IL-8 (reviewed in [10]). It is possible therefore that fibroblast-derived factors may also alter EC properties in ways that contribute to the development of scleroderma.
In addition to microvascular damage, lesional tissues in systemic sclerosis (SSc) demonstrate mononuclear cell infiltrate at times when the disease is clinically active [11]. These cells include T lymphocytes expressing a number of surface markers of activation [12], and recent studies suggest that cells of the monocyte/macrophage lineage are also present, especially at the early stages of the disease [13]. Infiltrating leucocytes are potential sources of a number of immunomodulatory or profibrotic cytokines. It seems likely that complex cytokine-mediated networks of interaction between different cell types are involved in the development of scleroderma.
This complexity limits the usefulness of conventional tissue culture methods which study cell types in isolation. Other fields have benefited from the use of co-culture and organotypic systems [14] and such approaches may also prove valuable in unravelling the interactions between different cell types in scleroderma. In the present study we use a three-cell co-culture system, together with complementary conditioned medium transfer experiments, to examine the hypothesis that scleroderma fibroblasts promote migration of mononuclear leucocytes across EC monolayers and modulate the expression of EC surface adhesion molecules. Our findings extend the data which we have previously reported relating to modulation of fibroblast properties in scleroderma by EC and provide further evidence for altered intercellular interactions in this disease.
MATERIALS AND METHODS
Fibroblast cultures
Fibroblasts were grown by explant culture from 4-mm3 punch skin biopsies taken from lesional skin of eight patients with active diffuse cutaneous scleroderma and from four normal healthy control individuals. Biopsies were taken for clinical or research purposes with full informed consent, and this study was approved by the Royal Free NHS Trust Ethics Committee. Scleroderma patients had a mean age of 51 ± 9 years (mean ± s.e.m.), four were female, and the mean durations of scleroderma and Raynaud's symptoms at biopsy were 48 ± 12 months and 60 ± 10 months months, respectively. The mean age of control subjects was 44 ± 4 years and all but one were female. For co-culture experiments fibroblasts were seeded at confluent density into 12-well culture plates and maintained in standard fibroblast culture medium (Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal calf serum (FCS)). In some studies fibroblasts were preincubated for 8 h with activating cytokines (IL-1α, TNF-α or interferon-gamma (IFN-γ) (R&D Systems, Oxford, UK)) at 100 U/ml final concentration, which has previously been shown to markedly up-regulate expression of ICAM-1 [8].
Endothelial cell cultures
The virally transformed line derived from human umbilical vein endothelial cells (1E-7) was used in these experiments. 1E-7 has been shown to maintain a differentiated EC phenotype [15]. Our own data (not shown) confirm that morphology and patterns of EC adhesion molecule expression are similar to those of non-transformed EC. This line has a number of practical advantages, including an ability to grow well on tissue culture inserts, and a lower serum requirement than native human EC. Cells were seeded at a density of 5–10 × 103 per 6.5-mm diameter transwell culture insert (8 μm pore size). After culture overnight these cells formed a confluent monolayer and the transwell culture insert was transferred to a well containing the fresh medium. Medium within the insert was replaced to remove non-adherent cells. For some experiments EC-seeded inserts were incubated for 6–8 h in medium containing the activating cytokine (TNF-α or IL-1α at 100 U/ml; R&D Systems) or lipopolysaccharide (LPS; 1 mg/ml; Sigma Chemical Co., St Louis, MO), prior to addition of leucocytes. For measurement of surface adhesion molecule expression 1E-7 cells were seeded at confluent density into 96-well tissue culture plates. These plates were cultured 24–48 h before treatment with recombinant cytokines or fibroblast-conditioned medium, and subsequent adhesion molecule assay.
Preparation of conditioned media
Monolayers of fibroblasts, in 75-cm2 tissue culture flasks, were rinsed twice with serum-supplemented (10% FCS) DMEM, then 10 ml of fresh medium were added. After a 24-h conditioning period, medium was harvested and cellular debris removed by centrifugation at 3000 g for 10 min. Medium was either used immediately or stored frozen at −70°C for later experiments.
Leucocyte culture
The human premonocytic cell line U937 [16] was chosen because it grows well in standard fibroblast growth medium and has been extensively used to investigate leucocyte–endothelial cell interactions [17]. To extend the data obtained from U937 cells, migration of the human lymphocytic line Jurkat-6 [18] or peripheral blood mononuclear cells (PBMC) isolated from a 32-year-old female patient with an 18-month history of diffuse cutaneous scleroderma were also studied. Isolation of PBMC was by density gradient centrifugation of buffy coat leucocytes using Lymphoprep solution, according to the manufacturer's instructions.
Migration assay
Leucocytes were seeded in the upper chamber of a transwell tissue culture insert which had been seeded with EC at confluent density 16 h earlier. Migration of cells into the lower chamber, across the EC monolayer, was determined by direct counting. Medium from the lower chamber was aspirated, the under-surface of the transwell insert and the floor of the lower chamber were washed gently with fresh DMEM to remove loosely adherent leucocytes, and the resulting suspension of cells was centrifuged at 3000 g for 5 min and resuspended in counting fluid. Cell number was determined by direct counting using a haemocytometer. Cells could be detected in the lower chamber from 2 h after seeding, and increased to a maximum by 24 h. After 24 h, the EC layer had sometimes started to detach from the tissue culture insert. Based on the results of initial experiments, a 16-h migration period was found to give consistent results, and this time point was selected for subsequent studies. The effect of fibroblasts on leucocyte migration was examined by co-culturing dermal fibroblast (control or scleroderma) monolayers in the tissue culture wells below the transwell insert.
Control experiments compared leucocyte migration in the absence of an EC layer, at 16 h and also after 2 h or 6 h, in case the large number of cells passing into the lower chamber at 16 h, in the absence of an EC monolayer, obscured differential effects of fibroblast co-culture which might have been apparent at earlier time points.
Co-cultures in which either the EC monolayer or the fibroblast monolayer had been activated using proinflammatory cytokines (IL-1α, TNF-α or IFN-γ at 100 U/ml final concentration; R&D Systems) were also undertaken. Later experiments investigated whether fibroblast-conditioned medium had the same effect as scleroderma fibroblast co-culture, and other studies compared recombinant human IL-8 or monocyte chemoattractant protein-1 (MCP-1; R&D Systems) with conditioned medium. These experiments confirmed that IL-8 or MCP-1 at a concentration of 50 ng/ml consistently increased migration of U937 cells into the lower chamber, and this concentration was used in subsequent studies. Later we examined whether preincubation with anti-IL-8 or anti-MCP-1 antibodies (500 μg/ml for 60 min at room temperature; R&D Systems) modulated the effect of fibroblast-conditioned medium or recombinant chemokines.
Migration and binding of radiolabelled U937 cells
Radiolabelled U937 cells were used to confirm data obtained by direct counting, and also to investigate binding of leucocytes to the EC layer. U937 cells were radiolabelled as described previously [17]. Briefly, 1 mCi/ml of 3H-thymidine was added to a suspension of U937 cells and culture continued for 24 h. The cells were then washed three times in fresh medium to remove unincorporated thymidine and the labelled cells used within 6 h for migration assays. Standard curves (data not shown) confirmed that the radioactivity in cell aliquots reflected cell number. Migration studies using labelled U937 followed a similar protocol to those outlined above, except that, after centrifugation of the cells collected from the lower culture chamber, the cell pellet was lysed using sodium hydroxide and 3H-thymidine measured by liquid scintillation counting in triplicate samples. In experiments using labelled cells, binding to the EC monolayer was also quantified by lysing the monolayer and adherent leucocytes and determining radioactivity as above. Resting and activated EC monolayers in the presence or absence of scleroderma fibroblasts were compared.
Measurement of cell surface adhesion molecule expression
Endothelial cell surface adhesion molecule expression was measured using a cell-bound ELISA [19]. Endothelial cells seeded in 96-well plates were incubated with test media or cytokines for between 6 h and 24 h. Control experiments (data not shown) suggested that, for 1E-7 cells in monolayer culture, time points of 6, 10 and 24 h coincided with maximal induction of E-selectin, vascular cell adhesion molecule-1 (VCAM-1) and ICAM-1, respectively. Following incubation, the cell layer was rinsed three times in PBS, fixed for 20 min at 4°C in 0.1% glutaraldehyde solution and then washed thoroughly with PBS. Non-specific protein binding was blocked using 10% fat-free dried milk solution for 1 h, murine anti-human ICAM-1, VCAM-1 or E-selectin antibody (R&D Systems) was added (1:1000 dilution) for 1 h, wells were washed for 30 min and adhesion molecule-specific antibody binding was quantified using horseradish peroxidase-conjugated goat anti-mouse IgG (1:2000) added for 1 h. The ELISA was developed by adding o-phenylenediamine/H2O2 solution with measurement of mean absorbance at 450 nm using an automatic plate reader.
RESULTS
Migration studies
In the absence of an EC monolayer there was substantial passage of U937 cells from the upper chamber into the underlying tissue culture well. At 2 h the mean (+ s.e.m.) proportion of the seeded U937 cells which had migrated across the insert into the lower culture chamber was 8.0 + 0.9%, increasing to 27.2 + 1.5% at 6 h and 76.4 + 23.6% at 16 h. There was no significant difference in U937 migration for tissue culture wells containing control or scleroderma dermal fibroblasts at each time point, compared with control wells without fibroblasts. When a confluent monolayer of EC was present on the culture insert, U937 cell migration at 16 h was reduced to around 14.4 ± 2.6% of cells seeded. The proportion of cells migrating was slightly greater (15.8 ± 3.4%) when a non-scleroderma fibroblast monolayer was present in the tissue culture well, but this difference was not statistically significant. In contrast, scleroderma fibroblasts significantly promoted migration of U937 cells across EC monolayers in a co-culture system (P = 0.02, Student's paired t-test). Thus, in the presence of scleroderma fibroblasts the proportion of U937 cells passing across into the lower chamber over 16 h was around 24.8 ± 4.6% of the total number added to the upper chamber.
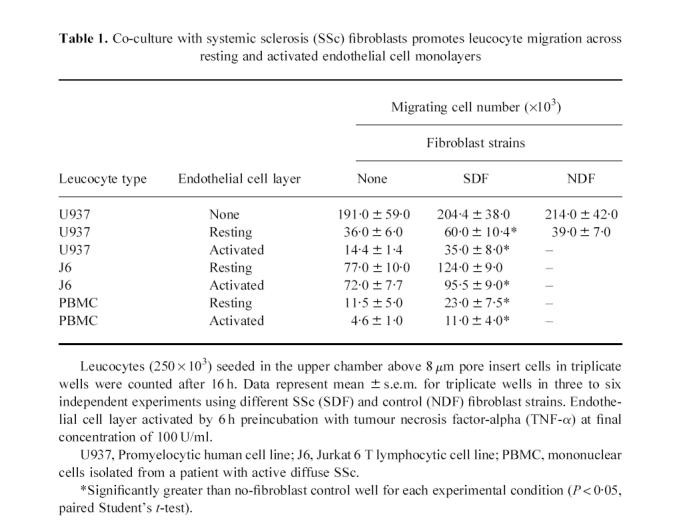
Prior cytokine-induced activation of the EC monolayer did not alter the selective ability of scleroderma fibroblasts to enhance migration. Interestingly, however, the number of cells migrating across activated monolayers was significantly below that for resting endothelium, at 5.8 ± 0.8% seeded U937 cells. In the presence of scleroderma fibroblasts this migration increased to 15.0 ± 3.4%. These data are summarized in Fig. 1. Qualitatively similar results were observed using the human T lymphocyte cell line J-6 and with mononuclear cells isolated from whole blood of a patient with active diffuse cutaneous scleroderma (Table 1). Overall, these results strongly suggest that scleroderma fibroblasts promote migration of mononuclear leucocytes across EC monolayers in vitro.
Fig. 1.
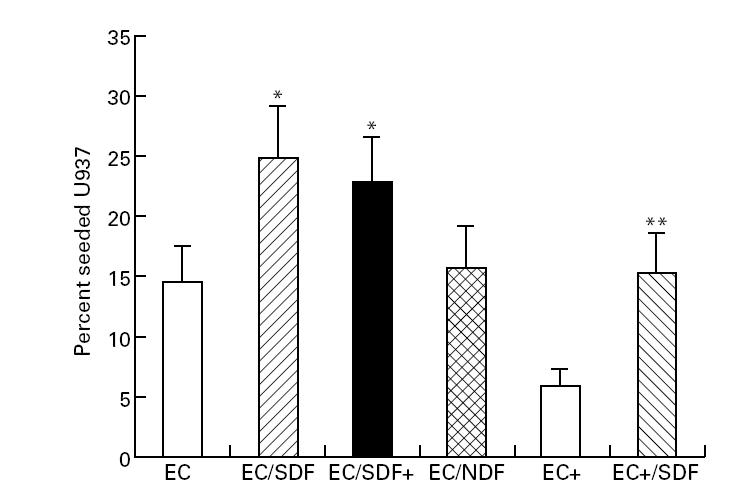
Co-culture with scleroderma fibroblasts promotes U937 cell migration across endothelial cell (EC) monolayers. Leucocyte migration is expressed as mean percentage (± s.e.m.) of added cells migrating across control EC monolayer over 16 h. Data are from replicate wells in six independent experiments using six different scleroderma (SDF) and three normal fibroblast (NDF) strains. Cells were counted directly using a haemocytometer. Cytokine activation (tumour necrosis factor-alpha (TNF-α) 100 U/ml for 8 h) is indicated by EC+ and SDF+. *Significantly greater than migration across resting EC layer; **greater than migration across activated EC layer (Student's paired t-test, P < 0.05).
Table 1.
Co-culture with systemic sclerosis (SSc) fibroblasts promotes leucocyte migration across resting and activated endothelial cell monolayers
Data for labelled U937 cells confirm those from direct cell counting (Fig. 2). The use of labelling also allowed the retention of U937 cells attached within the monolayer to be examined. Cytokine activation of the EC monolayer increased adhesion of U937 cells, and so we suspected that the apparent reduction in migration across activated EC layers might result from retention of leucocytes bound to the endothelial monolayer. Our results using radiolabelled U937 cells support this by showing reduced migration across the cell layer after EC activation and increased retention of the U937 cells in the EC layer (Fig. 2).
Fig. 2.
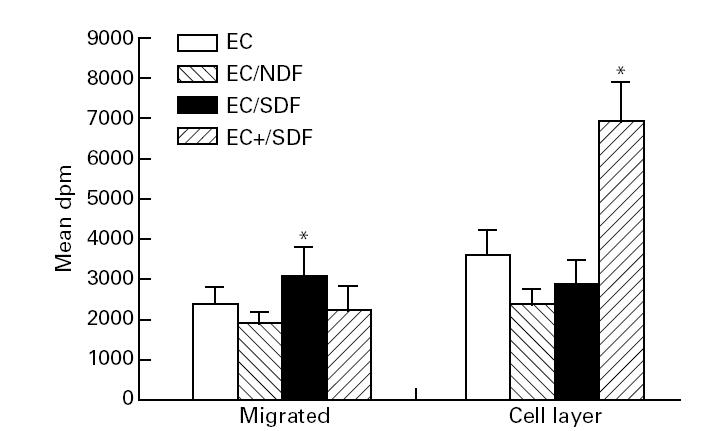
Effect of fibroblast co-culture on labelled U937 cell migration across endothelial cell (EC) monolayers. U937 cells were labelled using 3H-thymidine. Data from a representative of three independent experiments (using three scleroderma (SDF) or normal fibroblast (NDF) strains) are shown. Migration of U937 into the lower culture chamber and binding of U937 to the EC layer were examined in triplicate wells, after 16 h of co-culture. Scleroderma fibroblasts but not NDF increased migration of U937 across the EC monolayer. Activation of the EC layer (EC+) increased U937 cell binding and reduced the number of cells migrating across the monolayer. *P < 0.05 by Student's paired t-test compared with non-fibroblast wells.
The co-culture data suggest that a soluble factor derived from scleroderma fibroblasts promotes trans-endothelial leucocyte migration by altering the properties of the EC layer. To investigate this further we undertook a series of conditioned medium transfer experiments. The effect of scleroderma fibroblast-conditioned medium was similar to that of co-culture, with migration increasing to 25.2 ± 3.9% seeded U937 cells. Later experiments investigated whether promotion of transmigration of leucocytes might involve chemokine mediators. IL-8, a C-X-C chemokine, has been implicated as a promoter of leucocyte extravasation in scleroderma [20], whereas the C-C class of chemokine ligands predominantly affects the movement of mononuclear leucocytes [21], and include MCP-1. In our assay system recombinant human MCP-1 induced transendothelial migration of U937 cells into the lower chamber similarly to scleroderma fibroblast-conditioned medium (27.2 + 2.6% seeded cells, P = 0.003). U937 cell migration was also consistently greater in the presence of recombinant human IL-8 (19.6 + 2.5%), although this did not reach statistical significance (P = 0.10). Pre-incubation of recombinant chemokines with their respective neutralizing antibodies reduced U937 migration back to that seen in control wells (13.5 + 1.4% seeded cells, P = 0.02). Pre-incubation of scleroderma fibroblast-conditioned medium with anti-MCP-1 partially blocked the promotion of U937 cell migration, to 21.1 + 1.3% seeded U937 cells (P = 0.02). This was not observed for anti-IL-8 antibodies (migration 24.0 + 4.8% seeded cells). These results are shown in Fig. 3.
Fig. 3.
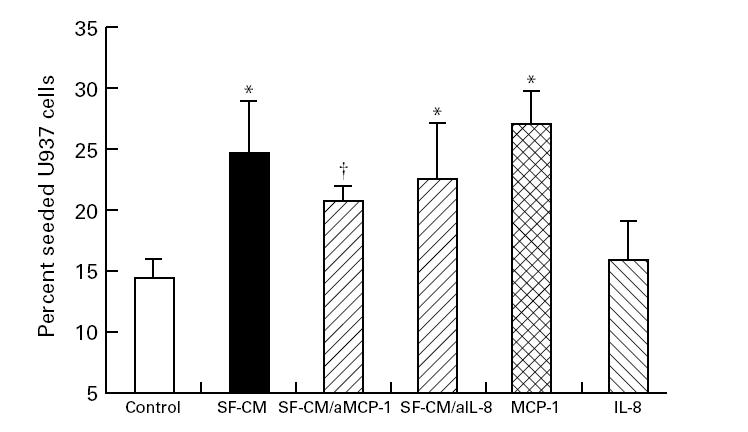
Promotion of U937 migration by scleroderma fibroblast-conditioned medium and recombinant monocyte chemoattractant protein-1 (MCP-1). Cell number in the lower chamber was counted in replicate wells 16 h after addition of 250 × 103 U937 cells into the tissue culture insert seeded with 1E-7 cells at confluent density, and preincubated with either scleroderma fibroblast conditioned medium (SF-CM) recombinant human MCP-1 or IL-8. Data are expressed as percentage (± s.e.m.) seeded cells. SF-CM and MCP-1 substantially increased leucocyte migration into the lower chamber. IL-8 consistently induced a modest increase in U937 cell migration compared with control wells, although this did not reach statistical significance (P = 0.10). Neutralizing antibodies blocked the effect of recombinant MCP-1 (P = 0.02) or IL-8 (P = 0.05) on leucocyte migration, and anti-MCP-1 (aMCP1) but not anti-IL-8 (aIL-8) reduced the promotion of U937 migration into the lower chamber. Data summarize a series of five independent experiments using different fibroblast strains. *Significantly above migration in control wells; †significantly below SF-CM wells (P < 0.05, Student's paired t-test).
Modulation of endothelial cell adhesion molecule expression by SSc fibroblasts
Neither scleroderma nor control fibroblast-conditioned media induced up-regulation of EC surface E-selectin or VCAM-1 (Fig. 4). Both were readily induced by recombinant TNF-α or IL-1α, with a threshold concentration of 10 U/ml. ICAM-1 was expressed by unstimulated 1E-7 cells at levels somewhat greater than those seen in non-transformed human umbilical vein endothelial cells (HUVEC) (data not shown) and expression was modestly, but consistently, up-regulated by medium conditioned by one scleroderma and two control fibroblast strains. In no cases was expression of ICAM-1 reduced below the basal level after exposure to fibroblast-conditioned medium.
Fig. 4.
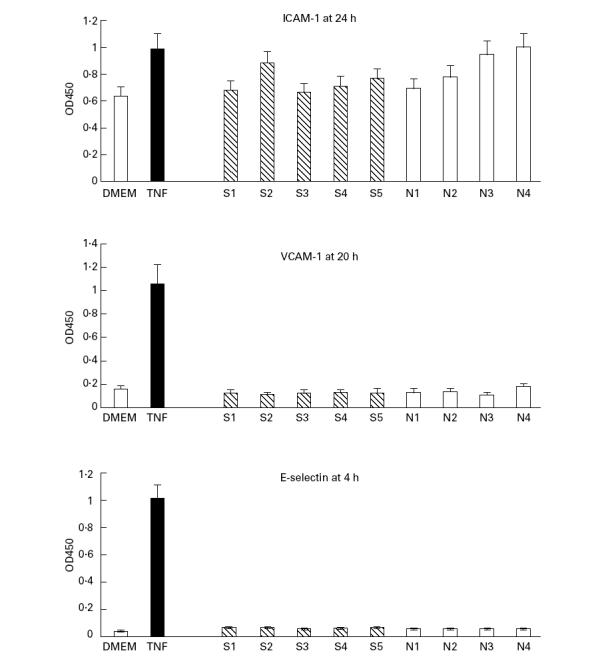
Fibroblast conditioned medium does not induce endothelial cell (EC) activation. A minimal volume (2 ml per 25-cm2 culture flask) of medium (Dulbecco's modified Eagle's medium (DMEM)) was conditioned for 24 h by scleroderma (S1–S5) or control (N1–N4) dermal fibroblasts and then added to confluent cultures of the human EC line IE-7. Neither E-selectin nor vascular cell adhesion molecule-1 (VCAM-1) were up-regulated. Some media induced modest up-regulation of intercellular adhesion molecule-1 (ICAM-1) but in no wells was ICAM-1 reduced below its constitutive level of expression. Quantification was by cell-bound ELISA using absorbance at 450 nm in triplicate wells (mean ± s.d.). Control wells had no cytokine added and reflect constitutive expression of the adhesion molecules. Adhesion molecules were readily up-regulated by tumour necrosis factor-alpha (TNF-α) at 100 U/ml final concentration. Similar data were obtained in two further experiments.
DISCUSSION
The emigration of circulating leucocytes across the endothelium into perivascular tissues has been extensively studied. Its initial phase appears to involve rolling of leucocytes along the vessel wall via a series of loose and reversible interactions between members of the selectin family (e.g. E-selectin, P-selectin) and their glycoprotein counter-receptors [22]. Rolling is facilitated by increased expression of selectins in response to inflammatory stimuli. Later, leucocytes attach more firmly to the luminal surface of the EC, a process termed tethering, which involves integrins. These are heterodimeric adhesion molecules which are modulated by cytokines, or other stimuli, to increase their affinity for specific ligands, which are usually members of the immunoglobulin superfamily of adhesion molecules [23]. Tethering fixes leucocytes to the EC lining layer of the blood vessel and is an essential precursor of transmigration across the endothelial monolayer and underlying basement membrane.
One potential mechanism by which scleroderma fibroblasts might promote the passage of leucocytes across EC layers is though modulation of endothelial adhesion molecule expression. However, our results do not suggest that this is important in these experiments, despite reports that scleroderma fibroblasts release a number of cytokines which can activate EC [4]. This may reflect a subthreshold concentration of proinflammatory cytokines in conditioned medium, or perhaps the interplay between multiple secreted cytokines, as has previously been observed for EC [24]. It is somewhat surprising that migration across activated EC layers was below that for resting EC, since most studies suggest that EC activation promotes leucocyte extravasation in vivo. Our results using labelled U937 cells show that this reduction in migration is associated with increased binding of leucocytes to the EC layer. Trapping of leucocytes within cell monolayers expressing ICAM-1 has also been reported in other tissue culture systems. For example, in a series of experiments in which ICAM-1 was over-expressed on fibroblasts, cells expressing increased ICAM-1 retained leucocytes within the fibroblast layer in a manner analogous to that seen in the present study [25].
Apart from changes in adhesion molecule expression, leucocyte extravasation also involves morphological changes in the transmigrating leucocyte or the EC layer. Endothelial permeability changes often involve breakdown of the underlying basement membrane by metalloproteinases, and opening of intercellular junctions [26]. A specific role for leucocyte-derived elastase in the breakdown of intercellular tight junctions has been proposed, and this is supported by experimental data derived from a similar system to that used in our experiments [27]. It is possible that proteases derived from scleroderma fibroblasts may subserve a similar function. One consequence of changes in endothelial cell shape or cell–cell interactions might be greater exposure of the culture insert membrane, thereby increasing the accessibility of the pores through which leucocyte passage occurs. Support for this mechanism is provided by studies using similar co-culture systems which have demonstrated marked changes in EC morphology induced by fibroblast-derived soluble factors [28,29].
The chemokine family of cytokines is believed to be important in the regulation of leucocyte trafficking, through their chemotactic activity and by activating specific subsets of inflammatory cells or modulating leucocyte–endothelial interactions [30]. Families of chemokines are distinguished by the arrangement of conserved cysteine residues in the mature protein. The C-X-C family includes the cytokine IL-8, and MCP-1 is a prototypic member of the C-C family of chemokines [21]. The release or activation of chemokines by scleroderma fibroblasts provides another plausible mechanism by which scleroderma fibroblasts might promote transendothelial leucocyte migration. Scleroderma fibroblasts have been shown to secrete IL-8 [31] and elevated circulating levels of this mediator have been reported in scleroderma sera [32]. Our findings confirm that both MCP-1 and IL-8 promote leucocyte migration in this experimental system. Moreover, the blocking effect of anti-MCP-1 on scleroderma fibroblast-conditioned medium suggests that MCP-1 is likely to be, at least partially, responsible for fibroblast-induced promotion of leucocyte migration. The source of MCP-1 cannot be directly determined from these studies, although fibroblast-derived MCP-1 has been shown to promote T cell activation in an analogous co-culture system with murine lung fibroblasts [33]. Another possibility is that fibroblast-derived factors may induce or increase the activity of MCP-1 released from EC or from U937 cells. Interestingly, IL-6 has been shown to induce MCP-1 in U937 cells [34] and elevated levels of IL-6 have been reported in culture medium from scleroderma fibroblasts [35]. Our antibody neutralization studies suggest that IL-8 is not a major mediator of fibroblast-induced leucocyte migration, although a recent study proposes that cytomegalovirus infection of fibroblasts induces IL-8 secretion and promotes migration of neutrophils across EC monolayers in a similar experimental system [36]. In addition to direct chemotactic effects or modulation of leucocyte–endothelial cell interactions, leucocyte trafficking might be promoted by chemokine-induced morphological changes in U937 cells [37].
Although most of our experiments used U937 cells, a cell line originally derived from a human histiocytic tumour, though showing many phenotypic features of monocytes [16], the similar pattern of results observed using the T lymphocytic Jurkat-6 cell line, and with PBMC taken from a patient with active scleroderma suggest a more general promotion of mononuclear leucocyte migration. The Jurkat-6 cell line has T lymphocyte properties and expresses surface markers of the T-helper cell subset including CD4 [18], which are often present in increased numbers in lesional scleroderma tissues [12].
In conclusion, our data suggest that scleroderma fibroblasts modulate leucocyte–endothelial cell interactions in ways that facilitate migration of leucocytes across EC layers, at least partly via an MCP-1-dependent mechanism. These results provide further support for the view that, in addition to their role in extracellular matrix homeostasis, fibroblasts may influence inflammatory disease processes through an effect on leucocyte trafficking.
Acknowledgments
The authors are grateful to Dr Ken Welsh for valuable advice. Financial support was provided by the Arthritis Research Campaign (UK) and the Raynaud's and Scleroderma Association (UK). The cell line 1E-7 was a gift from Glaxo Group Research Ltd.
References
- 1.Black CM. The aetiopathogenesis of systemic sclerosis: thick skin-thin hypotheses. The Parkes Weber Lecture 1994. J Royal Coll Physicians London. 1995;29:119–30. [PMC free article] [PubMed] [Google Scholar]
- 2.White B. The immunopathogenesis of systemic sclerosis (scleroderma) Rheum Dis Clin North Am. 1996;22:825–40. doi: 10.1016/s0889-857x(05)70296-9. [DOI] [PubMed] [Google Scholar]
- 3.LeRoy EC. Systemic sclerosis: a vascular perspective. Rheum Dis Clin North Am. 1996;22:675–94. doi: 10.1016/s0889-857x(05)70295-7. [DOI] [PubMed] [Google Scholar]
- 4.Postlethwaite AE. Connective tissue metabolism including cytokines in scleroderma. Curr Opin Rheumatol. 1995;7:535–40. doi: 10.1097/00002281-199511000-00012. [DOI] [PubMed] [Google Scholar]
- 5.Abraham D, Lupoli S, McWhirter A, Plater-Zyberg C, Piela TH, Korn JH, Olsen I, Black CM. Expression and function of surface antigens on scleroderma fibroblasts. Arthritis Rheum. 1991;34:1164–72. doi: 10.1002/art.1780340913. [DOI] [PubMed] [Google Scholar]
- 6.Worrall JG, Whiteside TL, Prince RK, Buckigham RB, Stachura I, Rodnan GP. Persistence of scleroderma-like phenotype in normal fibroblasts after prolonged exposure to soluble mediators from mononuclear cells. Arthritis Rheum. 1986;29:54–64. doi: 10.1002/art.1780290108. [DOI] [PubMed] [Google Scholar]
- 7.Denton CP, Shi-Wen X, Welsh KI, Pearson JD, Black CM. Scleroderma fibroblast phenotype is modulated by endothelial cell co-culture. J Rheumatol. 1996;23:633–8. [PubMed] [Google Scholar]
- 8.Denton CP, Shi-Wen X, Black CM, Pearson JD. Scleroderma fibroblasts show increased responsiveness to endothelial cell derived IL-1 and bFGF. J Invest Dermatol. 1997;108:269–74. doi: 10.1111/1523-1747.ep12286455. [DOI] [PubMed] [Google Scholar]
- 9.Scharffetter K, Lankat-Buttgereit B, Krieg T. Localisation of collagen mRNA in normal and scleroderma skin by in situ hybridisation. Eur J Clin Invest. 1988;18:9–17. doi: 10.1111/j.1365-2362.1988.tb01158.x. [DOI] [PubMed] [Google Scholar]
- 10.Fagundus DM, LeRoy EC. Cytokines and systemic sclerosis. Clinics Dermatol. 1994;12:407–17. doi: 10.1016/0738-081x(94)90293-3. [DOI] [PubMed] [Google Scholar]
- 11.Prescott RJ, Freemont AJ, Jones CJ, Hoyland J, Fielding P. Sequential dermal microvascular and perivascular changes in the development of scleroderma. J Pathol. 1992;166:255–63. doi: 10.1002/path.1711660307. [DOI] [PubMed] [Google Scholar]
- 12.Fleischmajer R, Perlish JS, Reeves JRT. Cellular infiltrates in scleroderma skin. Arthritis Rheum. 1977;20:975–84. doi: 10.1002/art.1780200410. [DOI] [PubMed] [Google Scholar]
- 13.Kraling BM, Maul GG, Jimenez SA. Mononuclear cellular infiltrates in clinically involved skin from patients with systemic sclerosis of recent onset predominantly consist of monocytes/macrophages. Pathobiology. 1995;63:48–56. doi: 10.1159/000163933. [DOI] [PubMed] [Google Scholar]
- 14.Tuan T-L, Keller LC, Sun D, Nimni ME, Cheung D. Dermal fibroblasts activate keratinocyte outgrowth on collagen gels. J Cell Sci. 1994;107:2285–9. doi: 10.1242/jcs.107.8.2285. [DOI] [PubMed] [Google Scholar]
- 15.Fickling SA, Tooze JA, Whitely GS. Characterisation of human umbilical vein endothelial cell lines produced by transfection with the early region of SV40. Exp Cell Res. 1992;201:517–21. doi: 10.1016/0014-4827(92)90303-p. [DOI] [PubMed] [Google Scholar]
- 16.Harris P, Ralph P. Human leukaemic models of myelomonocytic development: a review of the HL-60 and U937 cell lines. J Leucocyte Biol. 1985;37:407–22. doi: 10.1002/jlb.37.4.407. [DOI] [PubMed] [Google Scholar]
- 17.Carvalho D, Savage CO, Black CM, Pearson JD. IgG antiendothelial cell autoantibodies from scleroderma patients induce leukocyte adhesion to human vascular endothelial cells in vitro. Induction of adhesion molecule expression and involvement of endothelium-derived cytokines. J Clin Invest. 1996;97:111–9. doi: 10.1172/JCI118377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greenwood J, Wang Y, Calder VL. Lymphocyte adhesion and transendothelial migration in the central nervous system: the role of LFA-1, ICAM-1, VLA-4 and VCAM-1. Immunology. 1995;86:408–15. [PMC free article] [PubMed] [Google Scholar]
- 19.Pober JS, Bevilacqua MP, Mendrick DL, Lapiere LA, Fiers W, Gimbrone MA. Two distinct monokines IL-1 and TNF each independently induce biosynthesis and transient expression of the same antigen on the surface of cultured human vascular endothelial cells. J Immunol. 1986;136:1680–7. [PubMed] [Google Scholar]
- 20.Southcott AM, Jones KP, Li D, et al. Interleukin-8. Differential expression in lone fibrosing alveolitis and systemic sclerosis. Am J Resp Crit Care Med. 1995;151:1604–12. doi: 10.1164/ajrccm.151.5.7735620. [DOI] [PubMed] [Google Scholar]
- 21.Luster AD. Chemokines-chemotactic cytokines that mediate inflammation. N Engl J Med. 1998;338:436–45. doi: 10.1056/NEJM199802123380706. [DOI] [PubMed] [Google Scholar]
- 22.Carlos TM, Harlan JM. Leucocyte-endothelial adhesion markers. Blood. 1994;84:2068–101. [PubMed] [Google Scholar]
- 23.Dejana E, Breviario F, Caveda L. Leucocyte-endothelial cell adhesive receptors. Clin Exp Immunol. 1996;12(Suppl. 10):S25–S28. [PubMed] [Google Scholar]
- 24.Rival Y, Maschio AD, Rabiet M-J, Dejana E, Duperray A. Inhibition of PECAM-1 synthesis and leucocyte transmigration in endothelial cells by the combined action of TNFα and IFNγ. J Immunol. 1996;157:1233–41. [PubMed] [Google Scholar]
- 25.Gao JX, Issekutz AC. Mac-1 is the predominant beta 2 integrin mediating human neutrophil migration through synovial and dermal fibroblast barriers. Immunology. 1996;88:463–70. doi: 10.1046/j.1365-2567.1996.d01-662.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Romanic AM, Madri JA. The induction of a 72kD gelatinase in T-cells upon adhesion to endothelial cells is VCAM-1 dependent. J Cell Biol. 1994;125:1165–78. doi: 10.1083/jcb.125.5.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cepinskas G, Noseworthy R, Kvietys PR. Transendothelial neutrophil migration—role of neutrophil-derived proteases and relationship to transendothelial protein movement. Circulation Res. 1997;81:618–26. doi: 10.1161/01.res.81.4.618. [DOI] [PubMed] [Google Scholar]
- 28.Montesano R, Pepper MS, Orci L. Paracrine induction of angiogenesis in vitro by Swiss 3T3 fibroblasts. J Cell Sci. 1993;105:1013–24. doi: 10.1242/jcs.105.4.1013. [DOI] [PubMed] [Google Scholar]
- 29.Kuzuya M, Kinsella JL. Endothelial cell differentiation is induced by fibroblast-derived soluble factors. Exp Cell Res. 1994;215:310–8. doi: 10.1006/excr.1994.1347. [DOI] [PubMed] [Google Scholar]
- 30.Bacon KB, Flores-Romo L, Aubry JP, Wells TN, Power CA. Interleukin-8 and RANTES induce the adhesion of the human basophilic cell line KU-812 to human endothelial cell monolayers. Immunology. 1994;82:473–81. [PMC free article] [PubMed] [Google Scholar]
- 31.Kadono T, Kikuchi K, Takehara K, Tamaki K. Increased production of IL-6 and IL-8 in scleroderma fibroblasts. J Rheumatol. 1998;25:296–301. [PubMed] [Google Scholar]
- 32.Reitamo S, Remitz A, Varga J, Ceska M, Effenberger F, Jimenez S, Uitto J. Demonstration of IL-8 and autoantibodies to IL-8 in the serum of patients with systemic sclerosis and related disorders. Arch Dermatol. 1993;129:189–93. [PubMed] [Google Scholar]
- 33.Hogaboam CM, Lukacs NW, Chensue SW, Streiter RM, Kunkel SL. Monocyte chemoattractant protein-1 synthesis by murine lung fibroblasts modulates CD4+ T lymphocyte activation. J Immunol. 1998;160:4606–14. [PubMed] [Google Scholar]
- 34.Biswas P, Delfanti F, Bernasconi S, et al. Interleukin-6 induces monocyte chemotactic protein-1 in peripheral blood mononuclear cells and in the U937 cell line. Blood. 1998;91:258–65. [PubMed] [Google Scholar]
- 35.Feghali CA, Bost KL, Boulware DW, Levy LS. Mechanisms of pathogenesis of scleroderma I. Overproduction of interleukin-6 by fibroblasts cultured from affected skin sites of patients with scleroderma. J Rheumatol. 1992;19:1207–11. [PubMed] [Google Scholar]
- 36.Khabar KSA, Al-Zoghaibi F, Al-Ahdal MN, et al. The α chemokine, IL-8, inhibits the antiviral action of interferon α. J Exp Med. 1997;186:1077–85. doi: 10.1084/jem.186.7.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cross AK, Richardson V, Ali SA, Palmer I, Taud DD, Rees RC. Migration responses of human monocytic cell lines to alpha- and beta-chemokines. Cytokine. 1997;9:521–8. doi: 10.1006/cyto.1996.0196. [DOI] [PubMed] [Google Scholar]


