Abstract
IgG4 and IgE isotypes contribute marginally to the pool of circulating antibodies in healthy individuals, but are elevated during atopic diseases and particularly upon helminth infections. To examine whether the high levels of these isotypes in circulation are reflected in a higher capacity of PBMC to produce IgG4 and IgE, we examined cells from patients infected with filarial nematodes that exhibit high levels of IgG4 and IgE. Indeed, IgG4 production by PBMC correlated strongly with plasma levels of IgG4 (r = 0.534, P = 0.002), but such correlation was not found for IgE. The replacement of CD19+ cells from PBMC by IgD+ cells abrogated the high capacity of PBMC to make IgG4. This indicates that an altered B cell compartment accounts for the high IgG4-producing capacity of the PBMC. The high production of IgG4 in vitro was not dependent on IL-4 and IL-13, as neutralizing antibodies to these cytokines did not inhibit IgG4. However, IgE release by PBMC was dependent on IL-4 and IL-13. Anti-filarial IgG4 was detected in culture supernatants from filarial patients and its production was independent of IL-4 and IL-13. These results demonstrate that in individuals with elevated IgG4, the B cell compartment in PBMC carries cells that are already committed to IgG4 production and are independent of IL-4 and IL-13.
Keywords: IgG4, IL-4, IL-13, committed B cells, helminths
INTRODUCTION
Infections with helminth parasites are commonly associated with elevated serum IgG4 and IgE. Likewise, allergic diseases exhibit high levels of IgE and immunotherapy often leads to increasing production of IgG4, an effect that is achieved after long-term repeated stimulation with an antigen [1–3]. The synthesis of any immunoglobulin by mature B cells is primarily regulated by CD40 engagement and by B cell differentiation factors such as IL-6 and IL-10 [4,5]. The regulation of IgG4 and particularly IgE isotype synthesis has been intensively studied over the past few years. B cells stimulated with an antigen can be induced to proliferate after CD40 ligation by activated T helper (Th) cells [6–8]. These proliferating B cells can subsequently switch to IgG4 and IgE in the presence of IL-4 or IL-13 [9–12], cytokines typical of Th2 cells. Most studies so far have examined the production of IgG4 and IgE by B cells following switch induction using CD40 ligation and IL-4. Such a system provides information on the precise T cell signals that are essential for triggering of non-switched B cells. In contrast, less attention has been paid to the contribution of committed B cells to IgG4 and IgE secretion and their activation requirement. It becomes important to examine this in situations of persistent antigenic challenge such as during chronic infections that would be expected to generate a substantial pool of differentiated memory B cells.
Helminth-infected subjects who exhibit strong Th2 responses and high levels of IgG4 and IgE may provide a suitable model to study the contribution of the T and the B cell compartment to the production of these isotypes during an ongoing and continuous antigenic exposure. Indeed, Sμ/Sε recombined chromosomal DNA fragments have recently been demonstrated in PBMC of helminth-infected patients, suggesting that IgE committed cells are present in the circulation [13,14]. However, the IgE-producing capacity of the cells or their activation requirements were not analysed and IgG4 was not addressed in the study. Altogether, there is a paucity of information concerning IgG4. With increasing evidence that this isotype may be involved in functionally inhibiting IgE and hence affecting clinical outcome of helminth as well as allergic diseases, the study of IgG4 production and regulation becomes important. Here we sought to examine IgG4 in addition to IgE release by PBMC derived from individuals resident in a rural area in Sulawesi, Indonesia where brugian filariasis is endemic. These helminth-exposed subjects who show high levels of circulating IgE and IgG4 were studied alongside European controls with IgG4 and IgE levels at the lower end of the spectrum to obtain information on how increased levels of these isotypes may be maintained in vivo during repeated and chronic antigenic challenge.
SUBJECTS AND METHODS
Study subjects
Altogether serum samples from 26 filarial subjects (mean age 28 years) and 25 European control donors (mean age 34 years) were studied. PBMC were available from 26 filarial subjects and 11 European controls for cellular analysis. Filarial patients were residents of an area endemic for brugian filariasis in Central Sulawesi, Indonesia, and have been described before [15]. All study subjects were positive for anti-filarial IgG4, an indicator of active infection [16], and nine of these individuals harboured microfilariae (geometric mean of 408 microfilariae/ml) as detected by filtration of 1 ml night blood. European controls consisted of healthy volunteers who had not resided in the tropics.
Plasma and PBMC
Heparinized blood was diluted 1:1 with Hanks' buffered salt solution (HBSS) and centrifuged over Ficoll–Isopaque as described previously [17]. Plasma samples were taken and kept frozen at −30°C until use. PBMC were cryopreserved in Jakarta before transport to The Netherlands, as described earlier [17]. Plasma and cells from European donors were fractionated and stored as described for filarial patients.
Antibody production in vitro
For in vitro antibody production, cryopreserved PBMC were thawed and cultured at a concentration of 6 × 103 cells/well in 96-well round-bottomed plates (Nunc, Roskilde, Denmark) at a final volume of 0.2 ml of culture medium containing human transferrin (20 μg/ml, Life Technologies, Paisley, UK) as described before [18]. PBMC were stimulated with two MoAbs against CD2 (6G4 and B2H4; provided by René van Lier, CLB, Amsterdam, The Netherlands) and recombinant human IL-2 (CLB). In some experiments neutralizing antibodies to IL-4 or IL-13 were included (5 μg/ml [19,20]) (obtained from T. vd Pouw Kraan, CLB; this concentration of the antibodies has been shown to neutralize > 90% of the IL-4 and IL-13 present in culture supernatants). In a few experiments the production of IgE and IgG4 was stimulated with IL-4 (provided by M. Schreier; Sandoz Pharma Ltd, Basel, Switzerland). Experiments were carried out in replicates of 12 or 20 wells. After 12 days of incubation, supernatants were collected and kept frozen at −20°C until assayed.
For the determination of filarial-specific antibody production PBMC from 12 filarial patients and six European controls were examined. Cells (1.5 × 105 cells/well) were cultured in replicates of 20 or 30. The results are expressed as the frequency of positive wells (above background ± 3 s.d., see below).
ELISA for immunoglobulin measurements
To determine immunoglobulins in supernatants, IgG4 and IgE were measured as described before [18], except that all incubation steps were performed for 1 h at room temperature. For detection of IgE, the protocol was slightly modified, utilizing peroxidase-conjugated streptavidin (1:10 000) purchased from CLB, resulting in a detection limit of 10 pg/ml. For IgM measurements, plates were coated with rabbit anti-human IgM (1:1000; Dako, Glostrup, Denmark), subsequently incubated with culture supernatants, biotinylated goat anti-human IgM (1:5000; Jackson, West Grove, PA) and peroxidase-conjugated streptavidin (1:10 000). Finally, IgE and IgM plates were incubated with TMB (Sigma, St Louis, MO) (0.2 mg/ml) and H2O2 (0.003%) in 0.1 M sodium acetate pH 5.5 and the reaction was stopped and developed with 1.8 M H2SO4. Absorbance was subsequently read at 450 nm and immunoglobulins were quantified against known immunoglobulin standards (CLB and Behringwerke AG, Marburg, Germany).
The ELISA for Brugia malayi-specific IgG4 antibodies has been documented by Haarbrink et al. [16]. Briefly, plates were coated with crude extract from B. malayi worms, blocked and incubated with culture supernatants. The incubation was continued with mouse anti-human IgG4 (HP 6025; Sigma) followed by alkaline phosphatase-conjugated goat anti-mouse IgG (Sigma), after which the wells were stained by incubation with 4-Npp (Sigma). Samples were scored positive when absorbance was higher than background + 3 × s.d. The background was determined using IgG4-deficient sera [21].
Depletion of CD19+ B cells and replacement with IgD+ B cells
CD19+ B cells were depleted from PBMC using a magnetic activated cell sorter (MACS; Miltenyi Biotec, Bergisch Gladbach, Germany) according to the manufacturer's recommendations. Briefly, PBMC were incubated for 15 min with anti-CD19-coated magnetic beads at ± 6°C. Cells were washed and loaded onto a magnetically activated column. Negative cells flowing through had < 1% CD19-bearing cells as assessed by FACS analysis, and when stimulated produced only trace amounts of IgM. IgD+ B cells were purified from European control donors by using CD19+ B cells (purified by MACS) which were thereafter labelled with biotinylated goat anti-human IgD (Southern Biotechnology Associates, Inc., Birmingham, AL) and streptavidin–FITC (Dako). The IgD+ cells were subsequently purified to > 99% by FACS sorting (FACSstar; Becton Dickinson, Bedford, MA). The number of IgD+ cells added to PBMC cultures exactly corresponded to the number of CD19+ cells that were removed by depletion.
Polymerase chain reaction for detection of γ4-heavy chain
Reverse transcriptase-polymerase chain reaction (RT-PCR) was performed to detect the presence of constant γ4 mRNA. Total RNA from approx. 5 × 105 cells was isolated by the guanidinium thiocyanate method [22] and transcribed into cDNA by using a cDNA synthesis kit according to the manufacturer's recommendations (Life Technologies). Ten-fold dilution series of the cDNA were used in PCR analysis. PCR cycles (n = 35) consisted of 94° C for 60 s, 53°C for 60 s, and 72°C for 3 min. The following primers were used: γ4 sense 5′-GCTTCCACCAAGGGCCCATC-3′, γ4 anti-sense 5′-GCATGATGGGCATGGGGGACCATATTTGGA-3′, hypoxanthine phosphoribosyltransferase (HPRT) sense 5′-CGAGATGTGATGAAGGAGATGG-3′, HPRT anti-sense 5′-GGATTATACTGCCTGACCAAGG-3′. The internal probe used for hybridization of the amplified γ4 transcripts was: 5′-TCCTGAGGACTGTAGGACAGC-3′.
To allow quantification of the mRNA, PCR products were separated on agarose gel, blotted onto nylon filters (Hybond-N+; Amersham Pharmacia Biotech, Uppsala, Sweden) and hybridized against a 32P-labelled internal primer. The resulting radioactivity was measured by a phospho-imager (ImageQuant). The density values obtained by densitometry were plotted against the dilution of the cDNA. In the linear range, the slope was calculated to represent the relative amount of the γ4 message present in the PBMC of an individual. As control, HPRT primers were used for cDNA quantity and quality.
Statistical analysis
Differences in antibody isotype production, mRNA levels and cellular responses were analysed when appropriate by either Student's t-test or paired t-test. Correlations were calculated using Spearman's rank correlation.
RESULTS
Plasma IgG4 and IgE levels in study subjects
Levels of total IgG4 and IgE in plasma of individuals from filarial-endemic regions (geometric mean IgG4 = 1518 μg/ml and IgE 2758 ng/ml, n = 26) were significantly higher than in European controls (IgG4 = 115 μg/ml and IgE = 30 ng/ml, n = 25, P < 0.0001).
IgG4 and IgE production by PBMC in vitro
In order to stimulate the production of measurable IgE by PBMC in vitro, it was necessary to culture cells at low densities [23]. Without stimulation, there was no IgM, IgG4 or IgE production, indicating that no plasma cells were detectable in our cultures (not shown). To test the relation between serum immunoglobulin concentrations and immunoglobulin production by PBMC, PBMC were stimulated with anti-CD2 + IL-2 and immunoglobulin levels in the culture supernatant were plotted against plasma immunoglobulin levels. As shown in Fig. 1, the levels of IgG4 released in vitro correlated with plasma levels of IgG4 when considering both patients and controls (r = 0.534, P = 0.002), whereas no such correlation was found for IgE (r = 0.285, P = 0.107). Both IgM production and proliferation were equivalent in filarial subjects and European controls (not shown). The data above demonstrate that in vitro IgG4 production by PBMC reflects IgG4 levels in serum, suggesting that this system can be used for studying in vitro mechanisms that are responsible for high IgG4 levels in vivo. Moreover, it can be utilized to address the question of whether the high IgG4 production observed originates from switch or alternatively from already γ4 heavy chain-committed B cells.
Fig. 1.
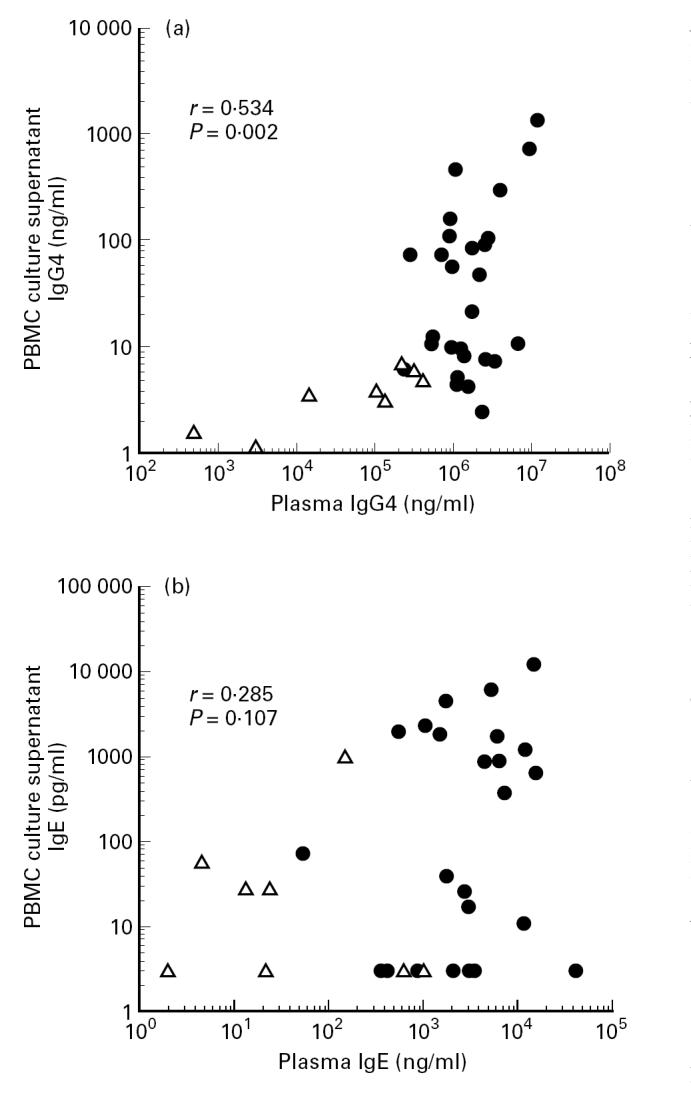
Correlation between plasma levels of IgG4 (a) or IgE (b) and in vitro IgG4 or IgE produced by anti-CD2 + IL-2-stimulated PBMC. IgG4 levels correlated significantly (r = 0.534, P = 0.002, n = 36), whereas IgE did not (r = 0.285, P = 0.107, n = 36). •, Filarial subjects; ▵, European control donors.
The elevated IgG4 production by PBMC is not caused by a high degree of class switch from IgD+ cells
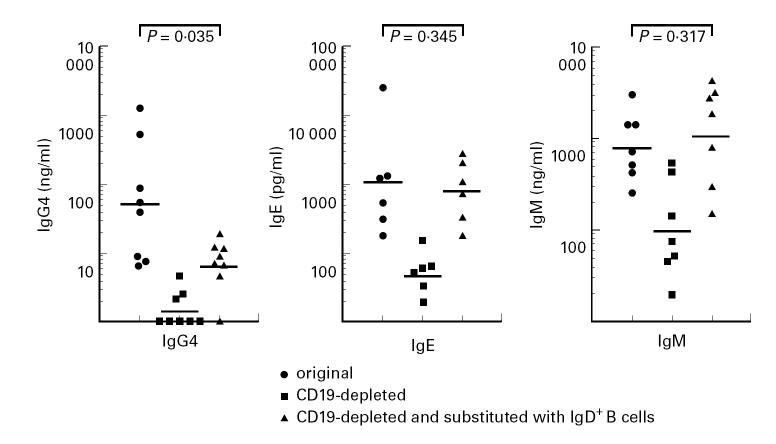
To determine whether class switching from IgD/IgM to IgG4 accounts for the high IgG4 production by PBMC, CD19+ cells were depleted by MACS, after which IgD+ B cells originating from European control donors were added to the CD19-depleted PBMC. As shown in Fig. 2, the depletion of CD19+ cells from PBMC abrogated both total IgG4 and IgE production. Interestingly, in IgD+ reconstituted PBMC, IgE production was restored, but IgG4 release was significantly reduced compared with the original intact PBMC. The measurable IgG4 indicates that IgD+ cells are capable of switching to IgG4, but the antibody levels generated are very low compared with intact PBMC. These results indicate that switching of IgD+ cells only can not account for the high IgG4 production in vitro that is observed in PBMC from our study subjects. It should also be noted that IgM production either remained the same or increased in PBMC supplemented with IgD+ B cells. The high IgM production, intact IgE synthesis and detectable IgG4 in cultures of IgD+ supplemented PBMC show that IgD+ cells are functionally intact and can undergo class switch. However, the switch from IgD/IgM to IgG4 can not account for the high IgG4 production in patient PBMC.
Fig. 2.
The role of endogenous CD19+ B cells in the production of IgG4, IgE and IgM by anti-CD2 + IL-2-stimulated PBMC. CD19+ cells were removed by MACS (see Subjects and Methods) and when indicated, replaced with purified IgD+ B cells. Each dot represents the average response (replicates of 20 wells) of one individual and horizontal lines represent geometric means.
IgG4 production is independent of IL-4 and IL-13
Having shown that switch from IgD+ cells does not seem to play a role in the high capacity of PBMC to produce IgG4 in our study subjects, we examined whether switch to IgG4 from other isotype-committed cells could account for the high total IgG4 production. IL-4 and IL-13 have been shown to induce class switch to IgG4 and IgE. In filariasis there is a strong skewing towards Th2, and patient PBMC, upon polyclonal stimulation, can release substantial amounts of IL-4 [24]. As anticipated, PBMC of filarial patients when stimulated with anti-CD2 + IL-2, produced higher amounts of IL-4 (15.7 pg/ml) and IL-13 (21.5 pg/ml) compared with PBMC from European controls (3.5 pg/ml and 8.1 pg/ml, respectively; not shown). In order to determine whether IL-4 and IL-13 production by PBMC can result in the high IgG4 production, experiments were performed in the presence of neutralizing antibodies to inhibit the biological activity of IL-4 and IL-13. It is clear from Fig. 3 that the presence of these neutralizing antibodies inhibited IgE production from 128.8 pg/ml to 14.4 pg/ml (P = 0.01), whereas the polyclonally elicited IgG4 response was independent of these two cytokines (from 21.6 ng/ml to 16.4 ng/ml, P = 0.20). Conversely, addition of IL-4 to PBMC cultures indeed enhanced IgE (from 114 pg/ml to 14 480 pg/ml; P = 0.02, n = 4; not shown) but did not show any significant effect on IgG4 (from 46.4 ng/ml to 52.0 ng/ml; P = 0.82, n = 8; not shown). Given that the neutralizing antibodies at 5 μg/ml will neutralize > 90% of the IL-4 and IL-13, the remaining 10% (1–2 pg/ml IL-4 and 1–3 pg/ml IL-13) is not sufficient to induce switch (unpublished results). These data indicate that in contrast to IgE, the majority of IgG4 production by PBMC cultures in vitro is not dependent on the process of isotype switching induced by IL-4 and IL-13.
Fig. 3.
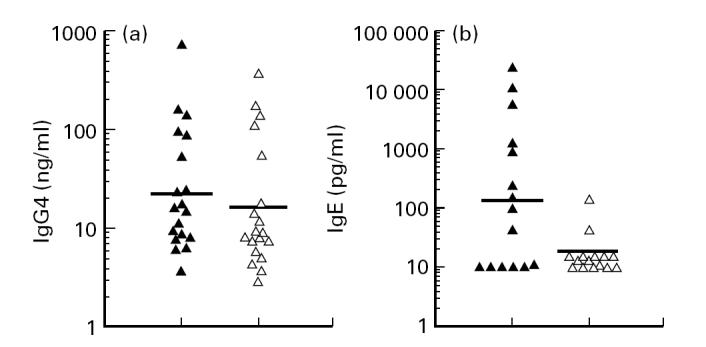
IgG4 (a) and IgE (b) production by PBMC stimulated with anti-CD2 + IL-2 in the absence (▴) or presence (▵) of neutralizing antibodies to IL-4 and IL-13. IgG4 production did not change significantly (P = 0.20, n = 19, paired t-test) after the addition of the neutralizing antibodies (from 21.6 ng/ml to 16.4 ng/ml). In contrast, IgE production was significantly inhibited by neutralizing antibodies to IL-4 and IL-13 (from 128.8 pg/ml to 14.4 pg/ml; P = 0.01, n = 15, paired t-test). Horizontal lines represent geometric means and each dot corresponds to one individual.
PBMC derived from filarial patients produce filarial-specific IgG4, a process which is independent of IL-4 and IL-13
To assess whether we are dealing with a true memory response in the in vitro cultures, we examined the filarial antigen-specific antibody responses in culture supernatants. Stimulation of 12 samples of PBMC derived from individuals living in a filarial-endemic area resulted in detectable production of filarial-specific IgG4 in 10 out of 12 cultures (Fig. 4a). In contrast, none of the supernatants from cultures of control donor PBMC showed detectable filarial-specific IgG4 (n = 6). When neutralizing MoAbs against IL-4 and IL-13 were used during culture of PBMC from seven donors, 8.1/25.1 wells (mean) in the absence and 5.3/22.8 wells in the presence of anti-IL-4 and anti-IL-13 were positive for anti-filarial IgG4 (P = 0.59). It is therefore apparent that individuals living in filarial-endemic areas have filarial-specific B cells in their circulation that are committed to IgG4 production and whose activation is independent of IL-4 and IL-13.
Fig. 4.
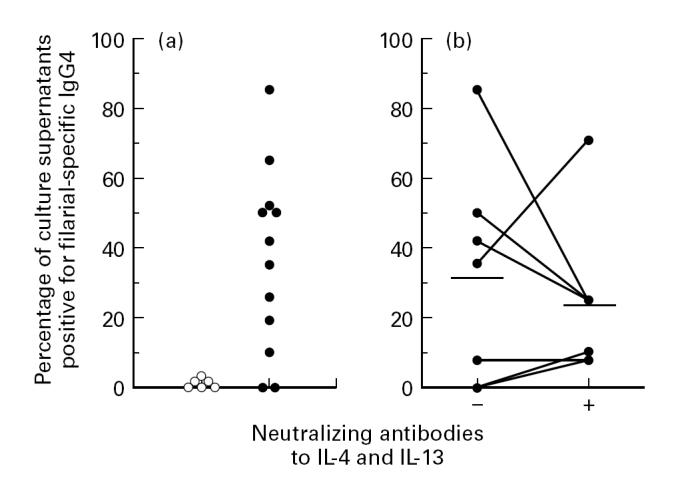
Filarial-specific IgG4 production by PBMC stimulated with anti-CD2 + IL-2. (a) Filarial-specific IgG4 production by Dutch control donors (○, n = 6) and by filarial patients (•, n = 12). (b) Comparison of filarial-specific IgG4 production by PBMC of filarial subjects (n = 7) in the absence (−) or presence (+) of neutralizing antibodies to IL-4 and IL-13. Cells were cultured at 1.5 × 105/well in replicates of 20–30. The results are given as the percentage of wells containing supernatants positive for anti-filarial IgG4. Each dot represents one subject and horizontal lines show geometric means. There was no significant difference in responses without or with neutralizing antibodies to IL-4 and IL-13 (32% versus 23%, respectively; P = 0.59, paired t-test).
Constitutive expression of mRNA for γ4 heavy chain in unstimulated PBMC
In order to study whether already committed IgG4+ B cells were present in ex vivo PBMC of the study subjects, RT-PCR was performed to specifically detect mRNA for γ4 heavy chains. It was possible to detect γ4 transcripts in unstimulated PBMC, suggesting that IgG4-committed cells were present in the circulation. Moreover, cells from filarial subjects tended to show higher amounts of γ4 than European cells (P = 0.09, Fig. 5).
Fig. 5.
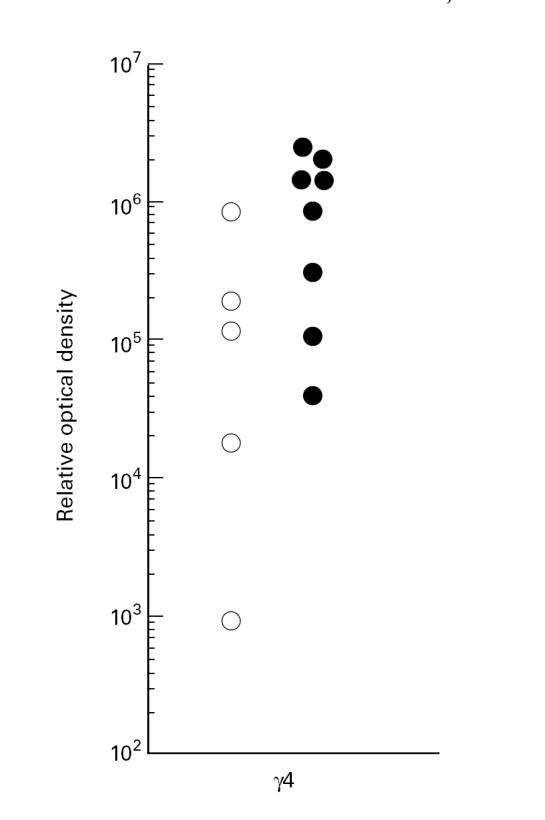
Expression of γ4 heavy chain transcripts in unstimulated PBMC. The relative amount of message was revealed after blotting of the reverse transcriptase-polymerase chain reaction (RT-PCR) product and subsequent probing with an internal probe (see Subjects and Methods). European controls (○) tended to have lower amounts of message than filarial patients (•) (P = 0.09).
DISCUSSION
In order to begin understanding how IgG4 and IgE responses during chronic diseases are regulated and sustained, we used a well described low cell density culture condition to study IgE and IgG4 production by PBMC of helminth-infected subjects as well as European controls [23]. The culture system relies on T cell-stimulated B cell activation and in our hands immunoglobulin production is superior to the co-culture of B cells with CD40-L transfectants plus IL-4 (not shown). In our experiments we observed no spontaneous IgG4 or IgE production. Despite this lack of plasma cell activity (confirmed by absence of immunoglobulin light chain staining in cytospins, not shown), it was clear that the in vitro IgG4 production was significantly correlated with serum levels of this isotype. However, no correlation between serum IgE levels and IgE produced by PBMC in vitro was found.
Given the association between in vitro produced IgG4 and IgG4 levels in vivo, we used this in vitro culture system to define cells that contribute to the high IgG4 levels in serum. It seems unlikely that IgG4 production is a result of a high rate of class switch in vitro. First, we showed that in PBMC capable of producing high levels of IgG4, the replacement of CD19+ cells by IgD+ cells strongly reduced IgG4 production, whereas IgE and IgM responses were not affected. Second, we observed that the high production of IgG4 by PBMC was independent of IL-4 and IL-13. This was in contrast to IgE production that was inhibited by neutralizing antibodies to IL-4 and IL-13. Altogether, the data indicate that in the PBMC of individuals with elevated IgG4, isotype-committed cells, that are not plasma cells, are the major source of in vitro produced IgG4. Upon T cell stimulation these IgG4-committed cells differentiate to produce IgG4 independently of IL-4 and IL-13. The analysis of mRNA in unstimulated PBMC indicates that the message for γ4 heavy-chain transcripts was higher in individuals with elevated plasma IgG4 than in European controls, suggesting that increased numbers of IgG4-committed cells were present in the periphery. Indeed by measuring anti-filarial IgG4 in culture supernatants, we confirmed that memory IgG4+ B cells circulate in the periphery of individuals with elevated IgG4 and that these cells do not require IL-4 and IL-13 for IgG4 production.
As already mentioned above, in contrast to IgG4, the production of IgE in our experiments was entirely dependent on IL-4 and IL-13, and IgE release in vitro did not correlate with plasma IgE levels. Furthermore, the addition of IgD+ cells to CD19-depleted PBMC reconstituted the IgE production to levels comparable to intact PBMC. It is therefore clear that IgE-committed B cells were not responsible for IgE production in our in vitro antibody assay. The analysis of mRNA for the presence of ε heavy chain message indicated that the message was detectable in unstimulated PBMC (not shown). It has to be concluded that IgE-committed B cells are present in peripheral circulation, but at a frequency too low to allow their detection in our culture system. However, it was not possible to test IgE using higher numbers of cells, as at high cell densities IgE release is inhibited. It should be noted that the number of cells used for the in vitro immunoglobulin production was approx. 50-fold lower than the number of cells utilized for detection of the ε transcript.
Earlier reports examining IgG4 and IgE production during helminth infections showed that antibody production in response to parasite antigen was clearly dependent on IL-4 and IL-13 [25,26]. This seems to be in contrast to our findings reported here. The difference may be explained by the length of time study subjects were infected with helminths. In the present study we analysed cells from subjects chronically infected with filarial worms, whereas the reports by Garraud et al. [24] may not have been life-long residents of endemic areas, but expatriates or travellers. The latter patient group may not possess a large pool of IgG4-committed B cells and the responses measured would be expected to result from new switch that is dependent on IL-4 and IL-13.
IgG4 antibodies are thought to play an important role in counteracting the biological activities of IgE [27]. This can have serious implications for allergy or helminth infections. The understanding of how high levels of this isotype are maintained can be important in clinical management of patients. Thus, what the precise requirements are for the activation of isotype-committed cells that when activated make a major contribution to the antibody pool will now have to be investigated. In an earlier study it was shown that the differentiation of human memory B cells into antibody-secreting cells is independent of CD40 [28], whereas Arpin et al. have shown that human CD40-activated memory B cells undergo terminal differentiation into plasma cells much more readily than do naive B cells [29]. Additionally, a study of murine isotype-switched B cells indicated that differentiation of these cells was dependent on CD40, among other T cell-derived signals [30]. The requirement for individual T cell signals has yet to be described specifically for IgG4- and IgE-committed cells. In this context, it would be interesting to analyse the functional properties/differentiation requirements of IgD+, IgG4+ and IgE+ B cells from our patients. However, the paucity of cells available from our patients, who reside in Indonesia, makes the isolation of these cells an impossible task.
In conclusion, individuals with chronic elevation of IgG4 have IgG4-committed cells in the periphery, that are not plasma cells, and can be stimulated to release large amounts of IgG4 in an IL-4- and IL-13-independent manner.
Acknowledgments
Informed consent was obtained from all patients before clinical and parasitological study and blood withdrawal. This work was supported by a grant from NWO (Dutch Organization for Scientific Research), contract number SLW 805.29.302. The authors thank Dr René van Lier, Dr Tineke vd Pouw Kraan and Dr M. Schreier for the kind provision of anti-CD2 antibodies, anti-IL-4 and anti-IL-13 antibodies and IL-4, respectively, and Dr Cor Verweij and Dr Cees van Kooten for reading the manuscript critically.
REFERENCES
- 1.Aalberse RC, van der Gaag R, van Leeuwen J. Serologic aspects of IgG4 antibodies. I. Prolonged immunization results in an IgG4-restricted response. J Immunol. 1983;130:722–6. [PubMed] [Google Scholar]
- 2.Oehling A, Sanz ML, Garcia BE. Immunological parameters in the immunotherapy follow-up. Int Arch Allergy Immunol. 1992;99:489–93. doi: 10.1159/000236317. [DOI] [PubMed] [Google Scholar]
- 3.Akdis CA, Blesken T, Akdis M, et al. Induction and differential regulation of bee venom phospholipase A2-specific human IgE and IgG4 antibodies in vitro requires allergen-specific and nonspecific activation of T and B cells. J Allergy Clin Immunol. 1997;99:345–53. doi: 10.1016/s0091-6749(97)70052-6. [DOI] [PubMed] [Google Scholar]
- 4.Rousset F, Garcia E, Defrance T, et al. Interleukin 10 is a potent growth and differentiation factor for activated human B lymphocytes. Proc Natl Acad Sci USA. 1992;89:1890–3. doi: 10.1073/pnas.89.5.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Banchereau J, Briere F, Liu YJ, et al. Molecular control of B lymphocyte growth and differentiation. [Rev] Stem Cells (Dayt) 1994;12:278–88. doi: 10.1002/stem.5530120304. [DOI] [PubMed] [Google Scholar]
- 6.Rousset F, Garcia E, Banchereau J. Cytokine-induced proliferation and immunoglobulin production of human B lymphocytes triggered through their CD40 antigen. J Exp Med. 1991;173:705–10. doi: 10.1084/jem.173.3.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lane P, Traunecker A, Hubele S, et al. Activated human T cells express a ligand for the human B cell-associated antigen CD40 which participates in T cell-dependent activation of B lymphocytes. Eur J Immunol. 1992;22:2573–8. doi: 10.1002/eji.1830221016. [DOI] [PubMed] [Google Scholar]
- 8.Noelle RJ, Ledbetter JA, Aruffo A. CD40 and its ligand, an essential ligand-receptor pair for thymus-dependent B-cell activation. Immunol Today. 1992;13:431–3. doi: 10.1016/0167-5699(92)90068-I. [DOI] [PubMed] [Google Scholar]
- 9.Lundgren M, Persson U, Larsson P, et al. Interleukin-4 induces synthesis of IgE and IgG4 in human B cells. Eur J Immunol. 1989;19:1311–5. doi: 10.1002/eji.1830190724. [DOI] [PubMed] [Google Scholar]
- 10.Gascan H, Gauchat J-F, Roncarolo M-G, et al. Human B cell clones can be induced to proliferate and to switch to IgE and IgG4 synthesis by IL-4 and a signal provided by activated CD4+ T cell clones. J Exp Med. 1991;173:747–50. doi: 10.1084/jem.173.3.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gascan H, Gauchat J-F, Aversa G, et al. Anti-CD40 monoclonal antibodies or CD4+ T cell clones and IL-4 induce IgG4 and IgE switching in purified human B cells via different signalling pathways. J Immunol. 1991;147:8–13. [PubMed] [Google Scholar]
- 12.Punnonen J, Aversa G, Cocks BG, et al. Interleukin-13 induces interleukin-4-independent IgG4 and IgE synthesis and CD23 expression by human B-cells. Proc Natl Acad Sci USA. 1993;90:3730–4. doi: 10.1073/pnas.90.8.3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baskin B, Islam KB, Evengard B, et al. Direct and sequential switching from μ to ε in patients with Schistosoma mansoni infection and atopic dermatitis. Eur J Immunol. 1997;27:130–5. doi: 10.1002/eji.1830270120. [DOI] [PubMed] [Google Scholar]
- 14.van der Stoep N, Korver W, Logtenberg T. In vivo and in vitro IgE isotype switching in human B lymphocytes: evidence for a predominantly direct IgM to IgE class switch program. Eur J Immunol. 1994;24:1307–11. doi: 10.1002/eji.1830240610. [DOI] [PubMed] [Google Scholar]
- 15.Sartono E, Kruize YCM, Kurniawan A, et al. Depression of antigen-specific interleukin-5 and interferon-γ responses in human lymphatic filariasis as a function of clinical status and age. J Infect Dis. 1997;175:1276–80. doi: 10.1086/593701. [DOI] [PubMed] [Google Scholar]
- 16.Haarbrink M, Terhell A, Abadi K, et al. IgG4 antibody assay in the detection of filariasis. Lancet. 1995;346:853–4. doi: 10.1016/s0140-6736(95)91676-8. [DOI] [PubMed] [Google Scholar]
- 17.Yazdanbakhsh M, Paxton WA, Kruize YCM, et al. T cell responsiveness correlates differentially with antibody isotype levels in clinical and asymptomatic filariasis. J Infect Dis. 1993;167:925–31. doi: 10.1093/infdis/167.4.925. [DOI] [PubMed] [Google Scholar]
- 18.de Boer BA, Kruize YCM, Rotmans JP, et al. Interleukin-12 suppresses immunoglobulin E but enhances immunoglobulin G4 by human peripheral blood mononuclear cells. Infect Immun. 1997;65:1122–5. doi: 10.1128/iai.65.3.1122-1125.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van der Pouw-Kraan T, Rensink I, Aarden L. Characterisation of monoclonal antibodies to human IL-4—application in an IL-4 ELISA and differential inhibition of IL-4 bioactivity on B cells and T cells. Eur Cytokine Netw. 1993;4:343–9. [PubMed] [Google Scholar]
- 20.van der Pouw Kraan Tctm, van der Zee JS, Boeije LCM, et al. The role of IL-13 in IgE synthesis by allergic asthma patients. Clin Exp Immunol. 1998;111:129–35. doi: 10.1046/j.1365-2249.1998.00471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bottaro A, de DeMarchi M, Lange GG, et al. Human IGHC locus restriction fragment length polymorphisms in IgG4 deficiency: evidence for a structural IGHC defect. Eur J Immunol. 1989;19:2159–62. doi: 10.1002/eji.1830191128. [DOI] [PubMed] [Google Scholar]
- 22.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–9. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 23.van der Pouw-Kraan T, van Kooten C, van Oers R, et al. Human transferrin allows efficient IgE production by anti-CD3-stimulated human lymphocytes at low cell densities. Eur J Immunol. 1991;21:385–90. doi: 10.1002/eji.1830210220. [DOI] [PubMed] [Google Scholar]
- 24.Yazdanbakhsh M, Sartono E, Kruize YCM, et al. Elevated levels of T cell activation antigen CD27 and increased interleukin-4 production in human lymphatic filariasis. Eur J Immunol. 1993;23:3312–7. doi: 10.1002/eji.1830231238. [DOI] [PubMed] [Google Scholar]
- 25.King CL, Nutman TB. IgE and IgG subclass regulation by IL-4 and IFN-gamma in human helminth infections—assessment by B-cell precursor frequencies. J Immunol. 1993;151:458–65. [PubMed] [Google Scholar]
- 26.Garraud O, Nkenfou C, Bradley JE, et al. Identification of recombinant filarial proteins capable of inducing polyclonal and antigen-specific IgE and IgG4 antibodies. J Immunol. 1995;155:1316–25. [PubMed] [Google Scholar]
- 27.Hussain R, Poindexter RW, Ottesen EA. Control of allergic reactivity in human filariasis. Predominant localization of blocking antibody to the IgG4 subclass. J Immunol. 1992;148:2731–7. [PubMed] [Google Scholar]
- 28.Silvy A, Lagresle C, Bella C, et al. The differentiation of human memory B cells into specific antigen-secreting cells is CD40 independent. Eur J Immunol. 1996;26:517–24. doi: 10.1002/eji.1830260303. [DOI] [PubMed] [Google Scholar]
- 29.Arpin C, Banchereau J, Liu Y-J. Memory B cells are biased towards terminal differentiation: a strategy that may prevent repertoire freezing. J Exp Med. 1997;186:931–40. doi: 10.1084/jem.186.6.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ehrhardt RO, Harriman GR, Inman JK, et al. Differential activation requirements of isotype-switched B cells. Eur J Immunol. 1996;26:1926–34. doi: 10.1002/eji.1830260838. [DOI] [PubMed] [Google Scholar]