Abstract
Neutrophil activation is thought to play a crucial role in the pathogenesis of sepsis. During activation, neutrophils adhere to and migrate through the endothelium. Therefore, the amount of circulating neutrophils does not adequately reflect the total amount of neutrophils that are involved in the pathophysiologic process of this condition. In this study we test the hypothesis that the severity of sepsis is associated with the total body mass of neutrophils as reflected in the plasma concentration of soluble Fcγ receptor type III (sFcγRIII). Nineteen patients with sepsis (12 male, seven female, median age of 69 years, range 29–87 years) were included in this study. Ten healthy volunteers served as controls. Plasma sFcγRIII concentrations were measured by ELISA. Other parameters that were studied were leucocyte count, plasma concentrations of lactoferrin and soluble l-selectin, and surface expression of CD11b and CD66b on circulating neutrophils. Disease activity was measured using the Acute Physiology and Chronic Health Evaluation (APACHE) II score. Soluble FcγRIII levels were elevated in sepsis patients whereas soluble l-selectin levels were moderately decreased compared with healthy controls. Markers of cell activation were significantly increased in sepsis patients. Soluble FcγRIII correlated with disease severity as measured by the APACHE score (P < 0.05, r = 0.53), whereas the other parameters did not correlate with the APACHE score. In conclusion, this study demonstrates that soluble FcγRIII is a useful marker for disease severity in patients with sepsis.
Keywords: sepsis, SIRS, soluble FcγRIII, APACHE, neutrophil
INTRODUCTION
Sepsis has become an increasingly important clinical syndrome in intensive care units. Mortality associated with this syndrome is unsatisfactorily high, despite modern intensive medicine and antibiotic therapy [1]. Better insight into the pathophysiology of sepsis could improve current treatment modalities, and possibly decrease its mortality [2].
Regarding the pathophysiology of sepsis it is hypothesized that local defence mechanisms are not sufficient in eliminating microorganisms, resulting in a systemic overwhelming inflammatory response. This initially proinflammatory reaction is followed by a compensatory, sometimes overwhelming, anti-inflammatory response [2,3].
Neutrophils play a central role in the inflammatory response in sepsis [4,5]. Neutrophils form the host's first line of defence against microorganisms. They kill invading microorganisms by phagocytosis and intracellular digestion. In sepsis, neutrophils are strongly activated and prepared to kill microorganisms, but they also cause severe damage to surrounding tissues by releasing toxic lysosomal enzymes and oxygen radicals [6]. It has been demonstrated that disease severity of sepsis correlates with the extent of neutrophil activation as measured by levels of elastase, a product released by activated neutrophils, in plasma [7], or by the expression of CD11b on circulating neutrophils [8]. Increased expression of CD11b and other adhesion molecules on neutrophils allows these cells to adhere to (activated) endothelial cells and to transmigrate through the endothelial monolayer into the tissues [9]. Therefore, the number of circulating leucocytes and/or neutrophils, as measured in daily clinical practice, does not reflect the total number of neutrophils involved in the disease process of sepsis, since activated adherent or transmigrated cells are not included. The total body mass of neutrophils may therefore better reflect the amount of neutrophils involved in the inflammatory process in sepsis than the number of circulating cells.
The total body mass of neutrophils consists of young neutrophils in the bone marrow, circulating neutrophils in the bloodstream, neutrophils from the marginating pool (mainly in the lungs), adherent activated neutrophils, and, finally, extravasated neutrophils in tissues [10]. As demonstrated by Huizinga et al. [11], the plasma concentration of the soluble Fc receptor III for IgG appears a good marker for estimating the total body mass of neutrophils.
The third Fcγ receptor or CD16 consists of two forms: the transmembrane FcγRIIIa on macrophages and natural killer (NK) cells which binds monomeric IgG, and the phosphatidyl-inositol-linked FcγRIIIb on neutrophils, which binds complexed IgG [12]. Soluble FcγRIII is mainly derived from neutrophils [13], and in vivo is thought to be cleaved from the cell surface in the process of neutrophil apoptosis [14,15]. The concentration of sFcγRIII is not affected by redistribution of neutrophils, and is correlated with the production of neutrophils in the bone marrow [11].
To test the hypothesis that the total number of neutrophils, as reflected in plasma levels of soluble FcγRIII, is a useful marker for disease severity in sepsis, we related those levels to clinical disease activity as measured by the Acute Physiology and Chronic Health Evaluation (APACHE) II scores. In addition, we related sFcγRIII levels to other possible markers, such as leucocyte counts, neutrophil surface expression of CD11b and CD66b, and plasma levels of soluble l-selectin and plasma lactoferrin.
PATIENTS AND METHODS
Patients and controls
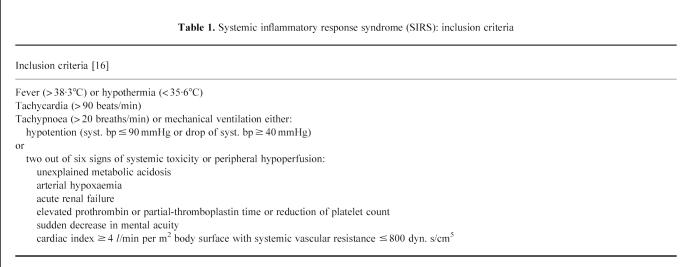
The patient group consisted of consecutive patients with sepsis admitted to our hospital's intensive care unit. Inclusion criteria were as previously described [16] (Table 1). Informed consent for collection of blood samples was obtained from each patient or an appropriate family member. All patients entered the study within hours after admittance to the intensive care unit. To assess disease severity, patients were scored with the APACHE II scoring system [17]. Additionally, 10 healthy volunteers (five male, five female, median age 57 years, range 43–61 years) were included in this study as healthy controls.
Table 1.
Systemic inflammatory response syndrome (SIRS): inclusion criteria
Methods
Blood was collected for the simultaneous measurement of leucocyte counts, flow cytometric parameters (see below), and plasma concentrations of sFcγRIII, lactoferrin and soluble l-selectin. Healthy laboratory personnel served as normal controls.
Soluble FcγRIII ELISA
Levels of sFcRIII in (EDTA) plasma were measured as previously described [18]. Briefly, ELISA plates (Nunc Immunoplate Maxisorp, Roskilde, Denmark) were coated with 5 μg/ml anti-CD16 MoAb (CLBFcRgran 1) in 0.1 m NaHCO3 pH 9.6. Unbound sites were blocked with PBS containing 2% (v/v) milk (PBS/milk). After washing the plates with PBS containing 0.02% Tween-20 (v/v), the wells were incubated with 25- and 50-fold dilutions of plasma samples in High Performance ELISA (HPE) buffer (CLB, Amsterdam, The Netherlands) at room temperature for 1 h in duplicate. Subsequently, the plates were incubated for 1 h with appropriate concentrations of biotin-labelled anti-FcγRIII polyclonal antibody (CLB), diluted in HPE buffer. After washing, the plates were then incubated for 30 min with horseradish peroxidase (HRP)-labelled streptavidin, diluted in PBS/milk, washed again, and 100 μl of substrate were added. The colourimetric reaction was stopped by addition of 2 m H2SO4, and the absorbance was measured in a Titretek multiscan ELISA reader (Flow Labs, Rockville, MD). Pooled plasma of 90 healthy individuals was used to obtain a calibration curve. The concentration of sFcγRIII in this pool was set at 100 arbitrary units (AU).
Soluble l-selectin ELISA
Soluble l-selectin (sl-selectin) was measured using a commercially available sl-selectin ELISA kit (BenderMed Systems, Vienna, Austria) [19]. In brief, microtitre plates, coated with a MoAb to human l-selectin, were incubated with different dilutions of either the sl-selectin standard (ranging from 0.4 to 25 ng/ml) or the patients' plasma samples. Plasma samples were two-fold diluted starting at a dilution of 1:100. Without washing, HRP-conjugated anti-l-selectin mouse MoAb was added and incubated for 2 h at room temperature. Plates were washed and the substrate, tetramethyl benzidine (TMB), was incubated for 15 min at room temperature. The enzyme reaction was stopped by addition of 4 n sulphuric acid. Optical density (OD) values were read at 450 nm.
Lactoferrin ELISA
Measurement of lactoferrin concentrations in plasma samples was performed as described previously [20]. Briefly, Hycult plates (Uden, The Netherlands) were coated with a F(ab′)2 rabbit anti-human lactoferrin polyclonal antibody (Jackson, Westgrove, PA) overnight at room temperature at a dilution of 1:750, then washed, and incubated with two-fold dilutions of the samples, starting at a dilution of 1:25, for 1 h at 37°C. After washing, a rabbit anti-human lactoferrin polyclonal antibody conjugated with HRP (Jackson) was incubated for 30 min at 37°C in a dilution of 1:500. Finally, o-phenylenediamine (OPD; Sigma, St Louis, MO) substrate was incubated for 15 min. The colour reaction was stopped with 100 μl per well of 2 n H2SO4. OD values were measured at 492 nm. As a reference sample, the supernatant of in vitro activated neutrophils was used at a concentration of 13 μg/ml.
Flow cytometric analysis of surface expression of CD11b and CD66b
Flow cytometric analysis was performed at time of admittance to the intensive care unit. To avoid in vitro activation of granulocytes we used a whole blood method [21]. EDTA anti-coagulated blood was kept on ice until sample preparation. Sample preparation was started always within 5 min after blood sampling. All steps were performed in Hanks' balanced salt solution (HBSS) without calcium and magnesium (Gibco, Life Technologies Ltd, Paisley, UK), supplemented with 1% bovine serum albumin (BSA; Boseral, Organon Teknika, Boxtel, The Netherlands). Cells were fixed with 1% paraformaldehyde in PBS for 10 min on ice, washed, followed by two times erythrocyte lysis with lysis buffer (155 mm NH4Cl, 10 mm KHCO3, 0.1 mm Na2EDTA.H2O) for 5 min at 37°C. The first antibody (anti-CD66b, CLB-B13.9, CLB; or anti-CD11b, 2LPM19c, Dako, Glostrup, Denmark) was incubated for 1 h at 4°C. After washing, the cells were incubated with a goat anti-mouse immunoglobulin polyclonal antibody conjugated with PE (Southern Biotechnology Associates Inc., Birmingham, AL) supplemented with 5% normal goat serum and 5% normal human serum, 1:20 diluted, for 1 h at 4°C in the dark. Subsequently, cells were washed and stored until flow cytometric analysis was performed.
Analysis of surface marker expression was performed on a Coulter Epics ELITE flow cytometer (Coulter, Hialeah, FL), the same day or, in some cases, the next day (always within 18 h). When the cell pellet contained erythrocytes, the intercalating dye, LDS751 (Exiton Chemical, Dayton, OH) was added before flow cytometry measurement. Erythrocytes could successfully be excluded from the leucocyte population in the LDS751/forward scatter dot plot, when combined with a lifegate. Neutrophils and monocytes were identified by forward and sideways scatter. Eosinophils were excluded from the neutrophil population by their high autofluorescence. Data were analysed using Immuno-4 software [22]. Blood samples from the controls were analysed in parallel.
The expression of surface markers was calculated as mean fluorescence intensity (MFI) in combination with the percentage of positive cells (pos. percentage). Data were expressed as a percentage of the expression (MFI) on neutrophils from healthy controls, and corrected for non-specific binding of an irrelevant antibody (of the same isotype) and the conjugate (NSB), and the percentage of positive cells, according to the following formula:
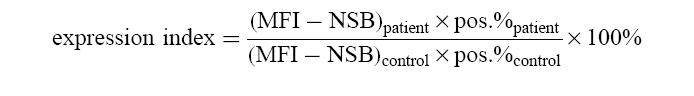 |
In order to assess the variability of the normal population, the age-matched controls were analysed simultaneously. Their individual data were expressed as a percentage of the mean of the healthy control population.
Statistical analysis
Groups were analysed for differences in surface expression by means of the Kruskal–Wallis test. Subsequently, differences between groups were analysed by the Mann–Whitney test. Correlation between parameters was analysed by the Spearman rank correlation test. A two tailed P value < 0.05 was considered to indicate statistical significance. These tests were performed by using GraphPad Instat2 Software.
RESULTS
Patients
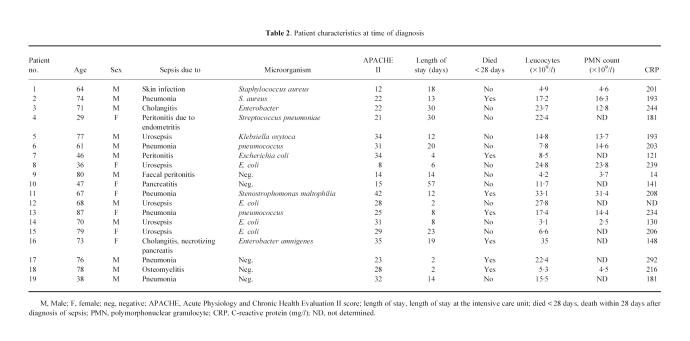
Nineteen consecutive patients with sepsis were included in this study (12 male, seven female, median age 69 years, range 29–87 years). All patients included with the systemic inflammatory response syndrome (SIRS) had a predisposing infection. Seven patients had normal leucocyte counts, whereas 12 patients had leukocytosis (> 11.0 × 109/l). Fourteen patients needed mechanical ventilation. Initially, 10 patients had impairment of renal function (serum creatinin levels > 120 μmol/l). Additionally, during follow up renal failure occurred in another two patients. Ten patients had abnormal liver test (alanine aminotransferase > 40 U/ml and/or alkaline phosphatase > 120 U/ml). Seven patients died within 28 days after the onset of sepsis. Median length of stay in the intensive care unit was 13 days, ranging from 2 to 57 days. Patient characteristics are given in Table 2.
Table 2.
Patient characteristics at time of diagnosis
Flow cytometry
As markers of neutrophil activation, the expression of CD66b and CD11b was used. Both markers are stored in granules of the neutrophil and are expressed at the cell surface upon activation and degranulation [23]. These markers have been shown to be sensitive markers for neutrophil activation [24,25].
Surface expression of both CD11b and CD66b on neutrophils was increased in patients with sepsis compared with the expression of these markers on cells from healthy controls (P = 0.0002 and P < 0.0001, respectively) (Fig. 1).
Fig. 1.
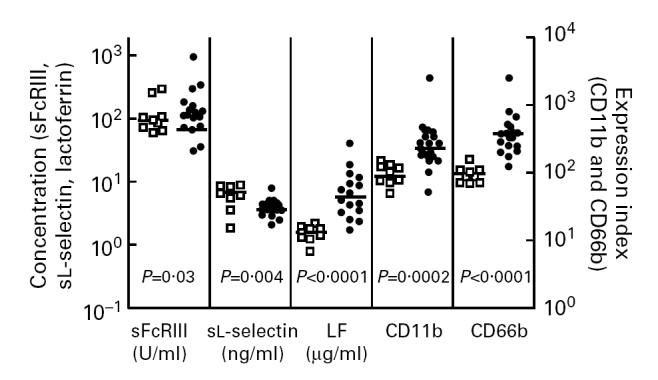
Dot blot of soluble FcγRIII, soluble l-selectin, and lactoferrin (LF) plasma concentrations, and neutrophil surface expression of CD11b and CD66b in patients with sepsis (•) and healthy controls (□). Horizontal bars represent median values.
Soluble l-selectin
l-selectin is an adhesion molecule which is expressed on most leucocytes and is shed upon cell activation [26,27].
Soluble l-selectin concentrations in plasma from patients with sepsis was decreased compared with plasma concentrations of healthy controls (P = 0.004) (Fig. 1).
Plasma lactoferrin
Lactoferrin (LF) is stored in the specific granules of neutrophils and is released upon neutrophil activation and subsequent degranulation. Therefore, LF plasma concentrations are a reflection of the extent of neutrophil degranulation [20].
Plasma concentrations of LF were increased in patients with sepsis compared with plasma concentrations in healthy controls (P < 0.0001) (Fig. 1).
Soluble FcγRIII
Soluble FcγRIII concentrations in plasma from patients with sepsis were increased compared with healthy controls (P = 0.03) (Fig. 1).
Correlations
No correlation could be found between leucocyte or the absolute polymorphonuclear neutrophil (PMN) count on the one hand and APACHE II score (P = 0.80, P = 0.76, respectively) on the other hand, nor between leucocyte or the absolute PMN count and levels of sFcγRIII (P = 0.64, P = 0.14, respectively) (Fig. 2).
Fig. 2.
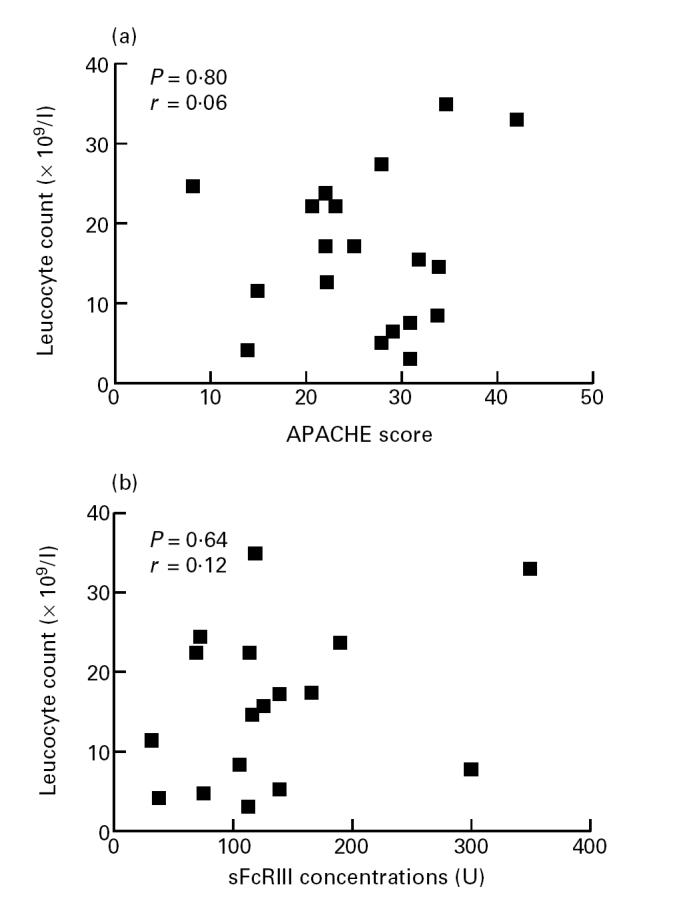
Relation between the numbers of circulating leucocytes (ordinate) and the Acute Physiology and Chronic Health Evaluation II (APACHE II) scores (a) and levels of soluble FcγRIII (b) (abscissa).
Levels of sFcγRIII correlated with the APACHE score (P = 0.02, and r = 0.53, Fig. 3). In addition, plasma levels of sFcγRIII correlated with levels of sl-selectin (P = 0.03, r = 0.66, Fig. 5).
Fig. 3.
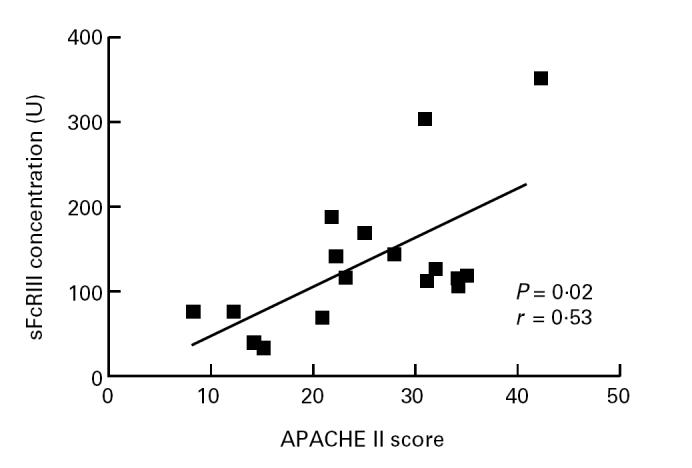
Relation between soluble FcγRIII concentrations (ordinate) and the Acute Physiology and Chronic Health Evaluation II (APACHE II) score (abscissa).
Fig. 5.
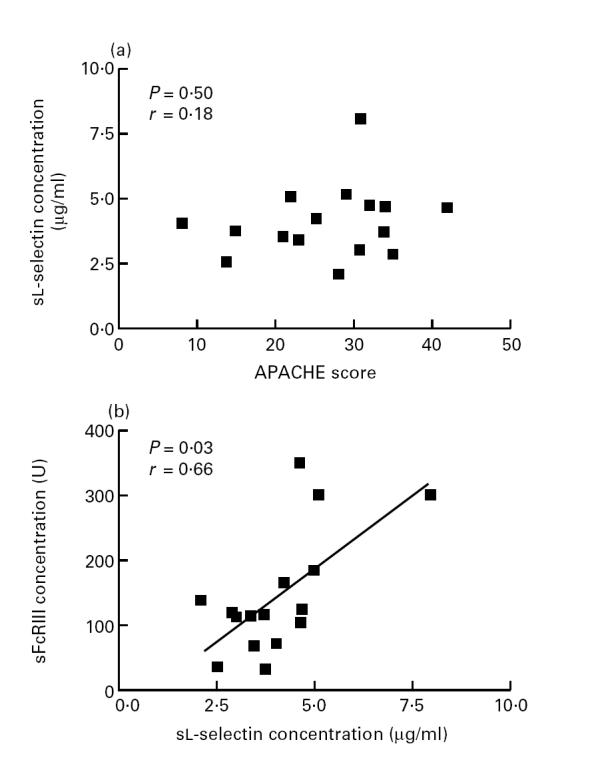
Relation between plasma levels of soluble l-selectin and Acute Physiology and Chronic Health Evaluation II (APACHE II) scores (a) and levels of soluble FcγRIII (b).
No correlation could be found between the expression of membrane markers of cell activation (CD66b or CD11b) on circulating cells and the APACHE II score, nor between these markers and sFcγRIII (Fig. 4). In addition, no correlation could be found between the expression of activation markers and the absolute number of circulating neutrophils or the leucocyte count.
Fig. 4.
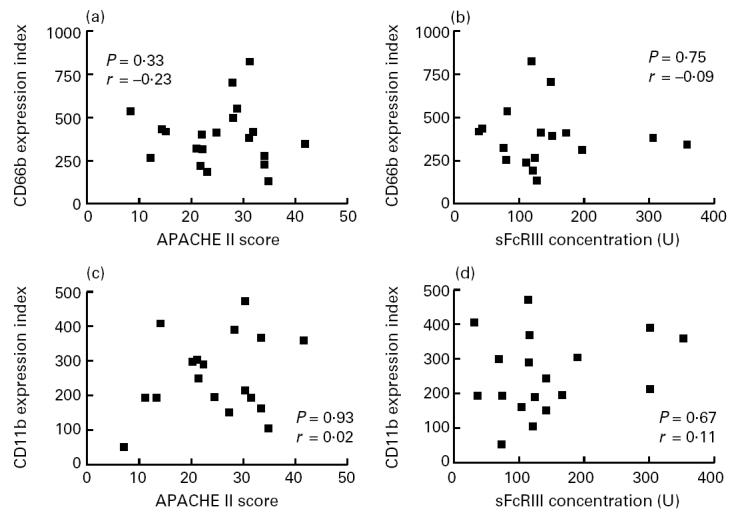
Relation between the expression of CD66b or CD11b on circulating neutrophils (ordinate) and the Acute Physiology and Chronic Health Evaluation II (APACHE II) scores (a and c, respectively) and levels of soluble FcγRIII (b and d, respectively) (abscissa).
Finally, sl-selectin concentrations did not correlate with the APACHE II score (P = 0.5), nor did plasma levels of LF (P = 0.48) (Fig. 5).
DISCUSSION
Massive systemic activation of neutrophils plays an important role in the pathophysiology of sepsis. Activated neutrophils express increased numbers of adhesion molecules that demonstrate a high-affinity binding state for endothelial cells, allowing these cells to adhere to and transmigrate through (activated) endothelium. These adherent and transmigrated cells no longer circulate in the blood and are therefore not measured when the number of circulating neutrophils is determined.
To obtain information about the total number of neutrophils that is involved in the inflammatory response, it may be useful to estimate the total body mass of neutrophils, since the total body mass reflects not only circulating neutrophils but also adherent and transmigrated cells. In the present study, we demonstrate that plasma concentrations of sFcγRIII, a marker of the total body mass of neutrophils, correlate with severity of disease in patients with sepsis.
We measured sFcγRIII with an ELISA in which antibodies were used that do not distinguish between sFcγRIIIa derived from macrophages and NK cells and sFcγRIIIb derived from neutrophils [18]. However, plasma analysis of FcγRIIIB gene-deficient donors has suggested that sFcγRIIIa contributes only marginally to total levels measured in healthy individuals [28]. Therefore, sFcγRIII is mainly derived from neutrophils.
Apoptosis is thought to be the major pathway by which neutrophils die. Neutrophils release FcγRIII upon apoptosis in vitro [14,15]. This, and the finding that a single dose of granulocyte colony-stimulating factor (G-CSF) induces increased neutrophil counts, followed by an increase in plasma sFcγRIII levels with a time lapse of 6 days [29], suggests that sFcγRIII is derived from apoptotic neutrophils. Therefore, if sFcγRIII is derived from apoptotic neutrophils in vivo, the level of sFcγRIII in plasma reflects the turnover of neutrophils. This would imply that severity of disease in sepsis patients is correlated with increased neutrophil turnover.
Soluble FcγRIII concentrations in plasma from patients with sepsis were higher compared with healthy controls. In patients with sepsis, sFcγRIII concentrations ranged, however, from lower normal levels to levels far above those found in healthy controls. This may suggest that in patients with sepsis a good prognosis, corresponding with a low APACHE II score, is associated with a decreased rate of apoptosis, whereas a poor prognosis is associated with an increased rate of apoptosis of neutrophils. Mediators released during infections, such as IL-6 and endotoxin, induce cell activation and at the same time suppress apoptosis, and therefore promote accumulation of neutrophils [30,31]. Indeed, in infections the accumulation of neutrophils is favourable for host defences.
Several studies have demonstrated increased expression of CD11b on circulating neutrophils in patients with sepsis, indicating that neutrophils are intravascularly activated [32–35]. In accordance with Rosenbloom et al. [8], we did not find a correlation between the expression of CD11b on neutrophils and the APACHE II score. Although in their study the Goris score correlated with the APACHE II score, a direct correlation between cell activation and the APACHE II score could not be found. In the present study we also measured the expression of CD66b as a marker for neutrophil activation. CD66b expression is successfully used in other studies to measure neutrophil activation in vivo [24,36]. The expression of CD66b on circulating neutrophils from patients with sepsis was increased compared with healthy controls, but did not, however, correlate with the APACHE II score in these patients. During activation of neutrophils, granule proteins such as LF are released. In line with the observed activation of neutrophils, we observed elevated levels of LF in our sepsis patients.
It has been hypothesized that during neutrophil activation l-selectin is shed in vivo [37]. Therefore, measurement of sl-selectin was included in our study as a possible marker for neutrophil activation. Surprisingly, we did not find elevated levels of sl-selectin in patients with sepsis, which may suggest that l-selectin shedding does not occur in vivo. Previously, Spertini et al. [37] and McGill et al. [38] found elevated sl-selectin levels in patients with sepsis. Since shedding of l-selectin may be induced by the handling of plasma samples, we took great care to avoid this process. Differences in handling of plasma samples possibly explains the observed differences. Soluble l-selectin was first discovered as being a product of activated leucocytes rather than of apoptotic leucocytes [26,27]. Recently, it has been demonstrated that l-selectin shedding also occurs during the process of apoptosis [39,40]. The findings in the present study suggest that in vivo sl-selectin may be generated in the process of neutrophil apoptosis. In line with this hypothesis, we found that levels of sFcγRIII were related to sl-selectin.
In conclusion, we found that sFcγRIII is a good marker of disease severity of sepsis. The total number of circulating neutrophils and/or markers of neutrophil activation on circulating neutrophils were not related to disease severity. These findings suggest that the total turnover of neutrophils, as reflected in the plasma concentrations of soluble FcγRIII, could be a useful marker for determining clinical outcome in this often fatal condition.
REFERENCES
- 1.Sands KE, Bates DW, Lanken PN, et al. Epidemiology of sepsis syndrome in 8 academic medical centers. JAMA. 1997;278:234–40. [PubMed] [Google Scholar]
- 2.Kox WJ, Bone RC, Krausch D, et al. Interferon gamma-1b in the treatment of compensatory anti-inflammatory response syndrome. A new approach: proof of principle. Arch Intern Med. 1997;157:389–93. [PubMed] [Google Scholar]
- 3.Bone RC. Sir Isaac Newton, sepsis, SIRS, and CARS. Crit Care Med. 1996;24:1125–8. doi: 10.1097/00003246-199607000-00010. [DOI] [PubMed] [Google Scholar]
- 4.Nuijens JH, Abbink JJ, Wachtfogel YT, et al. Plasma elastase alpha 1-antitrypsin and lactoferrin in sepsis: evidence for neutrophils as mediators in fatal sepsis. J Lab Clin Med. 1992;119:159–68. [PubMed] [Google Scholar]
- 5.Wakefield CH, Carey PD, Foulds S, Monson JR, Guillou PJ. Polymorphonuclear leukocyte activation. An early marker of the postsurgical sepsis response. Arch Surg. 1993;128:390–5. doi: 10.1001/archsurg.1993.01420160028003. [DOI] [PubMed] [Google Scholar]
- 6.Weiss SJ. Tissue destruction by neutrophils. N Engl J Med. 1989;320:365–76. doi: 10.1056/NEJM198902093200606. [DOI] [PubMed] [Google Scholar]
- 7.Nast Kolb D, Waydhas C, Gippner Steppert C, et al. Indicators of the posttraumatic inflammatory response correlate with organ failure in patients with multiple injuries. J Trauma. 1997;42:446–54. doi: 10.1097/00005373-199703000-00012. [DOI] [PubMed] [Google Scholar]
- 8.Rosenbloom AJ, Pinsky MR, Bryant JL, Shin A, Tran T, Whiteside T. Leukocyte activation in the peripheral blood of patients with cirrhosis of the liver and SIRS. Correlation with serum interleukin-6 levels and organ dysfunction. JAMA. 1995;274:58–65. [PubMed] [Google Scholar]
- 9.Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–14. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 10.Beck WS, Leukocytes I. Physiology. In: Beck, editor. Hematology. 5. Cambridge, MA: The MIT Press; 1991. pp. 339–58. [Google Scholar]
- 11.Huizinga TW, de Haas M, van Oers MH, et al. The plasma concentration of soluble Fc-gamma RIII is related to production of neutrophils. Br J Haematol. 1994;87:459–63. doi: 10.1111/j.1365-2141.1994.tb08298.x. [DOI] [PubMed] [Google Scholar]
- 12.Ravetch JV, Perussia B. Alternative membrane forms of Fc gamma RIII (CD16) on human natural killer cells and neutrophils. Cell type-specific expression of two genes that differ in single nucleotide substitutions. J Exp Med. 1989;170:481–97. doi: 10.1084/jem.170.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huizinga TW, de Haas M, Kleijer M, Nuijens JH, von Roos D, dem Borne AE. Soluble Fc gamma receptor III in human plasma originates from release by neutrophils. J Clin Invest. 1990;86:416–23. doi: 10.1172/JCI114727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dransfield I, Buckle AM, Savill JS, McDowall A, Haslett C, Hogg N. Neutrophil apoptosis is associated with a reduction in CD16 (Fc gamma RIII) expression. J Immunol. 1994;153:1254–63. [PubMed] [Google Scholar]
- 15.Homburg CH, de Haas M, dem Borne AE, Verhoeven AJ, Reutelingsperger CP, Roos D. Human neutrophils lose their surface Fc gamma RIII and acquire Annexin V binding sites during apoptosis in vitro. Blood. 1995;85:532–40. [PubMed] [Google Scholar]
- 16.Ziegler EJ, Fisher CJ Jr, Sprung CL, et al. Treatment of Gram-negative bacteremia and septic shock with HA-1A human monoclonal antibody against endotoxin. A randomized, double-blind, placebo-controlled trial. N Engl J Med. 1991;324:429–36. doi: 10.1056/NEJM199102143240701. [DOI] [PubMed] [Google Scholar]
- 17.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–29. [PubMed] [Google Scholar]
- 18.Koene HR, de Haas M, Kleijer M, von Roos D, dem Borne AE. NA-phenotype-dependent differences in neutrophil Fc gamma RIIIb expression cause differences in plasma levels of soluble Fc gamma RIII. Br J Haematol. 1996;93:235–41. doi: 10.1046/j.1365-2141.1996.4971038.x. [DOI] [PubMed] [Google Scholar]
- 19.Lampeter ER, Kishimoto TK, Rothlein R, et al. Elevated levels of circulating adhesion molecules in IDDM patients and in subjects at risk for IDDM. Diabetes. 1992;41:1668–71. doi: 10.2337/diab.41.12.1668. [DOI] [PubMed] [Google Scholar]
- 20.Martins CA, Fonteles MG, Barrett LJ, Guerrant RL. Correlation of lactoferrin with neutrophilic inflammation in body fluids. Clin Diagn Lab Immunol. 1995;2:763–5. doi: 10.1128/cdli.2.6.763-765.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuijpers TW, Tool AT, van der Schoot CE, et al. Membrane surface antigen expression on neutrophils: a reappraisal of the use of surface markers for neutrophil activation. Blood. 1991;78:1105–11. [PubMed] [Google Scholar]
- 22.Sladek TL, Jacobberger JW. Flow cytometric titration of retroviral expression vectors: comparison of methods for analysis of immunofluorescence histograms derived from cells expressing low antigen levels. Cytometry. 1993;14:23–31. doi: 10.1002/cyto.990140106. [DOI] [PubMed] [Google Scholar]
- 23.Ducker TP, Skubitz KM. Subcellular localization of CD66, CD67, and NCA in human neutrophils. J Leukoc Biol. 1992;52:11–16. doi: 10.1002/jlb.52.1.11. [DOI] [PubMed] [Google Scholar]
- 24.de Haas M, Kerst JM, van der Schoot CE, et al. Granulocyte colony-stimulating factor administration to healthy volunteers: analysis of the immediate activating effects on circulating neutrophils. Blood. 1994;84:3885–94. [PubMed] [Google Scholar]
- 25.Berger M, O'Shea J, Cross AS, et al. Human neutrophils increase expression of C3bi as well as C3b receptors upon activation. J Clin Invest. 1984;74:1566–71. doi: 10.1172/JCI111572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lundahl J, Hed J. Differences in altered expression of L-selectin and Mac-1 in monocytes and neutrophils. Inflammation. 1994;18:67–76. doi: 10.1007/BF01534599. [DOI] [PubMed] [Google Scholar]
- 27.Schleiffenbaum B, Spertini O, Tedder TF. Soluble L-selectin is present in human plasma at high levels and retains functional activity. J Cell Biol. 1992;119:229–38. doi: 10.1083/jcb.119.1.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Haas M, Kleijer M, Minchinton RM, von Roos D, dem Borne AE. Soluble Fc gamma RIIIa is present in plasma and is derived from natural killer cells. J Immunol. 1994;152:900–7. [PubMed] [Google Scholar]
- 29.Kerst JM, de Haas M, van der Schoot CE, et al. Recombinant granulocyte colony-stimulating factor administration to healthy volunteers: induction of immunophenotypically and functionally altered neutrophils via an effect on myeloid progenitor cells. Blood. 1993;82:3265–72. [PubMed] [Google Scholar]
- 30.Biffl WL, Moore EE, Moore FA, Barnett CC. Interleukin-6 delays neutrophil apoptosis via a mechanism involving platelet-activating factor. J Trauma. 1996;40:575–8. doi: 10.1097/00005373-199604000-00009. [DOI] [PubMed] [Google Scholar]
- 31.Colotta F, Re F, Polentarutti N, Sozzani S, Mantovani A. Modulation of granulocyte survival and programmed cell death by cytokines and bacterial products. Blood. 1992;80:2012–20. [PubMed] [Google Scholar]
- 32.Lin RY, Astiz ME, Saxon JC, Saha DC, Rackow EC. Relationships between plasma cytokine concentrations and leukocyte functional antigen expression in patients with sepsis. Crit Care Med. 1994;22:1595–602. [PubMed] [Google Scholar]
- 33.Lin RY, Astiz ME, Saxon JC, Rackow EC. Altered leukocyte immunophenotypes in septic shock. Studies of HLA-DR, CD11b, CD14, and IL-2R expression. Chest. 1993;104:847–53. doi: 10.1378/chest.104.3.847. [DOI] [PubMed] [Google Scholar]
- 34.Tellado JM, Christou NV. Critically ill anergic patients demonstrate polymorphonuclear neutrophil activation in the intravascular compartment with decreased cell delivery to inflammatory focci. J Leukoc Biol. 1991;50:547–53. doi: 10.1002/jlb.50.6.547. [DOI] [PubMed] [Google Scholar]
- 35.Repo H, Jansson SE, Leirisalo Repo M. Flow cytometric determination of CD11b upregulation in vivo. J Immunol Methods. 1993;164:193–202. doi: 10.1016/0022-1759(93)90312-u. [DOI] [PubMed] [Google Scholar]
- 36.Muller Kobold AC, Mesander G, Kallenberg CGM, Cohen Tervaert JW. Circulating leukocytes of anti-neutrophil cytoplasmic antibody (ANCA) associated vasculitis have increased expression of activation marker, but no increased expression of adhesion molecules. Sarcoidosis. 1996;13:259. (Abstr.): [Google Scholar]
- 37.Spertini O, Schleiffenbaum B, White Owen C, Ruiz P Jr, Tedder TF. ELISA for quantitation of L-selectin shed from leukocytes in vivo. J Immunol Methods. 1992;156:115–23. doi: 10.1016/0022-1759(92)90017-n. [DOI] [PubMed] [Google Scholar]
- 38.McGill SN, Ahmed NA, Hu F, Michel RP, Christou NV. Shedding of L-selectin as a mechanism for reduced polymorphonuclear neutrophil exudation in patients with the systemic inflammatory response syndrome. Arch Surg. 1996;131:1141–6. doi: 10.1001/archsurg.1996.01430230023005. [DOI] [PubMed] [Google Scholar]
- 39.Jones J, Morgan BP. Apoptosis is associated with reduced expression of complement regulatory molecules, adhesion molecules and other receptors on polymorphonuclear leucocytes: functional relevance and role in inflammation. Immunology. 1995;86:651–60. [PMC free article] [PubMed] [Google Scholar]
- 40.Dransfield I, Stocks SC, Haslett C. Regulation of cell adhesion molecule expression and function associated with neutrophil apoptosis. Blood. 1995;85:3264–73. [PubMed] [Google Scholar]