Abstract
The immunomodulatory properties of the vitamin D analogue MC 1288 (20-epi-1α,25-dihydroxycholecalciferol) were investigated in CIA in rats. The analogue was administered systemically at three different time points; (i) for 10 consecutive days before collagen (CII) immunization; (ii) for 10 consecutive days after CII immunization; or (iii) for 7 consecutive days from disease onset. Treatment initiated either 10 days before CII immunization or at the day of collagen immunization effectively suppressed the development of arthritis. Treatment initiated at the day of the onset of arthritis reduced the severity of joint inflammation. Significantly, doses which did not induce hypercalcaemia decreased the incidence and severity of arthritis. In vivo treatment with the 20-epi analogue of 1α,25-dihydroxycholecalciferol diminished the serum levels of antibodies to rat CII. Similarly, mitogen-induced proliferation of lymph node cells from rat CII-immunized animals was reduced. The experiments demonstrate that the vitamin D analogue MC 1288 has the ability to prevent, and furthermore to suppress, already established CIA by its immunomodulatory properties without inducing hypercalcaemia.
Keywords: vitamin D3, analogue, experimental, arthritis, treatment, hypercalcaemia immunomodulation
INTRODUCTION
1α,25-dihydroxycholecalciferol (1α,25(OH)2D3), the active form of vitamin D3, has been shown to possess immunomodulatory properties. However, the systemic use of 1α,25(OH)2D3 for treatment of immunological disorders is limited, as it may lead to the development of hypercalcaemia and hypercalcuria [1–3]. For this reason, new vitamin D analogues are being synthesized and investigated with regard to their potency on the immune system. One such analogue is MC 1288 (20-epi-1α,25-dihydroxycholecalciferol), which only differs from 1α,25(OH)2D3 in the altered stereochemistry at carbon 20. MC 1288 has much stronger effects than 1α,25(OH)2D3 on T cell activation. Receptors for 1α,25(OH)2D3 are expressed on monocytes and on activated T and B cells [4,5]. The substance inhibits the T cell production of IL-2 [6,7], interferon-gamma (IFN-γ) [8,9] and granulocyte-macrophage colony-stimulating factor (GM-CSF) [10] in vitro, and antigen- or mitogen-activated T cells incubated with 1α,25(OH)2D3 show a decreased proliferation rate [6].
One of the main goals of the development of new immunomodulatory drugs is their use in the treatment of immunological disorders. The drug MC 1288 has been used in experimental rat transplantation models, such as heart and small bowel transplantation, where it prolongs graft survival [11,12]. In experimental models of autoimmune diseases only few papers have been published, where a suppressive effect of 1α,25(OH)2D3 and its analogues on the development of experimental lupus [13], encephalitis [13,14], thyroiditis [15] and diabetes [16] has been demonstrated. Also in CIA in mice one study has been published in which CIA was suppressed with dietary supplementation of 1α,25(OH)2D3 [30].
The model we have chosen to use is the collagen-induced arthritis in the particularly arthritis-prone DA rat strain, where the rats develop a chronic, remitting peripheral polyarthritis, with many similarities to human rheumatoid arthritis (RA) [17]. CIA development is MHC class II-restricted and is dependent on functional T and B cells [18,19]. In situ hybridization studies have shown a dominance of Th1 versus Th2 cytokines as early as day 7 post-immunization (p.i.) [20]. Treatment with Th2-inducing adjuvants such as alum which reverses the Th1 dominance and induces IL-4 production ameliorates CIA [21].
Since 1α,25(OH)2D3 has profound immunomodulatory effects on B and T as well as antigen-presenting cells (APC), cells which are directly involved in the pathogenesis of CIA, we investigated the in vivo effects of one 20-epi analogue of vitamin D3 on CIA.
MATERIALS AND METHODS
Rats
Dark Agouti (DA) rats kept at the animal departments of Karolinska Hospital, Stockholm, and of the Biomedical Center, Uppsala, were used. All rats used were females and they were used at an age of 8–12 weeks. The animal investigations were performed with the approval of the ethical committee at Stockholm.
Collagen type II preparation
Rat collagen type II (CII) was used in all experiments. It was prepared from the Swarm rat chondrosarcoma. Chondrosarcoma tissue was grown subcutaneously and removed from exsanguinated rats and minced through a sterile steel net. The purification was performed according to Miller [22]. The purity and the intactness of the CII α helices was determined by SDS–gel electrophoresis. No degradation products (mol. wt < 90 kD) could be detected.
Induction of arthritis
Rats were immunized intradermally at the base of the tail with purified native CII dissolved in 0.1 m acetic acid and emulsified in Freund's incomplete adjuvant (FIA; Difco, Detroit, MI) on ice. CII (150 μg) emusified in FIA was injected at a total volume of 200 μl.
MC 1288 treatment
MC 1288 was obtained as a kind gift from Dr L. Binderup (Leo Pharmaceutical, Ballerup, Denmark). The drug was dissolved in propylene glycol, mol. wt 76, 10 g/mol (Riedel-de Haen, Seelze, Germany). Each rat was injected intraperitoneally with 0.05 μg/kg body wt MC 1288 twice daily (approx. 50 μl/rat). In the experiment where the doses of MC 1288 were titrated, group 1 received 0.05 μg/kg body wt MC 1288 twice daily, group 2 0.05 μg/kg body wt MC 1288 once daily, group 3 0.025 μg/kg body wt MC 1288 twice daily, group 4 0.025 μg/kg body wt MC 1288 once daily, and group 5 received 50 μl of the vehicle twice daily. The twice daily dosage was chosen to minimize the hypercalcaemic effect of the drug [23].
Determination of incidence and severity of arthritis
To determine the day of onset of arthritis the rats were checked each day from day 12 to day 20. After this time point rats were checked two to three times every week. Rats were individually scored according to a scheme previously used [24]. In short, 1 point signifies swelling of one group of joints, for example metatarsophalangeal (MTP) or proximal interphalangeal (PIP) joints, 2 points signifies two groups of swollen joints, 3 points signifies three (PIP, MTP and wrist or tarsal joints) groups of swollen joints, and 4 points signifies swelling of the entire paw. The maximum possible score for each animal is 16.
Mitogen-induced lymph node cell stimulation
Rats were immunized with rat CII in FIA as described above. Two groups of rats were treated either with MC 1288 0.05 μg/kg or with propylene glycol twice daily intraperitoneally for 10 consecutive days from the day of immunization. Draining lymph nodes were removed day 10 p.i., i.e. 24 h after the last MC 1288 injection, and single-cell suspensions were prepared from each rat. The cells (2.5 × 106/ml) were incubated in sterile 96-well microtitre plates in Dulbecco's modified Eagle's medium (DMEM) supplemented with 2.5% fetal calf serum (FCS) and polyethylene glycol with or without the addition of 2.5 μg/ml concanavalin A (Con A). The total cell culture time was 48 h. During the last 24 h of incubation 3H-thymidine was added and the incorporation of 3H-thymidine in the cells was measured in a liquid scintillation counter.
Anti-CII antibody determinations
Individual sera were collected from CII/FIA-immunized rats treated with MC 1288 or with the vehicle at day 28 p.i. The sera were stored at −20°C until analysed. ELISA microtitre plates (Dynatech, Plochingen, Germany) were coated with native rat CII at 4°C at a concentration of 10 mg/ml dissolved in 0.1 m acetic acid and incubated overnight at 4°C. Analysis of the IgG1, IgG2a and IgG2b anti-CII antibody levels was performed according to Mattsson et al. [21]. After washing with PBS–Tween the sera were added and serially diluted in 10-fold dilution steps from 1:10 to 1:10 000. As secondary antibodies, biotinylated goat anti-rat isotype-specific IgG1, IgG2a and IgG2b antibodies were used (Serotec, Oxford, UK). After incubation, streptavidin–alkaline phosphatase (Jackson ImmunoResearch Labs, West Groove, PA) was added and finally substrate buffer containing phosphatase substrate (Sigma, St Louis, MO) was added. Optical density (OD) was read at 405 nm. The titres of the anti-CII antibody sera were calculated by comparison with the steep portion of the titration curve of a pooled standard sera from rat CII-immunized DA rats with high anti-CII titres. Statistical analysis was performed on the levels of absorbance at a dilution of 1:1000. Sera from treated and control treated rats were compared.
To determine the change in antibody titre, a pooled serum collected from control treated arthritic rats was used for comparison. Its titration point was determined from the steep portion of the titration curve and was arbitrarily given the value 1.0. The individual sera from the four treated groups were expressed as the percentage of the titre of the control serum.
Monitoring
The animals were weighed before initiation of treatment, on day 10 p.i., and on the final day of the experiment. At these times the rats were also bled for anti-CII antibody and serum calcium measurements. The concentration of calcium was determined by complex formation with cresolpthalein.
Statistical analysis
The significance of differences in arthritic scores was evaluated by the Mann–Whitney rank test, the incidence of arthritis with the Fisher's exact test, and the significance of the difference in antibody titres and mitogen-induced proliferation with Student's t-test.
RESULTS
Effects of treatment with MC 1288 initiated prior to or immediately after CII immunization (prophylactic treatment)
The first experiment was designed to see whether the vitamin D analogue MC 1288 could inhibit the development of CIA. Treatment with 0.05 μg/kg twice daily was begun at the day of collagen immunization and continued for 10 consecutive days. A pronounced effect was seen in the MC 1288-treated group in regard to the incidence of arthritis. The incidence day 28 p.i. was 50% (4/8) in the MC 1288-treated group compared with 100% (8/8) arthritic rats in the control group (Table 1). The mean severity of the joint lesions, as determined by dividing the total arthritic score by the total number of rats in the group at day 28 p.i., was also reduced in the treated group (5.6 versus 12 (P < 0.05)).
Table 1.
Rats were treated intraperitoneally with 0.05 μg/kg 20-epi-1α,5-dihydroxycholecalciferol (MC 1288) twice daily, either before collagen type II (CII) immunization (day −10 to day 0), immediately after CII immunization (day 0 to day 10) or at the time of disease onset (day 15 to day 22).
Another experiment was designed to determine if treatment with MC 1288 before CII immunization would also down-regulate the disease. The rats were treated during 10 consecutive days intraperitoneally starting 10 days before immunization with CII/FIA. The incidence in the MC 1288-treated group was determined. At day 28 p.i. 50% (4/8) had developed arthritis compared with 87.5% (7/8) in the control group (not statistically significant). However, the severity of joint inflammation was significantly reduced at day 28 p.i. The mean arthritic score was 3.25 in the treated group compared with 9.9 in the control group (P < 0.05).
One striking effect observed in these experiments was a loss of weight among the drug-treated rats. The MC 1288-treated rats lost an average of 49 g of their body weight during treatment (from 152 g to 103 g), whereas the control treated rats lost only 2 g, despite the development of a more severe disease with higher arthritis incidence. Body weights were measured day 10 p.i., i.e. 1 day after the termination of treatment. This weight loss coincided with the development of hypercalcaemia in the treated rats day 10 p.i. (3.06 mmol/l in the MC 1288-treated group versus 2.46 mmol/l in the control group).
Effects of treatment with MC 1288 initiated at the time of disease onset (therapeutic treatment)
I.p. treatment with MC 1288 twice daily at the dose 0.05 μg/kg was initiated at the time when DA rats develop arthritis, i.e. 15 days after collagen immunization. This treatment did not affect the incidence of arthritis, since both the treated and the control group developed an incidence of 100% (8/8 versus 8/8 arthritic rats). However, the severity of the arthritic lesions was reduced in the MC 1288-treated rats. The mean arthritic score was 7.9 compared with a mean score of 14.3 (P = 0.01) in the group treated with vehicle only (Table 1) at day 28 p.i., 6 days after the termination of MC 1288 treatment.
Titration of the dose of MC 1288
Because of the negative effects on the weight and well being of the rats caused by high doses (0.05 μg/kg twice daily) of MC 1288, four groups of rats were treated with different doses of MC 1288, i.e. 0.05 μg/kg twice daily, 0.05 μg/kg once daily, 0.025 μg/kg twice daily and 0.025 μg/kg once daily. A fifth group received propylene glycol twice daily. The injections were given p.i. for 10 consecutive days starting at the day of CII immunization.
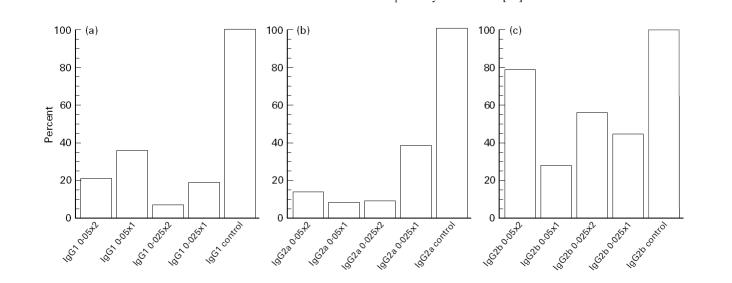
There was a decrease in arthritis susceptibility. In the two groups which received the lowest doses of the drug, i.e. 0.025 μg/kg once or twice daily, the rats had an arthritis incidence of 16.7% (1/6 rats in both groups developed joint swelling). The incidence in the groups treated with 0.05 μg/kg once or twice daily was 50% (3/6) and 63% (4/6), respectively, compared with an incidence of 83% (5/6) in the control treated group at day 28 p.i. (Fig. 1). The difference between the two groups treated with 0.025 μg/kg once or twice daily was statistically significant compared with the control group.
Fig. 1.
Incidence of arthritis. Five groups of rats (six animals in each group) were treated either with different doses of 20-epi-1α,25-dihydroxycholecalciferol (MC 1288) or with the vehicle (propylene glycol) at an approximate dose of 50 μl. Treatment was initiated at the day of collagen type II (CII) immunization and continued until day 9 p.i. Figure 1 (incidence) and Fig. 2 (severity) are based on data from the same experiment.
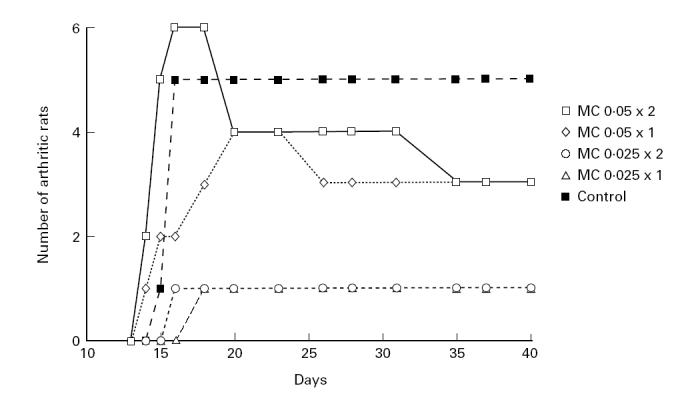
Similarly, the severity of arthritis was suppressed throughout the experiment (Fig. 2). The difference was significant in the group treated with the lowest dose of the analogue compared with the control treated group at day 28 p.i. (P < 0.05).
Fig. 2.
Severity of arthritis as measured by arthritic scores. Five groups of rats (six animals in each group) were treated either with 20-epi-1α,25-dihydroxycholecalciferol (MC 1288) at different doses or with the vehicle (propylene glycol). Treatment was initiated at the day of collagen type II (CII) immunization and continued until day 9 p.i. Figure 1 (incidence) and Fig. 2 (severity) are based on data from the same experiment.
It was significant that the animals which had received the low doses of the vitamin D analogue had a favourable arthritis outcome and an absence of hypercalcaemia (Fig. 3) and weight loss (Table 2).
Fig. 3.
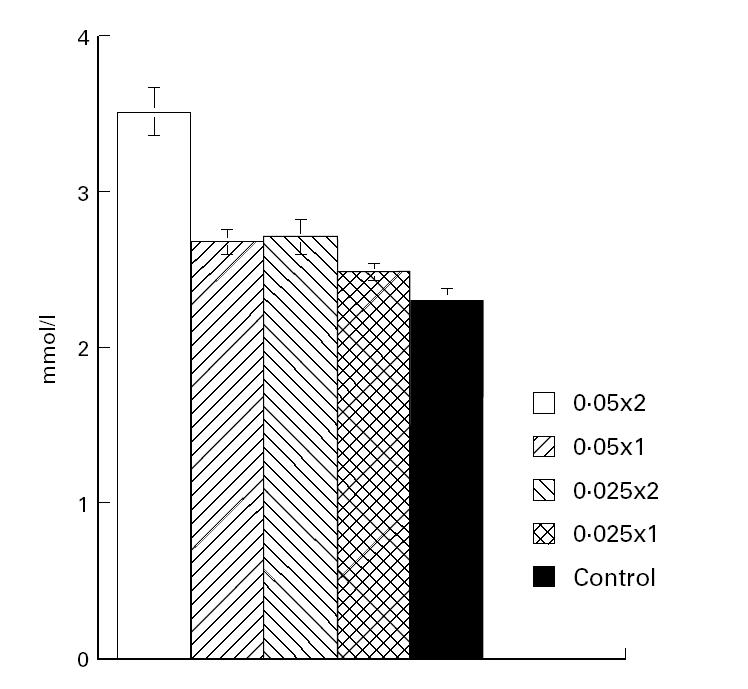
Calcium levels in serum (mmol/l, mean ± s.e.m.) of rats treated with different doses of 20-epi-1α,25-dihydroxycholecalciferol (MC 1288) (see legend to Fig. 1) from the day of collagen type II (CII) immunization until day 9 p.i.
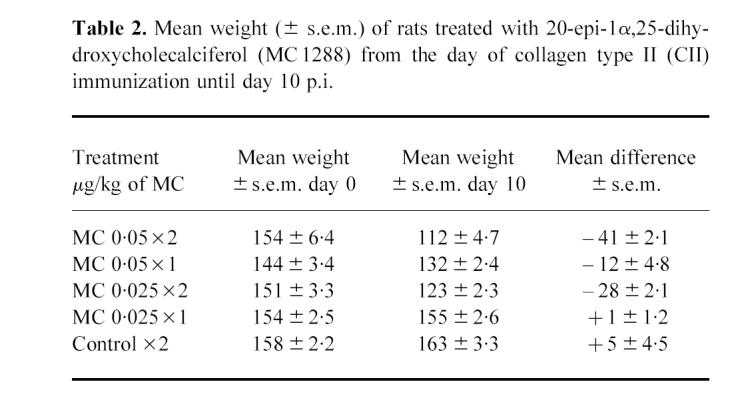
Table 2.
Mean weight (± s.e.m.) of rats treated with 20-epi-1α,25-dihydroxycholecalciferol (MC 1288) from the day of collagen type II (CII) immunization until day 10 p.i.
Treatment with MC 1288 diminishes mitogen-induced proliferation of lymph node cells and down-regulates the antibody response to CII
Con A-induced proliferation was estimated in lymph node cells from MC 1288-treated and control animals (Fig. 4). The mitogen-induced response of lymph node cells from these rats was depressed in the MC 1288-treated rats compared with rats treated with the vehicle (P < 0.05).
Fig. 4.
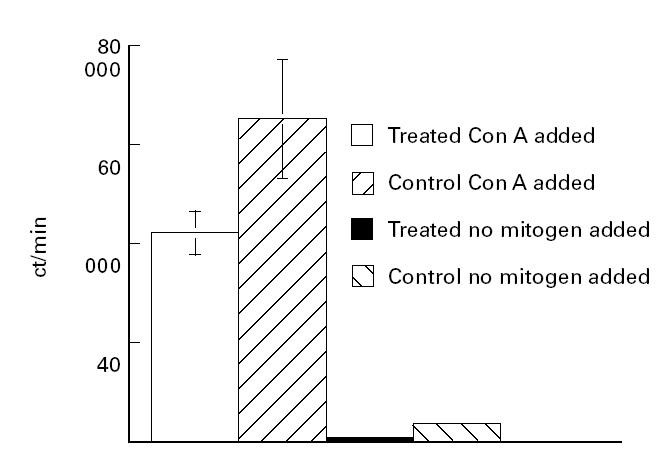
Mitogen-induced lymph node proliferation. Draining lymph node cells were obtained from collagen type II (CII)-immunized and 20-epi-1α,25-dihydroxycholecalciferol (MC 1288) (n = 3) or control (propylene glycol) treated (n = 3) rats. Lymph node cells (2.5 × 106/ml) were incubated with 2.5 μg/ml concanavalin A (Con A) for 48 h, after which 3H-thymidine incorporation was determined.
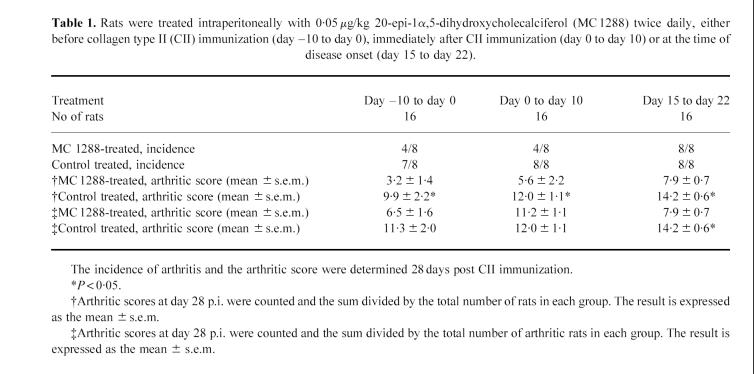
The serum antibody response to rat CII, in rat CII-immunized rats, was dominated by a strong IgG2a response with only little production of IgG1 and IgG2b antibodies. Treatment with MC 1288 significantly diminished the dominant IgG2a response to CII and also depressed the IgG1 response (Fig. 5a,b). The IgG2b response was small but unaffected (Fig. 5c).
Fig. 5.
Effects of 20-epi-1α,25-dihydroxycholecalciferol (MC 1288) treatment on anti-collagen type II (CII) antibody levels in serum at 28 p.i. The CII-reactive antibodies of the G1 (a), G2a (b) or G2b (c) subclasses were compared. A pooled serum collected from control treated arthritic rats was used for comparison and its titration point (see Materials and Methods) was arbitrarily given the value 1.0. The individual sera from the four treated groups were expressed as the percentage (%) of the titre of the control serum.
DISCUSSION
In this study, treatment of CIA with the vitamin D analogue MC 1288 at doses ranging from 0.1 μg/kg to 0.025 μg/kg body wt daily induced pronounced effects on the development of arthritis. Prophylactic treatment affected the incidence as well as the severity of the disease. Treatment given shortly after the onset of symptoms significantly reduced the severity of the arthritis.
The dose 0.1 μg/kg of MC 1288 was chosen because this dose has proved optimal for the prolongation of graft survival after experimental heart transplantation [11]. The major problem in the studies of experimental graft survival was, however, the induction of hypercalcaemia at therapeutic doses [11,23]. In our study the animals treated with this dose of MC 1288 developed a marked weight loss which coincided with the development of hypercalcaemia. However, in contrast to experimental transplantation, the dose of MC 1288 could be reduced to a point where no hypercalcaemia was induced and while reinforcing the clinical anti-arthritic effect of the drug. This finding needs to be emphasized, since it has been very difficult to produce significant effects in experimental models of autoimmunity or transplantation either with vitamin D3 or with vitamin D3 analogues without the development of hypercalcaemia. This finding may have important implications for therapy.
Vitamin D analogues with an altered stereochemistry at carbon 20—MC 1288 is one example of such analogues—have much more potent effects on cell growth regulation than 1α,25(OH)2D3 itself. For example, in inhibiting allogeneic T cell activation MC 1288 is about 300 times more potent than 1α,25(OH)2D3 [25]. The exact reason for this increased potency is not clear, but genomic post-receptor binding effects such as higher stability of the drug–receptor complex rather than increased affinity for the receptor may be involved [25].
Several investigators have shown that 1α,25(OH)2D3 affects the production of various cytokines by activated T cells. In vitro inhibition of IL-2 [6,7], IFN-γ [8,9] and GM-CSF [10] has been observed. In addition, the secretion of IL-12 by macrophages is inhibited [26]. Our data of a decreased responsiveness to the T cell mitogen Con A suggest that the down-regulation of arthritis may be due to an inhibition of T cell-induced proinflammatory pathways participating in the pathogenesis of the disease, and may also correspond to the finding of an increase of IL-4 expression in the lymph nodes day 7 p.i., which is associated with a decreased arthritis susceptibility in certain inbred rat strains [27] or in rats given antigen, i.e. CII, with the Th2 adjuvant alum [21]. MC 1288 treatment may also induce an enhancing effect on the production of IL-4 in CIA (own unpublished results). Furthermore, other researchers have found that in vivo treatment with IL-4 alone or in combination with IL-10 attenuates CIA [28,29].
Our data demonstrating a marked inhibition of CIA are in agreement not only with effects of 1α,25(OH)2D3 on the development of experimental autoimmune encephalomyelitis (EAE) [14], but also with the recently published results of Cantorna et al. on CIA in mice [30]. In this article CIA was suppressed by dietary supplementation with 1α,25(OH)2D3. Furthermore, we have demonstrated an inhibition of arthritis when the treatment was begun before CII immunization, after CII immunization, as well as after the arthritis onset. However, we regard the most important finding to be that low doses of MC 1288, doses which do not induce hypercalcaemia, still effectively suppress arthritis development.
Our results in the rat CIA model indicate that in the future vitamin D analogues with increased immunomodulatory and decreased hypercalcaemic effects may be further considered for therapy of immunological conditions such as human autoimmune arthritis.
Acknowledgments
Financial support was obtained from Swedish Medical Research Council, King Gustaf V 80-years fund and Nanna Swartz foundation. We thank Dr Gunnar Tufveson (Department of Transplantation Surgery, University Hospital, Uppsala, Sweden) for valuable advice and Dr Robert A. Harris for critical comments.
REFERENCES
- 1.Vieth R. The mechanism of vitamin D toxicity. Bone. 1990;11:267–72. doi: 10.1016/0169-6009(90)90023-9. [DOI] [PubMed] [Google Scholar]
- 2.Brown AJ, Dusso A, Slatopolsky E. Selective vitamin D analogs and their therapeutic applications. Sem Nephrol. 1994;14:156–74. [PubMed] [Google Scholar]
- 3.Casteels K, Bouillon R, Waer M, Mathieu C. Immunomodulatory effects of 1,25-dihydroxyvitamin D3. Current Op Nephrol Hyperten. 1995;4:313–8. doi: 10.1097/00041552-199507000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Bhalla AK, Amento EP, Clemens TL, et al. Specific high-affinity receptors for 1,25 dihydroxyvitamin D3 in human peripheral blood mononuclear cells presence in monocytes and induction in T lymphocytes following activation. J Clin Endocrinol Metab. 1983;57:1308–10. doi: 10.1210/jcem-57-6-1308. [DOI] [PubMed] [Google Scholar]
- 5.Provvedini DM, Tsoukas CD, Deftos LJ, Manolagas SC. 1,25-Dihydroxyvitamin D3 receptors in human leukocytes. Science. 1983;221:1181–-3. doi: 10.1126/science.6310748. [DOI] [PubMed] [Google Scholar]
- 6.Rigby WFC, Stacy T, Fanger MW. Inhibition of T lymphocyte mitogenesis by 1,25-Dihydroxyvitamin D3 (calcitriol) J Clin Invest. 1984;74:1451–5. doi: 10.1172/JCI111557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lemire JM, Adams JS, Kermani-Arab V, et al. 1,25-Dihydroxyvitamin D3 suppresses human T helper/inducer lymphocyte activity in vitro. J Immunol. 1985;134:3032–5. [PubMed] [Google Scholar]
- 8.Reichel H, Koeffler HP, Tobler A, Norman AW. 1,25-Dihydroxyvitamin D3 inhibits γ-interferon synthesis by normal human peripheral blood lymphocytes. Proc Natl Acad Sci USA. 1987;84:3385–9. doi: 10.1073/pnas.84.10.3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rigby WFC, Denome S, Fanger MW. Regulation of lymphokine production and human T lymphocyte activation by 1,25-Dihydroxyvitamin D3. Specific inhibition at the level of messenger RNA. J Clin Invest. 1987;79:659–64. doi: 10.1172/JCI113004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tobler A, Gasson J, Reichel H, Norman AW, Koeffler HR. Granulocyte-macrophage colony-stimulating factor. Sensitive and receptor-mediated regulation by 1,25-Dihydroxyvitamin D3 in normal human peripheral blood monocytes. J Clin Invest. 1987;79:1700–5. doi: 10.1172/JCI113009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnsson C, Binderup L, Tufveson G. The effects of combined treatment with the novel vitamin D analogue MC 1288 and cyclosporine A on cardiac allograft survival. Transpl Immunol. 1995;3:245–50. doi: 10.1016/0966-3274(95)80031-x. [DOI] [PubMed] [Google Scholar]
- 12.Johnsson C, Binderup L, Tufveson G. Immunosuppression with the vitamin D analogue MC 1288 in experimental transplantation. Transpl Proc. 1996;28:888–91. [PubMed] [Google Scholar]
- 13.Lemire JM, Archer DC, Beck L, et al. The role and mechanism of vitamin D analogs in immune-suppression. In: Norman AW, Bouillon R, Thomasset M, Vitamin D, editors. A pluripotent steroid hormone: structural studies, molecular endocrinology and clinical application. Berlin: Walter de Gruyter; 1994. pp. 531–9. [Google Scholar]
- 14.Lemire JM, Archer DC. 1,25-Dihydroxyvitamin D3 prevents in vivo induction of murine experimental autoimmune encephalomyelitis. J Clin Invest. 1991;87:1103–7. doi: 10.1172/JCI115072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fournier C, Gepner P, Sadouk M, Charreire J. In vivo beneficial effects of cyclosporin A and 1,25-dihydroxyvitamin D3 on the induction of experimental autoimmune thyroiditis. Clin Immunol Immunopathol. 1990;54:53–63. doi: 10.1016/0090-1229(90)90005-b. [DOI] [PubMed] [Google Scholar]
- 16.Mathieu C, Waer M, Bouillon R. Prevention of type I diabetes in NOD mice by 1,25 dihydroxyvitamin D3 and its analogues. In: Norman AW, Bouillon R, Thomasset M, Vitamin D, editors. A pluripotent steroid hormone: structural studies, molecular endocrinology and clinical application. Berlin: Walter de Gruyter; 1994. p. 540. [Google Scholar]
- 17.Larsson P, Kleinau S, Holmdahl R, Klareskog L. Homologous type II collagen-induced arthritis in rats. Characterization of the disease and demonstration of clinically distinct forms of arthritis in two strains of rats after immunization with the same collagen preparation. Arthritis Rheum. 1990;33:693–701. doi: 10.1002/art.1780330512. [DOI] [PubMed] [Google Scholar]
- 18.Klareskog L, Holmdahl R, Larsson E, Wigzell H. Role of T lymphocytes in collagen II induced arthritis in rats. Clin Exp Med. 1985;51:117–25. [PMC free article] [PubMed] [Google Scholar]
- 19.Ranges GE, Srinam S, Cooper SM. Prevention of type II collagen-induced arthritis by in vivo treatment with anti-L3T4. J Exp Med. 1985;162:1105–10. doi: 10.1084/jem.162.3.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Müssener Å, Klareskog L, Lorentzen JC, Kleinau S. TNF-α dominates cytokine mRNA expression in lymphoid tissues of rats developing collagen- and oil-induced arthritis. Scand J Immunol. 1995;42:128–34. doi: 10.1111/j.1365-3083.1995.tb03635.x. [DOI] [PubMed] [Google Scholar]
- 21.Mattsson L, Lorentzen JC, Svelander L, et al. The adjuvant alum used in conjunction with collagen type II suppresses the development of collagen induced arthritis in rats by skewing the immune response. Scand J Immunol. 1997;46:619–24. doi: 10.1046/j.1365-3083.1997.d01-163.x. [DOI] [PubMed] [Google Scholar]
- 22.Miller EJ. Structural studies on cartilage collagen employing limited cleavage and solubilization with pepsin. Biochemistry. 1972;11:4903–9. doi: 10.1021/bi00776a005. [DOI] [PubMed] [Google Scholar]
- 23.Johnsson C, Binderup L, Tufveson G. Vitamin D analogues for immunosuppression: a comparison between various routes of administration. Transpl Proc. 1995;27:3538–9. [PubMed] [Google Scholar]
- 24.Kleinau S, Erlandsson H, Holmdahl R, Klareskog L. Adjuvant oils induce arthritis in the DA rat. I. Characterization of the disease and evidence of an immunological involvement. J Autoimmun. 1991;4:871–80. doi: 10.1016/0896-8411(91)90050-M. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Binderup L, Latini S, Binderup E, et al. 20-Epi-vitamin D3 analogues: a novel class of potent regulators of cell growth and immune responses. Biochem Pharmacol. 1991;42:1569–75. doi: 10.1016/0006-2952(91)90426-6. [DOI] [PubMed] [Google Scholar]
- 26.Lemire JM. Immunomodulatory actions of 1,25-dihydroxyvitamin D3. J Steroid Biochem Molec Biol. 1995;53:599–602. doi: 10.1016/0960-0760(95)00106-a. [DOI] [PubMed] [Google Scholar]
- 27.Müssener Å, Lorentzen JC, Kleinau S, Klareskog L. Altered Th1/Th2 balance associated with non-major histocompatibility complex genes in collagen-induced arthritis in resistant and non-resistant rat strains. Eur J Immunol. 1997;27:695–9. doi: 10.1002/eji.1830270318. [DOI] [PubMed] [Google Scholar]
- 28.Joosten LA, Lubberts E, Durez P, et al. Role of interleukin-4 and interleukin-10 in murine collagen-induced arthritis. Protective effect of interleukin-4 and interleukin-10 treatment on cartilage destruction. Arthritis Rheum. 1997;40:249–60. doi: 10.1002/art.1780400209. [DOI] [PubMed] [Google Scholar]
- 29.Bessis N, Boissier MC, Ferrara P, et al. Attenuation of collagen-induced arthritis in mice by treatment of vector cells engineered to secrete interleukin-13. Eur J Immunol. 1996;26:2399–403. doi: 10.1002/eji.1830261020. [DOI] [PubMed] [Google Scholar]
- 30.Cantorna MT, Hayes CE, DeLuca HF. 1,25-Dihydroxycholecalciferol inhibits the progression of arthritis in murine models of human arthritis. J Nutr. 1998;128:68–72. doi: 10.1093/jn/128.1.68. [DOI] [PubMed] [Google Scholar]