Abstract
Cytotoxic T cells (CTL) may play an important role in host defence against mycobacterial infections. CD4 CTL are preferentially induced by mycobacteria, but both CD4 and CD8 CTL may be necessary components of a protective immune response. The 65-kD mycobacterium heat shock protein (hsp65) is a poor inducer of CTL in multibacillary leprosy (MB) patients. In this study we evaluate the possible role of cytokines in modulating the cytotoxic activity of CTL from leprosy patients and normal individuals (N) against autologous macrophages presenting Mycobacterium leprae hsp65. Our results show that hsp65-specific CTL were generated from both CD4 and CD8 lymphocytes. In N, individual cytokines as well as the combination of them were able to modify the hsp65-induced cytotoxic activity. The effect of cytokines on leprosy patients' lymphocytes was different in MB and paucibacillary (PB) patients. Thus, IL-6, IL-2, IFN-γ or TNF-α did not modify the generation of hsp65-CTL from either MB (with or without an erythema nodosum episode (ENL)) or PB. In all the patients the simultaneous addition of two cytokines was required in order to increase CTL generation. In MB, IL-6 plus IFN-γ or IL-2 increased both CD4 and CD8 CTL, while TNF-α plus IFN-γ up-regulated only CD4 CTL. In PB, CD8 CTL were prominent with IL-6 plus IFN-γ, while the increase was significant in CD4 CTL with IL-6 plus IL-2. Down-regulation of CTL was observed by addition of IL-4, IL-10, anti-IFN-γ or anti-TNF-α in N controls. Our data demonstrate that IFN-γ and TNF-α must be present for at least the first 60 h of the induction stage in order to generate full hsp65 CTL. Hence, IFN-γ and TNF-α would be key factors in the generation of hsp65 CTL.
Keywords: hsp65-M. leprae, CTL, interferon-gamma, tumour necrosis factor-alpha
INTRODUCTION
Intracellular parasites such as Mycobacterium leprae or M. tuberculosis are pathogenic bacteria that reside and replicate within macrophages. Lepromatous leprosy is characterized by a specific T cell non-responsiveness to M. leprae [1]. Several antigens involved in the immune response towards M. leprae have been identified. Analysis of T cell responses from subjects exposed to M. leprae have indicated that hsp of 10 kD, 18 kD, 65 kD and 71 kD, as well as the antigen 85 complex, are important T cell antigens [2–4]. However, the contribution of these antigen-specific T cell responses to protection or immunopathogenesis of leprosy remains unclear.
Mycobacterium leprae-specific cytotoxic T cell activity has been identified in leprosy patients [5–7]. Cytotoxic T cells may be beneficial for the host by elimination of M. leprae within Schwann cells and macrophages, but at the same time they participate in some of the adverse responses observed in leprosy patients. It is well known that cytotoxic T cell activity may be regulated by cytokines [8,9]. Type 1 and type 2 cytokines usually function in a balanced fashion, but exposure to various pathogens may result in one subset being dominant, as has been suggested in some infectious diseases such as leprosy [10]. In particular, cytokines are able to modulate M. leprae-induced cytotoxicity from T cells bearing both the CD4 and CD8 phenotype [8].
We have previously shown [11] that hsp from M. leprae and M. tuberculosis could induce cytotoxic T cells in paucibacillary (PB) patients and normal individuals (N). Lack of hsp65-induced cytotoxic activity was observed in multibacillary (MB) patients and only those MB patients undergoing an erythema nodosum leprosum (ENL) episode developed hsp65-specific effector cells (hsp-CTL) [11]. In the present study we analyse whether hsp65 induces cytotoxic CD4 and CD8 effector T cells in a differential way across the leprosy spectrum and whether the lack of CTL observed in MB patients may be due to an impaired balance of type 1 and type 2 cytokines. Our results show that hsp65 induces both CD4 and CD8 CTL across the leprosy spectrum, and that IFN-γ and TNF-α play a key role during hsp65 CTL activation.
PATIENTS AND METHODS
Patients
Twenty-five leprosy patients, diagnosed on the basis of clinical and bacteriological criteria and classified according to Ridley & Jopling [12], were studied: 17 lepromatous (LL), two borderline lepromatous (BL), four tuberculoid (TT) and two borderline tuberculoid (BT) patients. They were divided into two groups: PB (TT and BT) (three women, three men; 18–70 years) and MB (LL and BL) (10 women, nine men; 20–68 years) patients. All the patients included in this study were free of other infectious diseases and were receiving multidrug therapy (MDT) according to the recommendations of the World Health Organization. LL patients undergoing an ENL (MB-ENL) were receiving thalidomide. Most of the patients came from or were residing in endemic areas at the moment of the study.
Sixteen bacille Calmette–Guérin (BCG)-vaccinated N (10 women, six men; 30–55 years) were studied simultaneously.
Mononuclear cells
Heparinized blood was collected and peripheral blood mononuclear cells (PBMC) were isolated by Ficoll–Hypaque gradient centrifugation [13]. Cells were collected from the interphase and resuspended in RPMI 1640 tissue culture medium (Gibco Labs, Grand Island, NY) containing gentamycin 85 μg/ml and 15% heat-inactivated fetal calf serum (FCS; Gibco) (complete medium).
Effector cells for cytotoxicity assays
PBMC (2 × 106 cells/ml) were cultured in Falcon 2063 tubes at 37°C in an humidified 5% CO2 atmosphere, in complete medium with or without the 65-kD recombinant protein from M. leprae (hsp65) (batch ML65-6; the endotoxin concentration is 1.65 × 103 U/mg of protein according to Limulus amebocyte lysate assay; kindly provided by Dr M. Singh, GBF, Braunschweig, Germany, through the UNDP/World Bank/WHO) in the presence or absence of (i) IL-6 20 U/ml, (ii) IFN-γ 100 U/ml, (iii) IL-2 50 U/ml, (iv) TNF-α 10 μg/ml, (v) IL-10 10 or 20 ng/ml, (vi) IL-4 10 or 20 μg/ml, or (vii) combinations of IL-6 plus IFN-γ, IL-6 plus IL-2, or TNF-α plus IFN-γ (with or without IL-4 or IL-10). In order to assess the role of IFN-γ and TNF-α in the generation of hsp65-induced cytotoxic effector cells, different concentrations of neutralizing anti-IFN-γ or anti-TNF-α were added to the PBMC cultures. All cytokines employed in this work were purchased from Genzyme (Cambridge, MA) and were recombinant proteins. Anti-human IFN-γ MoAb (mouse class type IgG2a) was purchased from Genzyme and polyclonal rabbit anti-human TNF-α from Sigma (St Louis, MO). (Bioactivity: an antibody concentration of 0.4 μg/ml neutralizes 50% of the cytotoxicity of 1 U of r-human TNF-α.) On day 7, treated and control cells were washed three times with RPMI 1640, resuspended in complete medium (2 × 106 cells/ml) and tested for cytotoxic activity.
Isolation of CD4 and CD8 depleted effector cells
After 7 days of culture, PBMC were depleted of lymphocytes bearing the CD4 or CD8 antigen by treatment of hsp65-induced or control effector cells (stimulated or not with lymphokines) with anti-CD4 or anti-CD8 MoAb-conjugated magnetic beads (Dynal Inc, Oslo, Norway). Briefly, 2–4 × 106 mononuclear cells, resuspended in 100 μl of PBS containing 2% FCS, were mixed with 75 μl of anti-CD4 or 40 μl of anti-CD8 conjugated beads (anti-CD4 and anti-CD8 Dynabeads were supplied as a suspension of 1.4 × 108 beads/ml) and incubated for 45 min at 2–4°C. Then, PBS–FCS was added to a final volume of 5 ml and the cell suspensions were placed in a magnetic particle concentrator for 3 min to collect the rosetted cells and the unbound beads. The CD4-depleted (CD8) and CD8-depleted cells (CD4) were collected, washed three times, then resuspended with complete medium and tested for their cytotoxic activity. Purity of the CD4 or CD8 populations was verified by flow cytometry analysis. FITC- or PE-conjugated MoAbs, specific for CD3, CD4, CD8, CD16 and CD56 (Becton Dickinson, San Jose, CA) were employed.
Target cells
Monocytes were allowed to adhere to the bottom of 24-well flat-bottomed Falcon plates by incubation of PBMC (5 × 106/ml) for 2 h at 37°C. After removing non-adherent cells, cells remaining in the plates (10% of the original cell suspension) were incubated at 37°C in a humidified 5% CO2 atmosphere for 7 days. For the cytotoxic assays, on day 6 of incubation the cells were pulsed with hsp65 (10 μg/ml). Macrophages kept under the same conditions but without addition of antigen were used as controls. Plates were cooled for 2 h at 4°C to facilitate the detachment of adherent cells by vigorous pipetting using ice-cold medium. These cells were washed and pellets of 5–7 × 105 cells were labelled with 100 μCi of Na251CrO4 (NEN, Boston, MA) by incubation for 1 h at 37°C. Then the cells were washed three times and resuspended in complete medium at 1 × 105 cells/ml.
Cytotoxicity assay
Target cells (1 × 104) were seeded into each well of 96-well microtitre plates (Falcon, Becton Dickinson, Lincoln Park, NJ). Effector cells were added in triplicate at an effector:target (E:T) cell ratio of 40:1 in 0.2 ml final volume. The plates were centrifuged at 50 g for 5 min and incubated at 37°C in 5% CO2 for 4 h. After centrifugation at 500 g for 5 min, 100 μl of supernatants were removed from each well. The radioactivity of supernatants and pellets was measured in a gamma counter. Results were expressed as percentage of cytotoxicity:
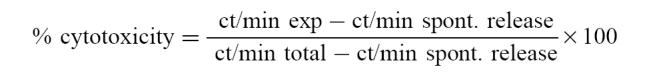 |
Spontaneous release is the radioactivity released from target cells incubated with complete medium alone. It ranged from 8% to 20%.
In all cases the cytotoxicity assays performed with PBMC cultured in the absence of hsp65 or with macrophages not pulsed with antigen (data not shown) rendered a negligible cytotoxicity (0–6%), even if cytokines were included in the cultures (2–7%). Mouse immunoglobulin IgG2a (Becton Dickinson) was used as isotype control for anti-IFN-γ in the cytotoxicity assay, neither inhibition nor enhancement was observed of hsp65 cytotoxic values. The cytotoxicity against non-hsp65-pulsed macrophages was subtracted from the experimental values determined using hsp65-pulsed targets.
PBMC incubated during 7 days with lipopolysaccharide (LPS; Escherichia coli 011:B4; Sigma), at the same concentration as endotoxin is present contaminating the hsp65 preparation, rendered negligible (1–2%) cytotoxicity against hsp65-pulsed macrophages. For blocking experiments, 5 μl of pure anti-class I (Immunotech, Marseille, France) or anti-class II (anti-DR; Becton Dickinson) MoAbs were added to the effector cells, or anti-CD56 (Caltag, San Francisco, CA) added to target cells. Reactions with isotype controls were also performed, and these antibodies did not modify the cytotoxic activity. Thereafter effector cells were added at an E:T cell ratio 40:1. Cells from PB patients could not be tested in this kind of experiment.
Natural killer activity
PBMC, fresh or cultured for 7 days, obtained from MB patients and N controls were employed as effector cells in order to test their natural killer (NK) cytotoxic activity. 51Cr-labelled K562 target cells (1 × 105 cells/ml) were mixed with the effector cells (1 × 106 cells/ml) in a total volume of 0.20 ml into each well of 96-well microtitre plates (Falcon) at an effector cell ratio of 40:1. Plates were centrifuged (200 g) for 1 min and incubated at 37°C for 4 h in a CO2 incubator. Then they were centrifuged at 500 g for 5 min, 100 μl of supernatant were removed and counted for radioactivity. Results were expressed as percentage of cytotoxicity:
 |
Spontaneous release from K562 target cells did not exceed 10%.
Statistical analysis
Comparisons of MB and PB patients and normal controls were performed using Student's t-test. Cytotoxicity values obtained from the different subsets of effector cells of each individual were compared using the Wilcoxon matched pairs signed rank test.
RESULTS
Hsp65 CTL raised from CD4 and CD8 T cells
In order to assess whether hsp65 could induce CD4 or CD8 CTL, hsp65-induced effector cells were depleted of T cells bearing the CD8 or CD4 phenotype before performing the cytotoxic assay. After culturing PBMC for 7 days with hsp65, 3–10% of the cells were CD3−CD56+CD16+. The purity of the CD4 population was: CD4 cells 80–94% CD4+ and 3–7% CD8+; CD8 cells 77–85% CD8+ and 5–9% CD4+. To analyse the contribution of CD4 and CD8 to the cytotoxic activity (CD4 CTL and CD8 CTL), values < 10% of cytotoxicity were considered as negative. This cut-off value results from subtracting 2 s.d. to the lowest level of N cytotoxicity. As shown in Table 1, only CD4 CTL were generated in two (nos 2 and 9) of the five MB patients that responded to hsp65 by giving rise to CTL, while in the remaining three patients CD4 and CD8 CTL were equally generated in patients 7 and 12, and one patient (no. 8) generated only CD8 CTL. In MB-ENL, hsp65 made no difference between CD4 or CD8 cytotoxic responses. PB patients developed a higher CD8 than CD4 CTL response, while in contrast, N controls presented higher CD4 than CD8 CTL activity. The development of CD4 and CD8 CTL by hsp65 was confirmed by adding anti-class I, anti-class II or anti-CD56 during the effector phase of the cytotoxicity assay (data not shown). In three N controls, anti-class I, anti-class II and anti-CD3 were added together. As a result, no cytotoxic activity could be detected in either case (data not shown)
Table 1.
Hsp65-induced cytotoxicity from CD4 and CD8 T cell subsets
Effect of IL-6, IFN-γ, IL-2 and TNF-α on hsp65-induced cytotoxic activity
To analyse the ability of cytokines to modulate the generation of hsp65-induced cytotoxicity, PBMC were stimulated with hsp65 in the presence of either IL-6, IL-2, IFN-γ or TNF-α.
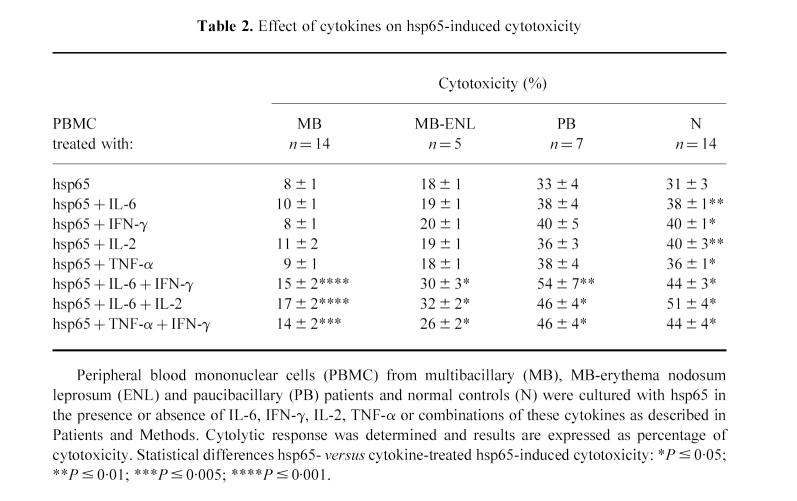
As shown in Table 2, none of these cytokines was able to increase the hsp65-induced cytotoxic activity in MB and MB-ENL patients. In PB patients, IL-6 and IFN-γ induced only a slight increase, whilst in N controls this cytotoxic activity was significantly enhanced by all four cytokines. On the other hand, the three combinations of cytokines tested significantly enhanced the CTL response from leprosy patients and N (Table 2).
Table 2.
Effect of cytokines on hsp65-induced cytotoxicity
We therefore examined whether this modulation was exerted on all the effector cells or on either the CD4 or CD8 T cells. As shown in Table 3, IL-6 and IFN-γ added together significantly enhanced the lytic activity of both CD4 and CD8 effector cells in the four groups studied. The combination of IL-6 and IL-2 was also able to enhance both CD4 and CD8 cytotoxic activity in MB, MB-ENL and N, while this positive effect was only exerted on CD4 cells in PB patients. CD4-mediated cytotoxicity was increased more than CD8-mediated activity in N by the addition of IL-6 plus IFN-γ or IL-2 (P < 0.05), while in PB patients a higher increase in CD8 cytolytic activity was observed when IL-6 + IFN-γ were simultaneously employed (P < 0.05).
Table 3.
Effect of cytokines on CD4 and CD8 hsp65-induced effector cells
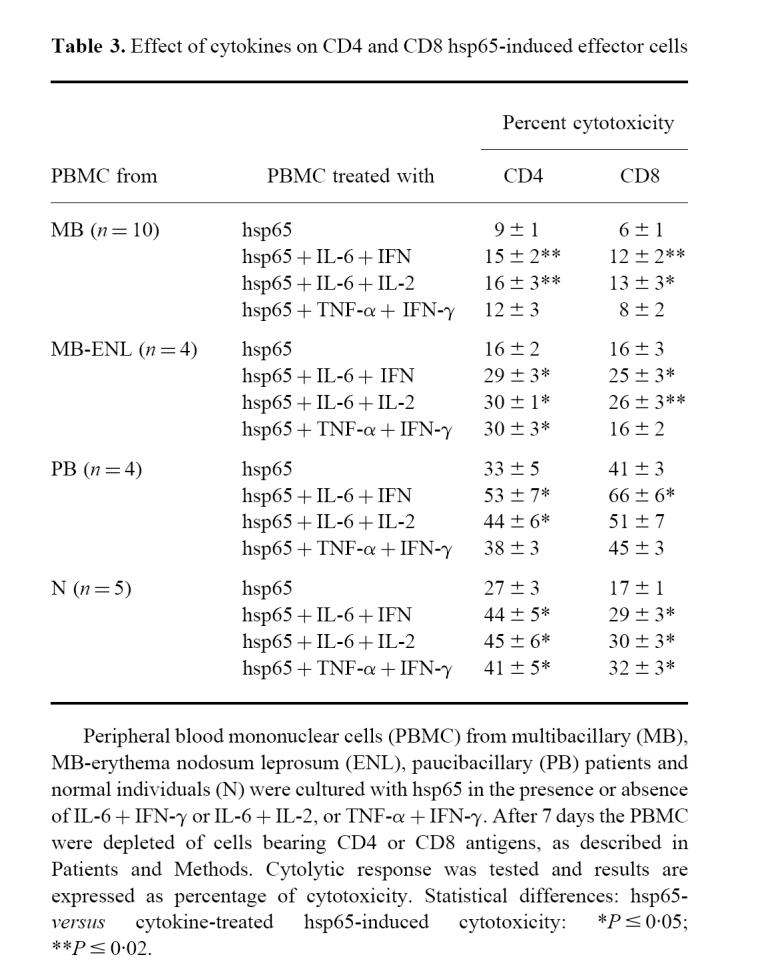
The addition of TNF-α together with IFN-γ increased CD4 CTL effector cells in MB-ENL (MB-ENL: CD4 versus CD8 = P < 0.05), and the same trend was observed in MB. Only in PB patients was the cytolytic activity not modified. An enhancement of both CD4 and CD8 CTL was observed in N controls by this combination of cytokines.
NK activity induced by hsp65 and cytokines
In order to determine whether the differences observed in hsp65-induced cytotoxicity between MB patients and N controls could be due to NK activity, assays were performed using the classical NK target, K562. As shown in Table 4, MB patients developed lower NK cytotoxicity than N controls when fresh PBMC were employed. After 7 days of culture, control cells diminished their NK activity, but it could be enhanced by addition of cytokines. In the presence of hsp65 and cytokines a higher NK activity was observed with respect to non-stimulated cells in MB as well as N controls, but no significant differences were observed between both groups.
Table 4.
Natural killer (NK) cytotoxic activity from fresh and 7 days cultured peripheral blood mononuclear cells (PBMC)
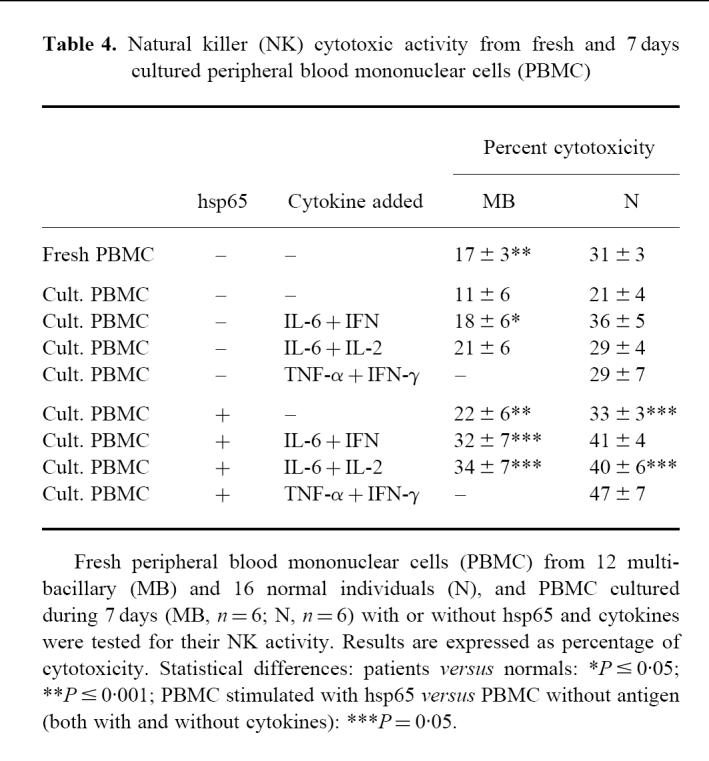
IL-4 and IL-10 down-regulate hsp65-induced cytotoxicity
The ability of IL-4 and IL-10 to exert an inhibitory effect on T cell-mediated responses [14,15] and the preferential expression of IL-4 and IL-10 mRNA in lesions of LL patients [10] have been well documented. In order to determine whether these two cytokines also inhibit the generation of hsp65-induced CTL activity, IL-4 or IL-10 were added during stimulation of PBMC with hsp65. Studies were carried out in normal controls due to the low cytotoxic activity against hsp65 observed in MB patients. Both IL-4 (20 μg/ml) and IL-10 (20 ng/ml) abrogated hsp65 CTL activity (Fig. 1). However, when IL-6 plus IL-2 or IFN-γ, or TNF-α plus IFN-γ were added together with IL-4 or IL-10 to PBMC cultures, the inhibitory effect of IL-4 or IL-10 was partially overcome.
Fig. 1.
Peripheral blood mononuclear cells (PBMC) from five normal controls (N) were cultured with hsp65 or hsp65 with IL-6 plus IFN-γ, IL-6 plus IL-2 or TNF-α plus IFN-γ in the presence or absence of IL-4 or IL-10 during 7 days. Then cultured mononuclear cells were tested for their cytotoxic activity and results are expressed as percentage of cytotoxicity.
On the other hand, the addition of IL-10 at 10 ng/ml to the PBMC cultures of MB and MB-ENL patients was enough to abrogate CTL activity (cytotoxicity (%), MB, n = 3: hsp65 CTL = 9 ± 1, (hsp65 + IL-10) CTL = 3 ± 1; MB-ENL, n = 2: hsp65 CTL = 17–14, (hsp65 + IL-10) CTL = 2–0), while in N this concentration of IL-10 only produced a partial inhibition of the cytotoxicity (hsp65 CTL = 35 ± 3, (hsp65 + IL-10) CTL = 16 ± 5).
IFN-γ and TNF-α are necessary to develop hsp65-induced CTL activity
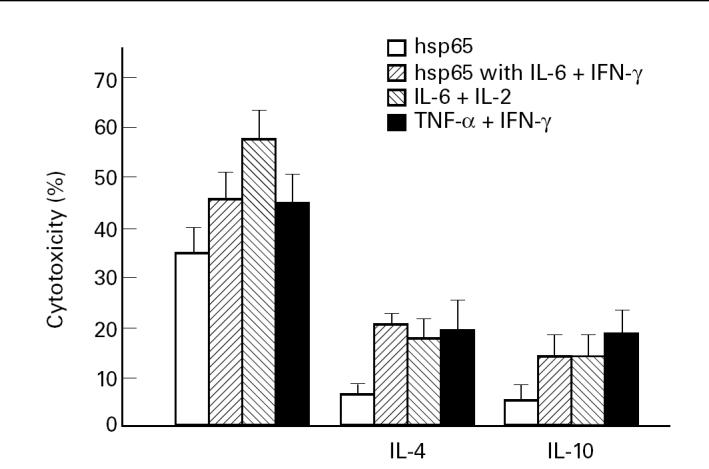
Taking into account the key role of IFN-γ and TNF-α in protection against mycobacterial infections, we analysed the effect of anti-IFN-γ and anti-TNF-α on the generation of hsp65-induced effector cells. PBMC from normal controls were used for this set of experiments.
As shown in Fig. 2, the neutralization of IFN-γ or TNF-α produced a marked inhibition of the hsp65-induced cytotoxicity. Moreover, the CTL inhibition could not be reversed by addition of exogenous cytokines when anti-IFN-γ or anti-TNF-α were present at the beginning of the antigen stimulation.
Fig. 2.
Peripheral blood mononuclear cells (PBMC) from five normal controls (N) were cultured with hsp65 or hsp65 with IL-6 plus IFN-γ, IL-6 plus IL-2 or TNF-α plus IFN-γ in the presence or absence of anti-IFN-γ (0.4 μg/ml) or anti-TNF-α (30 ng/ml) antibodies during 7 days. Then cultured mononuclear cells were tested for their cytotoxic activity and results are expressed as percentage of cytotoxicity.
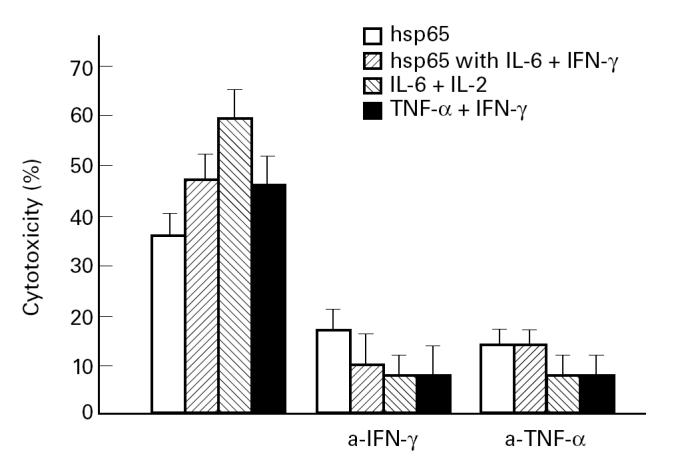
In order to analyse whether the presence of endogenous production of TNF-α or IFN-γ was required during the 7 days of culture, the antibodies were added at different time points of the induction stage and remained in the culture medium until the end of CTL generation. As shown in Fig. 3, either TNF-α or IFN-γ were necessary during the first 48 h of CTL induction, but for complete activation IFN-γ and TNF-α had to be present for at least 60 h. The inhibitory effect observed with anti-TNF-α or anti-IFN-γ was dose-dependent (data not shown). Moreover, an abrogation of hsp65-induced cytotoxicity was observed upon simultaneous addition of both neutralizing antibodies during the first 24 h. A partial inhibitory effect was observed up to 60 h after the beginning of the culture. These results suggest that both TNF-α and IFN-γ play a key role in the generation of hsp65 CTL.
Fig. 3.
Peripheral blood mononuclear cells (PBMC) from six normal controls (N) were cultured during 7 days with hsp65 (□), hsp65 and anti-IFN-γ (0.2 μg/ml) (▴), anti-TNF-α (15 /) (•) or both antibodies (○). These antibodies were added at different time points. Then mononuclear cells were tested for their cytotoxic activity and results are expressed as percentage of cytotoxicity.
DISCUSSION
Several studies have suggested that CTL could play an important role in the immune response to mycobacterial infections such as leprosy and tuberculosis (TB) [5,7,16]. In a previous work we have demonstrated that M. leprae hsp65 could induce cytotoxic effector T cells from PBMC of PB patients and normal controls; in MB patients a marked deficiency in developing hsp65-specific CTL activity was found [10]. The 65-kD protein is one of the most extensively studied mycobacterial hsp antigens [17,18]. T cells as well as T cell clones reactive against mycobacterial hsp65 have been identified [19]. The human immune response to this antigen appears to be directed mostly against cross-reactive antigenic epitopes, since they are widely distributed among many mycobacterial species [20].
Although mycobacteria induce preferentially CD4 CTL [16,21], we [8] as well as others [5] have demonstrated that both CD4 and CD8 cytotoxic effector cells can be generated from PBMC. An important role has been recently suggested for class I-restricted CD8 T cells in TB [22]. However, how these cells contribute to the control of mycobacterial infection has not yet been elucidated. Our study demonstrated that hsp65 induced a higher CD8 cytolytic activity in PB patients and more CD4 CTL in N controls. This is in agreement with our previous data, when whole M. leprae were employed as stimulus [8]. On the other hand, the behaviour of MB and MB-ENL was different to that observed in our previous report [8], since hsp65 did not induce preferentially CD4 CTL in MB-ENL, and neither CD4 nor CD8 CTL were generated in most MB patients. The generation of both CD4 and CD8 cytotoxic responses by hsp65, in the presence or absence of cytokines, was confirmed by blocking class I or class II antigens during the effector phase (data not shown). Several recent reports question the classical view that cytoplasmic antigens require necessarily a class I presentation pathway, and soluble antigens a class II one [23]. Culture filtrate antigens as well as soluble polypeptides chaperoned by hsp can also be efficiently presented in an MHC class I context [23,24]. It has been demonstrated that a subset of antigen-presenting cells (APC) can also present exogenous antigens or proteins transferred from phagosomes to the cytoplasm on class I molecules. This presentation pathway may be important in the initiation of a CTL response against pathogens such as intracellular bacteria [25].
It has been suggested that cell-mediated immunity to mycobacteria is closely associated with the induction of a Th1 response. mRNA coding for IL-2, IFN-γ, IL-1β, TNF-α, granulocyte-macrophage colony-stimulating factor (GM-CSF) and IL-6 were found to be more abundant in lesions of TT patients. In contrast, IL-4 and IL-10 mRNAs appeared more prominent in LL [15]; during ENL reactions, a selective increase in the expression of IL-6, IL-8 and IL-10 was found [26]. On the other hand, those studies carried out in PBMC did not show this clear cut-off in the pattern of cytokines: Th0, Th1 or Th2 patterns were detected irrespective of the antigen used and the clinical type of leprosy [27–29].
Both CD4 and CD8 hsp65 CTL could be modulated only by a combination of cytokines (Table 2). It is of note that in the presence of TNF-α plus IFN-γ a strong CTL CD4 response was generated in MB-ENL (Table 3), and IL-6 plus IFN-γ made CD8 CTL more prominent in PB patients. In our study, no antigen-independent CTL activity was observed in PBMC incubated with cytokines. Although NK activity was generated in PBMC during culture in the presence of hsp65 by addition of cytokines (Table 4), the differences in the hsp65 cytotoxic response cannot be attributed to NK activity, since in both MB and N controls the levels of lytic activity observed in a classical NK cytotoxic assay after 7 days of culture were not significantly different (except in the case of IL-6 + IFN-γ), while remarkable differences were observed when hsp65-pulsed autologous macrophages were the target cells of CTL (Table 2). Although NK cells are important effectors of cytotoxic activity as a first line of defence, they must also be taken into account for their role in immune regulation by virtue of their cytokine production [30,31], and that may be the role they play in our experimental model.
The suppressive function of IL-4 and IL-10 on T cell-mediated responses is well known [14], and the opposing effect of IFN-γ [32] has been demonstrated employing M. leprae [33–35]. We observed an inhibition of cytolytic activity when normal PBMC were stimulated with hsp65 in the presence of high concentrations of type 2 cytokines (Fig. 1). Moreover, the inhibitory effect of IL-4 or IL-10 was partially counteracted by addition of type 1 cytokines. One could speculate that high concentrations of type 2 cytokines, produced during antigen stimulation, could down-regulate not only hsp65-induced cytotoxicity but also the enhancing effect of type 1 cytokines, as observed in MB patients. On the other hand, the higher hsp65 cytotoxicity observed in MB-ENL could be due to a shift in the pattern of cytokines observed during ENL episodes, such as increased levels of TNF-α or IL-6 [26,27].
IFN-γ and TNF-α play an important role in the development of the protective response against M. leprae and M. tuberculosis [36–38]. IFN-γ enhances the microbicidal activity through the release of TNF-α, IL-1 and IL-6 [39], and it also modulates IL-12 and IL-10 secretion [35,40]. On the other hand, TNF-α enhances HLA expression and induction of IL-6 production [41], and appears to be involved in the formation and maintenance of granulomas. TNF-α together with IFN-γ can act as an autocrine signal to enhance the microbicidal activity of macrophages [42]. However, the role of this monokine in leprosy is unclear; high TNF-α titres have been detected in lepromatous leprosy as well as during ENL episodes [6,43].
One striking finding in our study is that IFN-γ and TNF-α are necessary during the early stages of CTL induction, and that their presence would be mandatory for complete activation of CTL from PBMC. When IFN-γ or TNF-α were neutralized at the beginning of CTL generation, exogenously added cytokines did not modify the inhibitory effect of cytolytic activity (Fig. 2). The fact that an almost total inhibition of CTL was obtained by the neutralization of only one of the cytokines, either IFN-γ or TNF-α, or both, at the beginning of CTL generation from PBMC (Fig. 3) clearly demonstrated that both TNF-α and IFN-γ act co-ordinately and not in an individual way. These results would exclude the involvement of other cytokines at this early stage in order to develop a full hsp65 cytotoxicity. NK and/or γδ cells could be the source of TNF-α and IFN-γ at this stage. The lack of these two cytokines could not be compensated for by others that perhaps would act at later stages of activation [44]. It is well known that MB patients have low in vitro production of IFN-γ, and on the other hand an elevated in vivo production of TNF-α has been demonstrated in MB and MB-ENL [6,43]. One would expect from the data obtained with N controls a higher cytotoxic activity from MB patients' cells than the one observed. On the other hand, IL-4 could abrogate hsp65-induced cytotoxicity in PBMC derived from N (Fig. 1), so in MB patients the presence of IL-4 during antigen stimulation could down-regulate the production and/or the effect of TNF-α or IFN-γ, hence encumbering the development of an appropriate CTL response, which might result in an increase of the bacterial load.
Our observation concerning the role of TNF-α in the generation of specific CTL would be one of the first descriptions with M. leprae antigens. Hence, the requirement for IFN-γ and TNF-α could be a general feature in response to mycobacterial infections, as has already been proven for M. tuberculosis and M. bovis BCG [37,45], suggesting an interaction between T cells and monocytes-macrophages rather than T cell subsets. An altered balance in the production of cytokines would result in failure of monocyte activation, including the secretion of monokines, antigen presentation and lymphocyte activation. The regulatory effect of IFN-γ modulating the production or activity of other cytokines such as IL-12 or IL-10 as seen in leprosy [35] may lead to either the perpetuation of the disease, as in the case of MB, or enhanced cell-mediated immunity, as observed in PB patients. Therefore, monocyte activation together with lysis of host cells harbouring M. leprae would be beneficial, but at the same time it may lead to nerve damage, as seen in PB patients and type 1 lepra reactions. Further studies are necessary to outline the mechanisms of activation of hsp65 CTL in healthy individuals in order to understand the pathways of activation in leprosy patients.
Acknowledgments
This work was supported by grants from the UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases (TDR), from CONICET and Fundación ‘Alberto J. Roemmers’. We gratefully acknowledge Dr Javier Anaya for histopathological classification and Dr María Marta de E. de Bracco and Dr Martin Isturiz for helpful discussion and revision of the manuscript.
References
- 1.Bloom BR, Mehra V. Immunological unresponsiveness in leprosy. Immunol Rev. 1984;80:5–28. doi: 10.1111/j.1600-065x.1984.tb00493.x. [DOI] [PubMed] [Google Scholar]
- 2.Mehra V, Bloom BR, Bajardi AC, et al. A major T cell antigen of Mycobacterium leprae is a 10-kD heat-shock protein. J Exp Med. 1992;175:275–84. doi: 10.1084/jem.175.1.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dockrell HM, Stoker NG, Lee SP. T cell recognition of the 18 kDa protein of M. leprae. Infect Immun. 1989;57:1879–983. doi: 10.1128/iai.57.7.1979-1983.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ottenhoff THM, Converse PJ, Gebre N, et al. T cell responses to fractioned Mycobacterium leprae antigens in leprosy. The lepromatous unresponder defect can be overcome in vitro by stimulation with fractionated M. leprae components. Eur J Immunol. 1988;19:707–13. doi: 10.1002/eji.1830190421. [DOI] [PubMed] [Google Scholar]
- 5.Kale Ab B, Kiessling R, Van Embden JDA, et al. Induction of antigen-specific CD4+ HLA-DR restricted T lymphocytes as well as non-specific non-restricted killer cells by the recombinant mycobacterial 65 kD hsp. Eur J Immunol. 1990;20:369–77. doi: 10.1002/eji.1830200221. [DOI] [PubMed] [Google Scholar]
- 6.Parida S, Grau GE, Zaher SA, Mukherjee R. Serum tumour necrosis factor and interleukin 1 in leprosy and during lepra reactions. Clin Immunol Immunopathol. 1992;63:23–27. doi: 10.1016/0090-1229(92)90088-6. [DOI] [PubMed] [Google Scholar]
- 7.Sasiain M del C, de la Barrera S, Minnucci F, et al. T cell-mediated cytotoxicity against Mycobacterium antigen-pulsed autologous macrophages in leprosy patients. Infect Immun. 1992;60:3389–95. doi: 10.1128/iai.60.8.3389-3395.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de la Barrera S, Finiasz M, Fink S, et al. Differential development of CD4 and CD8 cytotoxic T cells (CTL) in PBMC across the leprosy spectrum. IL-6 with IFNγ or IL-2 generate CTL in multibacillary patients. Int J Lepr. 1997;65:45–55. [PubMed] [Google Scholar]
- 9.Quentmeier H, Klauche J, Muhlradt P, Drexler H. Role of IL-6, IL-2 and IL-4 in the in vitro induction of cytotoxic T cells. J Immunol. 1992;149:3316–20. [PubMed] [Google Scholar]
- 10.Yamamura M, Uyemura K, Deans R, et al. Defining protective responses to pathogens: cytokine profiles in leprosy lesions. Science. 1991;254:277–9. doi: 10.1126/science.254.5029.277. [DOI] [PubMed] [Google Scholar]
- 11.de la Barrera S, Fink S, Finiasz M, et al. Lack of cytotoxicity against Mycobacterium leprae 65-kD heat shock protein (hsp) in multibacillary leprosy patients. Clin Exp Immunol. 1995;99:90–97. doi: 10.1111/j.1365-2249.1995.tb03477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ridley DA, Jopling W. Classification of leprosy according to immunity. A five-group system. Int J Lepr. 1966;34:255–73. [PubMed] [Google Scholar]
- 13.Boyum A. Isolation of mononuclear cells and granulocytes from human blood. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- 14.Parronchi P, de Carli M, Manetti R, et al. IL-4 and IFN (α and γ) exert opposite regulatory effects on the development of cytolytic potential by Th1 or Th2 T cell clones. J Immunol. 1992;149:2977–83. [PubMed] [Google Scholar]
- 15.Sieling PA, Abrams JS, Yamamura M, et al. Immunosuppressive roles for IL-10 and IL-4 in human infection. J Immunol. 1993;150:5501–10. [PubMed] [Google Scholar]
- 16.Kumararatne DS, Pithie AS, Drysdale P, et al. Specific lysis of mycobacterial antigen-bearing macrophages by class II MHC-restricted polyclonal cell lines in healthy donors or patients with tuberculosis. Clin Exp Immunol. 1990;80:314–23. doi: 10.1111/j.1365-2249.1990.tb03287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thole JER, Hinderson P, de Bruyn J. Antigenic relatedness of a strongly immunogenic 65 kDa mycobacterial protein antigen with a similarity sized ubiquitous bacterial common antigen. Microb Pathol. 1988;4:71–83. doi: 10.1016/0882-4010(88)90049-6. [DOI] [PubMed] [Google Scholar]
- 18.Shinnick TM, Vodkin MH, Williams JC. The M. tuberculosis 65 kDa antigen is a heat shock protein which corresponds to common antigen and to E. coli GroEl protein. Infect Immun. 1988;56:446–51. doi: 10.1128/iai.56.2.446-451.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thole JER, Janson AAM, Kifle A, et al. Analysis of T-cell and B-cell responses to recombinant M. leprae antigens in leprosy patients and in healthy contacts: significant T cell-responses to antigens in M. leprae nonresponders. Int J Lepr. 1995;63:369–80. [PubMed] [Google Scholar]
- 20.Kaufmann SHE. Heat shock protein and the immune response. Immunol Today. 1990;11:129–36. doi: 10.1016/0167-5699(90)90050-j. [DOI] [PubMed] [Google Scholar]
- 21.Mutis T, Cornelisse YE, Ottenhoff THM. Mycobacteria induce CD4+ T cells that are cytotoxical and display Th1-like cytokine secretion profile: heterogeneity in cytotoxic activity and cytokine secretion levels. Eur J Immunol. 1993;23:2189–95. doi: 10.1002/eji.1830230921. [DOI] [PubMed] [Google Scholar]
- 22.Flynn J, Goldstein M, Triebold K, et al. Major histocompatibility complex class I-restricted T cells are required for resistance to Mycobacterium tuberculosis infection. Proc Natl Acad Sci USA. 1992;89:1213–7. doi: 10.1073/pnas.89.24.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Denis O, Lozes E, Huygen K. Induction of cytotoxic T-cell responses against culture filtrate antigens in Mycobacterium bovis Bacillus Calmette–Guérin infected mice. Infect Immun. 1997;65:676–84. doi: 10.1128/iai.65.2.676-684.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suto R, Srivastava PK. A mechanism for the specific immunogenicity of heat-shock-chaperoned peptides. Science. 1995;269:1585–8. doi: 10.1126/science.7545313. [DOI] [PubMed] [Google Scholar]
- 25.Rock KL. A new foreign policy: MHC class I molecules monitor the outside the world in human infection. Immunol Today. 1996;17:131–7. doi: 10.1016/0167-5699(96)80605-0. [DOI] [PubMed] [Google Scholar]
- 26.Yamamura M, Wang XH, Ohmen JS, et al. Cytokine patterns of immunologically mediated tissue damage. J Immunol. 1992;149:1470–5. [PubMed] [Google Scholar]
- 27.Misra N, Murtaza A, Walker B, et al. Cytokine profile of circulating T cells in leprosy patients reflects both indiscriminate and polarized T-helper subsets: T-helper phenotype is stable and uninfluenced by related antigens of Mycobacterium leprae. Immunol. 1995;86:97–103. [PMC free article] [PubMed] [Google Scholar]
- 28.Mutis T, Kraakman EM, Cornelisse YE, et al. Analysis of cytokine production by Mycobacterium-reactive T cells. Failure to explain M. leprae-specific nonresponsiveness of peripheral blood T cells from lepromatous leprosy patients. J Immunol. 1993;151:4641–51. [PubMed] [Google Scholar]
- 29.Fink S, Finiasz M, Valdez R, et al. Evaluación de la producción de citoquinas en enfermos de lepra. Medicina (Buenos Aires) 1996;56:705–8. [PubMed] [Google Scholar]
- 30.Horwitz DA, Gray JD, Ohtsuka K, Hirokawa M, Takahashi T. The immuneregulatory effects of NK cells: the role of TGF-β and implications for autoimmunity. Immunol Today. 1997;18:538–42. doi: 10.1016/s0167-5699(97)01149-3. [DOI] [PubMed] [Google Scholar]
- 31.Kos FJ, Engleman EG. Immune regulation: a critical link between NK cells and CTLs. Immunol Today. 1997;17:174–6. doi: 10.1016/0167-5699(96)80616-5. [DOI] [PubMed] [Google Scholar]
- 32.Nakamura T, Lee RK, Nam SY, et al. Roles of IL-4 and IFNγ in stabilizing the T helper cell type 1 and 2 phenotype. J Immunol. 1997;158:2648–53. [PubMed] [Google Scholar]
- 33.Fink S, de la Barrera S, Minucci F, et al. IFNγ, IL-6 and IL-4 modulate M. leprae- or PPD-specific cytotoxic T cells in leprosy patients. Scand J Immunol. 1993;38:551–8. doi: 10.1111/j.1365-3083.1993.tb03240.x. [DOI] [PubMed] [Google Scholar]
- 34.de Waal Malefyt R, Abrams RJ, Bennett B, et al. Interleukin-10 (IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med. 1991;174:1209–20. doi: 10.1084/jem.174.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Libraty D, Airan LE, Uyemura K, et al. Interferon γ differentially regulates interleukin-12 and interleukin-10 production in leprosy. J Clin Invest. 1997;99:336–41. doi: 10.1172/JCI119162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Andersen P. Host responses and antigens involved in protective immunity to Mycobacterium tuberculosis. Scand J Immunol. 1997;45:115–31. doi: 10.1046/j.1365-3083.1997.d01-380.x. [DOI] [PubMed] [Google Scholar]
- 37.Cooper AM, Dalton DK, Stewart AT, et al. Disseminated tuberculosis in IFNγ gene-disrupted mice. J Exp Med. 1993;178:2243–8. doi: 10.1084/jem.178.6.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Flesch IEA, Hess JH, Oswald IP, Kaufmann SHE. Growth inhibition of Mycobacterium tuberculosis by IFNγ stimulated macrophages: regulation by endogenous tumour necrosis factor-alpha and by IL-10. Int Immunol. 1994;6:693–700. doi: 10.1093/intimm/6.5.693. [DOI] [PubMed] [Google Scholar]
- 39.Hart PH, Whitty GA, Piccoli DS, Hamilton JA. Control by IFNγ and PGE2 of TNFα and IL-1 production by human monocytes. Immunol. 1989;66:376–83. [PMC free article] [PubMed] [Google Scholar]
- 40.Trinchieri G. Cytokines acting on or secreted by macrophages during intracellular infection (IL-10, IL-12, IFNγ) Curr Opin Immunol. 1997;9:17–23. doi: 10.1016/s0952-7915(97)80154-9. [DOI] [PubMed] [Google Scholar]
- 41.Muñoz-Fernández MA, Pimentel-Muiños FX, Alonso MA, et al. Synergy of tumour necrosis factor with protein kinase C activators on T cell activation. Eur J Immunol. 1990;20:605–10. doi: 10.1002/eji.1830200321. [DOI] [PubMed] [Google Scholar]
- 42.Langermans JAM, van der Hultz MEB, Nibbering PH, et al. Endogenous tumour necrosis factor alpha is required for enhanced antimicrobial activity against Toxoplasma gondii and Listeria monocytogenes in recombinant gamma interferon-treated mice. Infect Immun. 1992;60:5107–12. doi: 10.1128/iai.60.12.5107-5112.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sarno EN, Grau GE, Vieira LMM, Nery JA. Serum levels of tumour necrosis factor and interleukin-1β during leprosy reactional states. Clin Exp Immunol. 1991;84:103–8. [PMC free article] [PubMed] [Google Scholar]
- 44.Flesch IEA, Hess JH, Hang S, et al. Early interleukin-12 production by macrophages in response to mycobacterial infection depends on interferonγ and tumour necrosis factor α. J Exp Med. 1995;181:1615–21. doi: 10.1084/jem.181.5.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Flynn JL, Chan J, Triebold KJ, et al. An essential role for IFNγ in resistance to Mycobacterium tuberculosis infection. J Exp Med. 1993;178:2249–54. doi: 10.1084/jem.178.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]