Abstract
We investigated the kinetics of allergen-induced eotaxin expression and its relationship to eosinophil accumulation and activation in the airways of patients with allergic asthma. Twenty-four patients with allergic asthma and late asthmatic responses to allergen inhalation were randomly allocated into three groups of eight patients each, who received bronchoscopy with bronchial biopsies and BAL at 2, 4 and 24 h, respectively, after the inhalation of the diluent and the allergen. The expression of eotaxin mRNA and protein and eotaxin release were evaluated by in situ hybridization, immunohistochemistry, immunocytochemistry, and radioimmunoassay. Increased transcription from the eotaxin gene preceded the appearance of the late asthmatic response and the influx of activated eosinophils in bronchial tissue and BAL fluid (BALF). This was followed by increased cell expression of eotaxin protein (P < 0.001) and increased eotaxin release (P < 0.001), which correlated with the numbers of total and activated eosinophils and the level of airflow obstruction at 4 h after allergen exposure (P < 0.05 for all correlations). At 24 h after allergen inhalation, enhanced eotaxin expression declined without a similar reduction in the numbers of eosinophils in bronchial biopsies and when there was a further increase in the number of these cells in BALF (P < 0.05). These results indicate that eotaxin contributes to the early phase of allergen-induced recruitment of activated eosinophils into the airways of patients with allergic asthma and that other factors are implicated in the persistence of eosinophil infiltration.
Keywords: asthma, airway allergy, eotaxin, chemokines, eosinophils
INTRODUCTION
Allergic bronchial asthma is an inflammatory disease of the airways characterized by the recruitment of a large number of eosinophils into the bronchial mucosa [1–5]. Toxic and proinflammatory agents released from these cells are thought to contribute to many of the structural and functional changes observed in asthmatic patients [1–5].
The selective accumulation of eosinophils in asthmatic airways suggests that there might be a local release of chemoattractants specific for these leucocytes [6], and their identification would allow the development of more specific anti-asthmatic drugs. There is growing evidence that the C-C chemokine, eotaxin, may represent one of those chemoattractants.
Eotaxin has shown potent and specific chemotactic activity for eosinophils in vitro and in vivo [7–10]. Several studies in animal models of allergic inflammation have demonstrated that allergen challenge enhances eotaxin gene and protein expression [8,11–15] and that the kinetics of eotaxin production parallels the kinetics of eosinophil accumulation at the tissue site [13,14]. These findings have suggested the potential involvement of eotaxin in human allergic diseases.
More recent studies have indeed reported increased transcription from the eotaxin gene and increased eotaxin production in bronchial mucosa [16–18] and bronchoalveolar lavage fluid (BALF) [17] of patients with allergic asthma compared with non-asthmatic subjects. This up-regulation of eotaxin expression correlated with the number of airway eosinophils [16–18], and with clinical parameters of disease severity, particularly symptom scores [16] and level of airway hyper-responsiveness [16,18]. A variety of cell types was responsible for eotaxin production, including macrophages, T lymphocytes and eosinophils themselves [16–18]. However, airway resident cells represented the most important sources, particularly bronchial epithelial cells [16–18] and the endothelium of small vessels [18]. Taken together, these results have suggested that eotaxin may make an important contribution to the pathogenesis of asthma by promoting the selective recruitment of eosinophils into the airway. If this is the case, increased eotaxin production should precede eosinophil infiltration during an acute exacerbation of the disease, and it should be limited to cells that are strategically positioned to attract eosinophils from the peripheral blood and promote their movement towards the bronchial lumen, particularly endothelial cells and airway epithelium.
The present study was designed to test this hypothesis by using an experimental model that mimics the inflammatory and functional changes of an exacerbation of asthma due to natural allergen exposure, the late asthmatic response (LAR) induced by allergen inhalation in the laboratory [19,20]. The use of that model allowed us to compare the kinetics of allergen-induced eotaxin generation with the time course and magnitude of eosinophil accumulation in the airways of patients with allergic asthma, and see which cells contribute to the production of this chemokine at different time points after allergen inhalation.
PATIENTS AND METHODS
Subjects
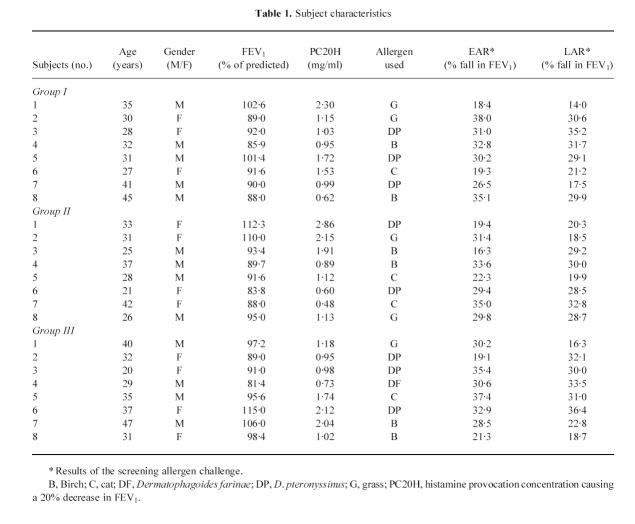
We tested 24 patients with allergic asthma. They were recruited from the patients of the Diagnostic Centre for Respiratory and Allergic Diseases and from patients responding to advertisements or referred to the out-patient clinic after contacts with local general practitioners.
Recruitment criteria were based on published international guidelines [21]. The patients showed the following characteristics: one or more positive skin weal-and-flare response to skin prick testing with a range of local allergens; history indicating several episodes of bronchoconstriction over a period of at least 2 years and in relation to the exposure to one or more of those allergens; reversible airflow obstruction with > 20% increase in the forced expiratory volume in 1 s (FEV1) after inhaled β2-receptor agonists; and airway hyper-responsiveness, as demonstrated by a non-cumulative histamine provocation concentration causing a 20% decrease in FEV1 (PC20H) of < 8 mg/ml on more than one occasion during the previous 30 days.
Exclusion criteria were the following: age < 18 years or > 60 years, FEV1 < 70% of predicted value, abnormal routine blood analysis for bronchoscopic examination, including platelets and clotting parameters, evidence of any other pulmonary disease in addition to asthma, respiratory tract infections during the 2 months preceding the study, abnormal chest radiography, abnormal electrocardiography, pregnancy, breast feeding, and treatment with oral corticosteroids in the 2 months preceding the study.
None of the tested patients was a current smoker. They were stable at the time of testing, requiring only intermittent use of inhaled short-acting β2-receptor agonists.
The study was approved by the Ethics Committee of the Diagnostic Centre for Respiratory and Allergic Diseases and the Ethical Committee of the Italian Foundation of Experimental Medicine, Milano. All the patients gave informed consent.
Study design
At the initial visit, baseline spirometry, histamine inhalation test and standard skin prick tests were performed. Within 1 week, patients undertook an inhalation challenge with the diluent of the allergen extracts. After a period of 3 weeks, patients returned to the laboratory to have spirometry, histamine inhalation tests and skin prick titration tests with the relevant allergen. Within 1 week after that, they underwent the allergen inhalation test (screening test) to verify the occurrence of both early asthmatic response (EAR) and LAR.
Patients were then randomly allocated into three groups. They returned to the laboratory 4 weeks after the allergen challenge to repeat spirometry and the histamine inhalation test. If FEV1 was > 80% of predicted value and PC20H was within two doubling concentrations of the initial value, they had again a diluent inhalation challenge within 1 week. Otherwise, the challenge was postponed until FEV1 and PC20H had returned within the initial values. The patients underwent fibreoptic bronchoscopy with biopsies and BAL at 2 h (group I), 4 h (group II) or 24 h (group III) after the inhalation of the allergen diluent. After a period of 3 weeks, patients returned to the laboratory to have spirometry, histamine inhalation test and skin-prick titration test with the relevant allergen. Within 1 week after that, they had another allergen challenge and underwent fibreoptic bronchoscopy with biopsies and BAL at 2 h (group I), 4 h (group II) or 24 h (group III) after the inhalation of the allergen. After the last bronchoscopic procedure, the patients received a short course of oral corticosteroids to induce a rapid resolution of the allergen-induced inflammatory reaction and to restore the stable clinical and functional conditions they had before their participation in the study.
Histamine inhalation test and skin tests
The histamine inhalation test was performed according to a standard protocol [22], and PC20H was calculated. The skin prick titration test was done using doubling dilutions of the relevant allergen in duplicate. The dilution giving a 2-mm weal response at 15 min was retained for calculation of the predicted allergen provocation concentration causing a 15% decrease in FEV1 (PC15A) [22].
Diluent inhalation challenge
Three doses of saline solution were aerosolized by a Wright nebulizer (Ferraris Medical Inc., Holland, NY) and inhaled for 2 min by tidal breathing with clipped nose at intervals of 10 min [22]. FEV1 was measured after each inhalation, at 10, 20, 30, 45, 60, 90 and 180 min after the final inhalation, and then every hour for the following 7 h.
Allergen inhalation challenge
Predicted PC15A (in protein nitrogen U/ml) was calculated using the results from the histamine inhalation test and the skin prick titration test, according to the formula published by Cockcroft and colleagues [22]. The starting concentration of the allergen to be inhaled was three doubling concentrations below the predicted PC15A. Increasing doubling concentrations of the allergen were then inhaled at intervals of 10 min, as reported for the diluent inhalation, until there was a 15% decrease in FEV1. Measurements of FEV1 were repeated at 10, 20, 30, 45, 60, 90 and 180 min after the final inhalation, and then every hour for the next 7 h. The EAR was defined as the maximal decrease in FEV1 occurring between 0 and 2 h after allergen inhalation [19,22]. The LAR was defined as the maximal decrease in FEV1 occurring between 3 and 7 h after allergen inhalation [19,22].
Bronchoscopic procedures and sample processing
Fibreoptic bronchoscopy was performed under local anaesthesia, according to a protocol [16,23,24] based on published international guidelines [25].
The bronchoscope (Olympus, Co., Tokyo, Japan) was inserted into the left lung and at least three mucosal biopsies were obtained from the lobar carinae and carinae of the basal segments, via separate alligator forceps (Olympus Co.). Then the bronchoscope was inserted into the middle lobe of the right lung and wedged into one segment. Five 20-ml aliquots of prewarmed saline solution (37°C) were infused and gently aspirated into polypropylene tubes kept at 4–5°C.
Biopsy samples were fixed in a solution containing 4% paraformaldehyde in PBS for 2 h, washed repeatedly in 15% sucrose/PBS, embedded in Tissue-Tek OCT compound (Miles Inc., Elkhart, IN), snap-frozen and stored at −80°C until processed for in situ hybridization and immunohistochemistry [16,24].
The recovered BALF was centrifuged at 400 g for 10 min at 4°C. Total protein and albumin concentrations in the supernatant were measured as previously described [23]. The supernatant was then concentrated 10-fold by the Centricon system (Amicon Inc., Beverley, MA) and stored at −80°C until use to measure the levels of immunoreactive eosinophil cationic protein (ECP) and eotaxin by radioimmunoassay (RIA), as reported below. Cells in the pellet were suspended in PBS and centrifuged onto Superfrost slides (Fisher Scientific Co., Pittsburgh, PA). Cytospin preparations were fixed in 4% paraformaldehyde for 20 min, washed repeatedly in PBS, air-dried and stored at −80°C until further processing for in situ hybridization and immunocytochemistry.
In situ hybridization
The 35S-labelled eotaxin riboprobes and the in situ hybridization procedure were the same as described in a previous study [16]. Cryostat tissue sections (6 μm) and cytospin preparations were prehybridized with 50% formamide in 2 × standard saline citrate (SSC) for 20 min at 37°C. Hybridization was carried out with the labelled antisense probe, complementary to human eostaxin messenger ribonucleic acid (mRNA) at 40°C for 12 h. Unhybridized single-stranded RNA was removed by treatment with ribonuclease (RNase A; Promega, Madison, WI) after washing the slides in decreasing concentrations of SSC (4–0.1 × SSC). Hybridization signal was visualized using standard autoradiographic procedures and haematoxylin counterstaining. For negative controls, slides were hybridized with the labelled sense probe, identical to human eotaxin mRNA, or pretreated with RNase A before hybridization with the labelled antisense probe. Positive controls were sections of nasal polyps [10].
Immunohistochemistry and immunocytochemistry
These procedures were performed according to standard protocols [23,24]. Total and activated eosinophils were identified by immunospecific labelling with the MoAb BMK13 (Cymbus Bioscience Ltd, Chilworth, UK) [26] (1:10 dilution) and EG2 (Kabi Pharmacia Diagnostics AB, Uppsala, Sweden) [27] (1:5 dilution), respectively. Eotaxin-positive cells were identified by using a specific polyclonal antibody (R&D Systems Europe Ltd, Abingdon, UK) (1:20 dilution). Immunostaining was detected by the alkaline phosphatase anti-alkaline phosphatase (APAAP) method as previously described [23], using commercially available reagents and an enzyme substrate that stains positive cells red (LSAB; Dako, Glostrup, Denmark). Controls for specificity of the BMK13 and EG2 immunostaining were sections incubated with a mouse IgG2a myeloma protein (Dako) as substitute for each primary MoAb. Controls for specificity of eotaxin immunostaining were biopsy sections and BAL cytospin preparations stained with the anti-eotaxin antibody preincubated with excess amounts of recombinant human eotaxin (R&D Systems).
To identify cell sources of eotaxin, double immunostaining was accomplished as described elsewhere [23,24]. Tissue sections and cytospin preparations were first incubated with the anti-eotaxin antibody. Immunoreactive cells were revealed by the streptavidin-biotin immunoperoxidase staining system (Immustain Kit; DPC Co., Los Angeles, CA) that stains positive cells brown. Slides were then incubated with the pan eosinophil marker, BMK13, or with each of the following antibodies: the MoAb against CD68 (Dako), human neutrophil elastase (Dako), tryptase (Dako), CD3 (Dako) and CD31 (Dako), and an anti-pancytokeratin polyclonal antibody (Dako). These label, respectively, total eosinophils, macrophages, neutrophils, mast cells, T lymphocytes, endothelial cells and epithelial cells. Antibody binding was demonstrated using the APAAP method and an enzyme substrate that labels positive cells blue (Substrate Kit III; Vector Labs Inc., Burlingame, CA). Controls for specificity were irrelevant isotype-matched mouse IgG or normal rabbit immunoglobulin (all from Dako), as appropriate.
Histological and cytological quantification
Mucosal tissue sections were coded and examined blind by two investigators under a light microscope. At least four sections were analysed from each tissue sample. Eotaxin mRNA-positive cells and eotaxin immunoreactive cells in the epithelium were scored on the basis of the percentage of the epithelium showing positive signal as follows: 0, no staining; 1, 0.1–10%; 2, 10–20%; 3, 20–30%; 4, 30–40%; 5, 40–50%; 6, 50–60%; 7, 60–80%; 8, 80–100%. In the subepithelial tissue, cells expressing eotaxin mRNA or protein and eosinophils were counted in a zone 115 μm deep along the entire length of the epithelial basement membrane (BM), using a squared eyepiece graticule, and expressed as numbers of positive cells in that zone per unit length (1 mm) of BM. A computerized morphometric tablet was employed to determine the length of the BM as previously described [24], and a total BM length of at least 8 mm per patient was assessed. The mean intra-observer coefficients of variation for three repeated measurements ranged from 4% to 8% for epithelial scores and from 5% to 11% for cell count in the subepithelial zone. The interobserver correlation coefficients ranged from 0.83 to 0.89.
BAL cell count was performed on coded slides by two observers. At least 1000 nucleated cells were counted on triplicate cytospins, and the numbers of cells positive for eotaxin mRNA and protein, or specifically stained with the BMK3 and EG2 antibodies, were expressed as percentage of the total nucleated cells. The mean intra-observer coefficients of variation for three repeated measurements were < 5%, and the interobserver correlation coefficients ranged from 0.94 to 0.97.
Measurement of BAL immunoreactive ECP and eotaxin
Levels of ECP in BALF were measured with a double-antibody RIA (Kabi Pharmacia Diagnostics AB). The sensitivity of this assay was 2 ng/ml.
Levels of eotaxin were determined by RIA, using a competitive binding assay protocol similar to that previously described for endothelin-1 assay [28], with the following modifications. Human eotaxin (R&D Systems) was iodinated using the Bolton Hunter reagent (DuPont NEN, Boston, MA) [29]. The specific activity of 125I-labelled eotaxin was calculated to be 1800 Ci/mm. BALF (100 μl) was mixed with 100 μl of PBS/azide/10% polyethylene glycol/0.5% protamine sulphate. This mixture was incubated for 24 h with the eotaxin tracer (2300 ct/min) and the anti-eotaxin antiserum (R&D Systems) (1:2000 dilution) diluted in a 0.2 m sodium phosphate buffer containing 0.5% bovine serum albumin (BSA), and 10 mm ethylenediaminetetraacetic acid/azide pH 7.4. Separation of the antibody-bound from free eotaxin was achieved using an immunobead second antibody reagent (0.8 mg) (BioRad Labs, Richmond, CA). After incubation for 6 h, the mixture was centrifuged, supernatant was discarded, and bound radioactivity in the precipitate was quantified in a gamma counter. Non-specific binding was determined in replicate samples incubated in absence of the antiserum or incubated with control rabbit immunoglobulin. Eotaxin concentrations were calculated by comparison with human eotaxin standard curve. All BAL samples were assayed in triplicate. The limit of adequate measurement, determined as the concentration required to inhibit the specific binding of 125I-ligand by 20%, was 14.5 pg/ml. The interassay and intra-assay coefficients of variation were < 5%. Cross-reactivity of human RANTES, MIP-1α, MIP-1β and chemokines of the MCP family was < 1%.
Statistical analysis of group data
Results were expressed as median and range. For unpaired data, differences across groups were tested by the Kruskal–Wallis analysis and comparisons were made by the Mann–Whitney U-test [30]. Paired data were compared by the Wilcoxon's signed rank test [30]. Associations between variables were tested by calculating the Rs coefficient with the Spearman's rank correlation test [30]. Significance was accepted when P < 0.05.
RESULTS
The clinical and demographic data of the tested subjects are given in Table 1, along with the EAR and LAR observed during the screening allergen challenge. The maximal percentage fall in FEV1 of the EAR was comparable to that obtained during the second allergen challenge when bronchoscopy was performed (group I, 29.5%, 17.6–36.2%; group II, 30.5%, 18.4–37.3%; group III, 31.9%, 20.8–36.1%), with P > 0.05 for all comparisons.
Table 1.
Subject characteristics
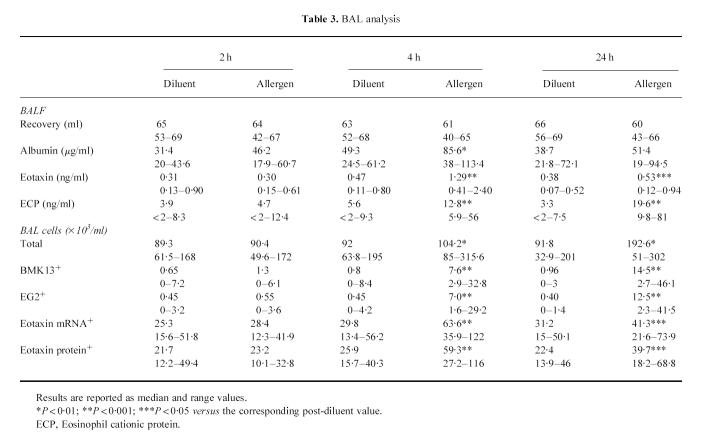
At 2 h after allergen inhalation (group I), all the patients had recovered from the EAR. Increased transcription from the eotaxin gene was already detectable in bronchial biopsies, both in the epithelium and in the subepithelial area (Table 2). Although there was a trend for a concomitant increase in eotaxin immunoreactivity, this did not reach statistical significance (Table 2). The expression of both eotaxin mRNA and protein in BAL was similar to that observed after the inhalation of the diluent (Table 3). At this time, no allergen-induced increase in the numbers of total and activated eosinophils was evident in bronchial biopsies (Table 2) or in BAL (Table 3).
Table 2.
In situ hybridization and immunohistochemical analysis in bronchial biopsies
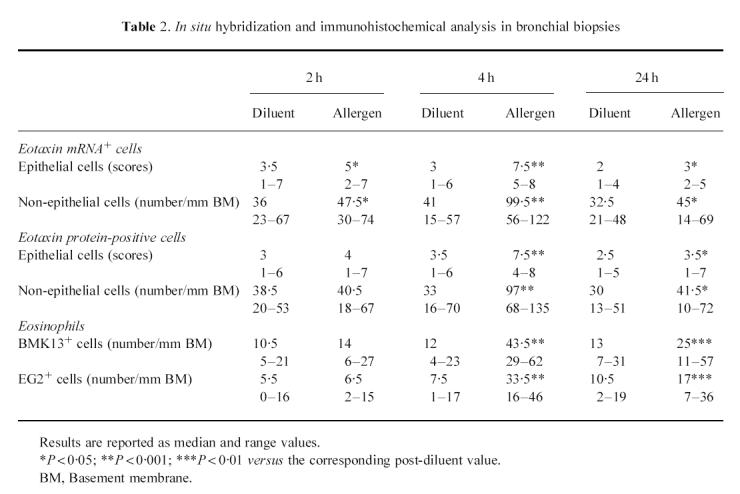
Table 3.
BAL analysis
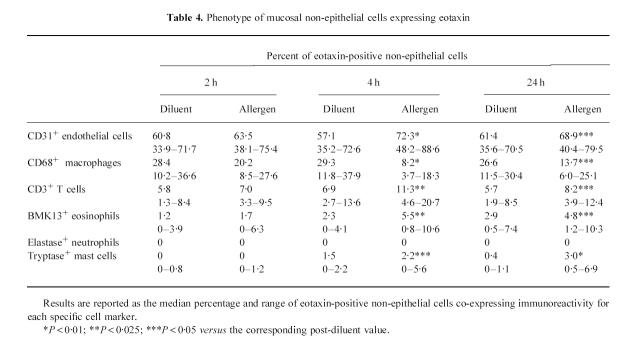
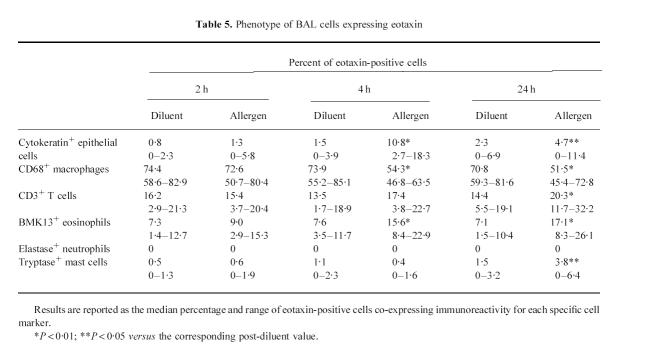
At 4 h after allergen inhalation (group II), the patients showed a prebronchoscopy percentage fall in FEV1 significantly greater than the corresponding post-diluent value (–9.7%, from −12.7 to −8.3%, versus−0.1%, from −5 to +4.9%, P < 0.01), indicating that the LAR had begun. At this time point there was a marked allergen-induced increase in the number of cells expressing eotaxin mRNA and eotaxin immunoreactivity, both in bronchial biopsies (Table 2) and in BAL (Table 3). The distribution of eotaxin mRNA followed a pattern similar to the distribution of the protein (Figs 1 and 2). In bronchial biopsies, positive hybridization signal mainly localized to bronchial epithelial cells, but many cells in the subepithelium were also positive. Non-epithelial cell sources of eotaxin were identified by double immunostaining. They were predominantly endothelial cells, followed by macrophages, T lymphocytes, eosinophils and mast cells (Table 4). In BAL cytospin preparations the most important sources of eotaxin were macrophages (Table 5). The relative contribution of T lymphocytes, eosinophils and mast cells to the total eotaxin immunoreactivity in bronchial tissue and BAL cells was significantly enhanced by allergen inhalation (Tables 4 and 5). However, it still appeared quite low compared with the contribution of mucosal epithelial and endothelial cells, which increased more markedly (Tables 2 and 4).
Fig. 1.
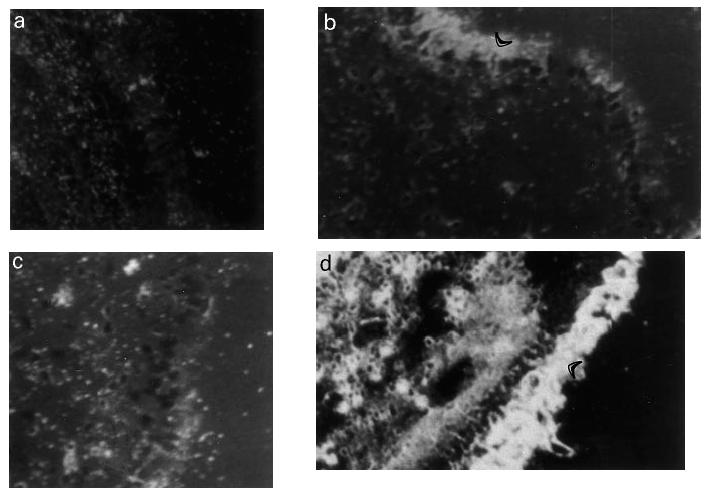
Representative photomicrographs of in situ hybridization showing the expression of eotaxin mRNA in sections of bronchial tissue from one asthmatic patient at 4 h after the inhalation of the diluent (a,b) and the allergen (c,d). Replicate sections were hybridized either with the antisense probe (b,d) or with the sense probe (a,c) as control. Note the marked increase in the hybridization signal both in the epithelium and in the subepithelial area of the antisense-probed section after allergen inhalation. The arrowheads indicate the epithelium. (Original mag. × 400.)
Fig. 2.
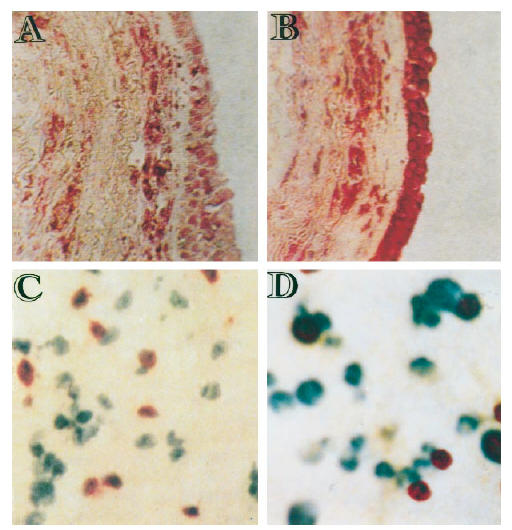
Representative photomicrographs showing eotaxin immunostaining in bronchial tissue (A,B) and in BAL cells (C,D) of one asthmatic patient at 4 h after the inhalation of the diluent (A) and the allergen (B,C,D). (A–C) Cells expressing eotaxin protein are stained red. (D) Expression of eotaxin protein in BAL macrophages by double immunostaining. In this case, cells expressing eotaxin immunoreactivity are stained brown whereas macrophages (CD68+) are stained blue. Thus, cells with mixed blue/brown colour are macrophages producing eotaxin. (Original mag.: × 200 (A–C) and × 400 (D).
Table 4.
Phenotype of mucosal non-epithelial cells expressing eotaxin
Table 5.
Phenotype of BAL cells expressing eotaxin
At the same time point, allergen inhalation induced a significant increase in the number of total and activated eosinophils both in bronchial biopsies (Table 2) and BAL (Table 3). Increased eosinophil activation was confirmed by the increased release of ECP in BALF (Table 3). The number of eosinophils in bronchial mucosa correlated with eotaxin immunoreactivity in the epithelium (Rs = 0.76, P < 0.05) and with the numbers of cells stained with the anti-eotaxin antibody in the subepithelial area (Rs = 0.79, P < 0.05). The prebronchoscopy percentage fall in FEV1 correlated with the number of total and activated eosinophils in bronchial tissue (Rs = 0.81, P < 0.05; Rs = 0.85, P < 0.025) and with eotaxin immunoreactivity in the epithelium and in the subepithelial area (Rs = 0.75, P < 0.05; Rs = 0.77, P < 0.05).
At 24 h after allergen inhalation (group III), a significant increase in the numbers of total and activated eosinophils was still detectable in bronchial biopsies (Table 2) and in BAL (Table 3). The increase in BAL was even greater than that observed in the patients tested at 4 h after allergen inhalation (P < 0.05), and it was associated with enhanced release of ECP (Table 3). The magnitude of the preceding LAR (percentage fall in FEV1: 29.2%, 15.8–32.7%) correlated with the numbers of total and activated eosinophils in bronchial tissue (Rs = 0.79, P < 0.05; Rs = 0.83, P < 0.05) and with the numbers of total and activated eosinophils in BAL (Rs = 0.78, P < 0.05; Rs = 0.80, P < 0.05). Nonetheless, the allergen-induced expression of eotaxin mRNA and protein in bronchial mucosa (Table 2) and in BAL cells (Table 3), and the increased contents of immunoreactive eotaxin in BALF (Table 3), were much lower than at 4 h. In addition, no correlation was found between eotaxin production and the number of eosinophils or magnitude of the preceding LAR.
DISCUSSION
During the last 10 years, the relationship between airway inflammation and the changes in airway function associated with asthma has been investigated in the laboratory using allergen challenge. LAR to inhaled allergen predominantly occurs in allergic individuals with asthma, and it is characterized by the development of airflow obstruction and temporary increase in airway hyper-responsiveness [19,20]. These functional changes mimic the functional changes occurring spontaneously during the exacerbation of the disease due to natural allergen exposure. They are the consequence of an inflammatory process specifically induced in asthmatic airways [19,20], where eosinophil accumulation and activation predominate and play an important pathogenic role [19,20,31]. We therefore used the allergen-induced LAR as a model of allergic airway inflammation to investigate the dynamics of eotaxin expression in relation to the kinetics of eosinophil accumulation and activation in asthmatic airways.
The number of total and activated eosinophils in bronchial mucosa increased concomitantly with the appearance of LAR, and these cells retained the ability to actively secrete ECP until 24 h after allergen inhalation. At this time point, there was a further increase in the number of eosinophils in BAL and in the amounts of ECP released in the BALF, indicating the movement of activated eosinophils from the mucosa to the airway lumen. These results are in keeping with those reported in previous kinetic studies in BAL [32–34] and bronchial biopsies [33]. In accord with our findings, Aalbers and colleagues [33] observed increased EG2 staining in bronchial biopsies obtained at 3 and 24 h after allergen challenge. This increase declined at 24 h, when EG2 staining of mucosal tissue inversely correlated with the numbers of EG2+ cells in BAL.
In the present study, increased transcription from the eotaxin gene preceded the appearance of the LAR and the concomitant increase in the number of total and activated eosinophils in bronchial tissue. The subsequent increase in eotaxin generation and release observed at 4 h after allergen inhalation correlated with the degree of eosinophil infiltration and activation. In addition, the concentrations of immunoreactive eotaxin measured in BALF at this time point were much higher than those required to induce optimal eosinophil chemotaxis in vivo in non-human primates (10–1000 pmol) [10]. These results suggest the involvement of eotaxin in the early phase of eosinophil recruitment during the LAR. The correlation observed between the level of airflow obstruction and eotaxin immunoreactivity in bronchial tissue may simply reflect this fact, since the level of airflow obstruction also correlated with mucosal eosinophilia. During that phase, eotaxin may work in concert with other less eosinophil-specific chemokines that are also released in excess in the airways of patients with allergic asthma at similar time points after allergen challenge [34].
The allergen-induced increase in eotaxin expression at 4 h was not merely due to the influx of inflammatory cells producing eotaxin, because eotaxin hybridization signal and immunoreactivity mainly localized to the bronchial epithelium and endothelial cells. In addition, the overall contribution of T lymphocytes, eosinophils, and other infiltrating cells to total eotaxin production by non-epithelial cells was < 30% (Table 4). The enhanced expression of eotaxin in the epithelium might contribute to the movement of eosinophils towards the airway lumen once they have migrated in the bronchial tissue from the peripheral blood. This may explain the progressive increase of eosinophils in BAL between 4 h and 24 h after allergen inhalation, which paralleled its decline in bronchial tissue.
The minimal increase in eotaxin expression in the patients tested at 24 h after allergen inhalation, and the lack of correlation with the number of eosinophils, suggest that other factors contribute to the persistence of eosinophil infiltration and activation at this time point. Granulocyte-macrophage colony-stimulating factor (GM-CSF) and IL-5 represent the most likely candidates, because they are known to promote eosinophil activation and survival [35,36] and are indeed produced in increased amounts in the airways of asthmatic patients at 24 h after allergen challenge [37–41] and later [42].
In conclusion, the results of this study show that increased eotaxin production is an early event in the endothelium of bronchial vessels and in the bronchial epithelium of patients with allergic asthma who have an exacerbation of the disease due to allergen exposure. They also provide evidence that this increased production may be involved in the allergen-induced inflammatory reaction and airflow obstruction by promoting the migration of eosinophils from the blood stream, their movement towards the epithelium, and their activation. Finally, they confirm the important effector role of bronchial epithelial cells [24,36], whose function and response to allergic stimuli are clearly altered in asthma [43].
Acknowledgments
We would like to thank all the subjects who volunteered to participate in this study. We are grateful to Drs M. Bianchini and R. Renzi, University of Milano, Italy, for providing surgical specimens from nasal polyps. This study was supported in part through extramural funds from the National Research Council and Cariplo Foundation, Italy.
REFERENCES
- 1.Bousquet J, Chanez P, Lacoste JY, et al. Eosinophilic inflammation in asthma. N Engl J Med. 1990;323:1033–9. doi: 10.1056/NEJM199010113231505. [DOI] [PubMed] [Google Scholar]
- 2.Busse WW, Sedgwick JB. Eosinophils in asthma. Ann Allergy. 1992;68:286–90. [PubMed] [Google Scholar]
- 3.Busse WW, Calhoun WF, Sedgwick JB. Mechanisms of airway inflammation in asthma. Am Rev Respir Dis. 1993;147:S20–S24. doi: 10.1164/ajrccm/147.6_Pt_2.S20. [DOI] [PubMed] [Google Scholar]
- 4.Seminario M-C, Gleich G. The role of eosinophils in the pathogenesis of asthma. Curr Opin Immunol. 1994;6:860–4. doi: 10.1016/0952-7915(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 5.Wardlaw AJ, Moqbel R, Kay AB. Eosinophils: biology and role in disease. Adv Immunol. 1995;60:151–266. doi: 10.1016/s0065-2776(08)60586-6. [DOI] [PubMed] [Google Scholar]
- 6.Resnick MB, Weller PF. Mechanisms of eosinophil recruitment. Am J Respir Cell Mol Biol. 1993;8:349–55. doi: 10.1165/ajrcmb/8.4.349. [DOI] [PubMed] [Google Scholar]
- 7.Griffiths-Johnson DA, Collins PD, Rossi AG, Jose PJ, Williams TJ. The chemokine, eotaxin, activates guinea-pig eosinophils in vitro and causes their accumulation into the lung in vivo. Biochem Biophys Res Commun. 1993;197:1167–72. doi: 10.1006/bbrc.1993.2599. [DOI] [PubMed] [Google Scholar]
- 8.Jose PJ, Griffiths-Johnson DA, Collins PD, et al. Eotaxin: a potent eosinophil chemoattractant cytokine detected in a guinea pig model of allergic airways inflammation. J Exp Med. 1994;179:881–7. doi: 10.1084/jem.179.3.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garcia-Zepada EA, Rothenberg ME, Ownbey RT, Celestin J, Leder P, Luster AD. Human eotaxin is a specific chemoattractant for eosinophil cells and provides a new mechanism to explain tissue eosinophilia. Nature Med. 1996;2:449–56. doi: 10.1038/nm0496-449. [DOI] [PubMed] [Google Scholar]
- 10.Ponath PD, Qin S, Ringler DJ, et al. Cloning of the human eosinophil chemoattractant, eotaxin. Expression, receptor binding, and functional properties suggest a mechanism for the selective recruitment of eosinophils. J Clin Invest. 1996;97:604–12. doi: 10.1172/JCI118456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jose PJ, Adcock IM, Griffiths-Johnson DA, Berkman N, Wells TNC, Williams TJ, Power CA. Eotaxin: cloning of an eosinophil chemoattractant cytokine and increased mRNA expression in allergen-challenged guinea-pig lungs. Biochem Biophys Res Commun. 1994;205:788–94. doi: 10.1006/bbrc.1994.2734. [DOI] [PubMed] [Google Scholar]
- 12.Rothenberg ME, Luster AD, Lilly CM, Drazen JM, Leder P. Constitutive and allergen-induced expression of eotaxin mRNA in the guinea-pig lung. J Exp Med. 1995;181:1211–6. doi: 10.1084/jem.181.3.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gonzalo JA, Jia G-Q, Aguirre V, et al. The expression of mouse eotaxin parallels eosinophil accumulation during lung allergic inflammatory reactions but is not restricted to a TH2 type response. Immunity. 1996;4:1–14. doi: 10.1016/s1074-7613(00)80293-9. [DOI] [PubMed] [Google Scholar]
- 14.Humbles AA, Conroy DM, Marleau S, et al. Kinetics of eotaxin generation and its relationship to eosinophil accumulation in allergic airway disease: analysis in a guinea pig model in vivo. J Exp Med. 1997;186:601–12. doi: 10.1084/jem.186.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li D, Wang D, Griffiths-Johnson DA, Wells TNC, Williams TJ, Jose PJ, Jeffery PK. Eotaxin protein and gene expression in guinea-pig lungs: constitutive expression and upregulation after allergen challenge. Eur Respir J. 1997;10:1946–54. doi: 10.1183/09031936.97.10091946. [DOI] [PubMed] [Google Scholar]
- 16.Mattoli S, Stacey MA, Sun G, Bellini A, Marini M. Eotaxin expression and eosinophilic inflammation in asthma. Biochem Biophys Res Commun. 1997;236:299–301. doi: 10.1006/bbrc.1997.6958. [DOI] [PubMed] [Google Scholar]
- 17.Lamkhioued B, Renzi PM, Abi-Younes S, et al. Increased expression of eotaxin in bronchoalveolar lavage and airways of asthmatics contributes to the chemotaxis of eosinophils to the site of inflammation. J Immunol. 1997;159:4593–601. [PubMed] [Google Scholar]
- 18.Ying S, Robinson DS, Meng Q, et al. Enhanced expression of eotaxin and CCR3 mRNA and protein in atopic asthma. Association with airway hyperresponsiveness and predominant co-localization of eotaxin mRNA to bronchial epithelial and endothelial cells. Eur J Immunol. 1997;27:3507–16. doi: 10.1002/eji.1830271252. [DOI] [PubMed] [Google Scholar]
- 19.O'Byrne PM, Dolovich J, Hargreave FE. Late asthmatic responses. Am Rev Respir Dis. 1987;136:740–51. doi: 10.1164/ajrccm/136.3.740. [DOI] [PubMed] [Google Scholar]
- 20.Lemanske RF, Kaliner MA. Late phase allergic reactions. In: Middleton E Jr, Reed CE, Ellis EF, Adkinson NF, Yuninger JW, Busse WW, editors. Allergy: principles and practice. 4. St Louis: Mosby Yearbook; 1993. pp. 320–61. [Google Scholar]
- 21.Guidelines for the diagnosis and management of asthma. National Heart, Lung, and Blood Institute, National Education program. Expert Panel Report. J Allergy Clin Immunol. 1991;88:425–534. [PubMed] [Google Scholar]
- 22.Cockcroft DW, Murdock KY, Kirby J, Hargreave FE. Prediction of airway responsiveness to allergen from skin sensitivity to allergen and airway responsiveness to histamine. Am Rev Resp Dis. 1987;135:264–8. doi: 10.1164/arrd.1987.135.1.264. [DOI] [PubMed] [Google Scholar]
- 23.Mattoli S, Mattoso VL, Soloperto M, Allegra L, Fasoli A. Cellular and biochemical characteristics of bronchoalveolar lavage fluid in symptomatic nonallergic asthma. J Allergy Clin Immunol. 1991;87:794–802. doi: 10.1016/0091-6749(91)90125-8. [DOI] [PubMed] [Google Scholar]
- 24.Ackerman V, Marini M, Vittori E, Bellini A, Vassalli G, Mattoli S. Detection of cytokines and their cell source in bronchial biopsy samples from asthmatic patients. Relationship to the atopic status, symptoms and level of airway hyperresponsiveness. Chest. 1994;105:687–96. doi: 10.1378/chest.105.3.687. [DOI] [PubMed] [Google Scholar]
- 25.Workshop summary and guidelines: investigative use of bronchoscopy, lavage, and bronchial biopsies in asthma and other airway diseases. J Allergy Clin Immunol. 1991;88:808–14. doi: 10.1016/0091-6749(91)90189-u. [DOI] [PubMed] [Google Scholar]
- 26.Moqbel R, Barkans J, Bradley BL, Durham SR, Key AB. Application of monoclonal antibodies against major basic protein (BMK-13) and eosinophil cationic protein (EG1 and EG2) for quantifying eosinophils in bronchial biopsies from atopic asthma. Clin Exp Allergy. 1992;22:265–73. doi: 10.1111/j.1365-2222.1992.tb03082.x. [DOI] [PubMed] [Google Scholar]
- 27.Tai P-C, Spry CJF, Peterson C, Venge P, Olssen I. Monoclonal antibodies distinguish storage and secreted forms of eosinophil cationic protein. Nature. 1984;309:182–4. doi: 10.1038/309182a0. [DOI] [PubMed] [Google Scholar]
- 28.Mattoli S, Mezzetti M, Riva G, Allegra L, Fasoli A. Specific binding of endothelin to human bronchial smooth muscle cells in culture and secretion of endothelin-like material from human bronchial epithelial cells. Am J Respir Cell Mol Biol. 1990;3:145–51. doi: 10.1165/ajrcmb/3.2.145. [DOI] [PubMed] [Google Scholar]
- 29.Coligan JE, Kruisbeek AM, Margulies DH, Shevach EM, Strober W. Current protocols in immunology. New York: John Wiley and Sons; 1992. [Google Scholar]
- 30.Conover WJ. New York: John Wiley and Sons; 1980. Practical non-parametric statistics. [Google Scholar]
- 31.Chung KF. The eosinophil in airway inflammation: potential role and pharmacologic modulation. In: Chung KF, Barnes PJ, editors. Pharmacology of the respiratory tract. New York: Marcel Dekker; 1993. pp. 303–29. [Google Scholar]
- 32.De Monchy JGR, Kauffman HF, Venge P, Koeter GH, Jansen HM, Sluiter HJ, De Vries K. Bronchoalveolar eosinophilia during allergen-induced late asthmatic reactions. Am Rev Respir Dis. 1985;131:373–6. doi: 10.1164/arrd.1985.131.3.373. [DOI] [PubMed] [Google Scholar]
- 33.Aalbers R, De Monchy JGR, Kauffman HF, Smith M, Hoekstra Y, Vrugt B, Timens W. Dynamics of eosinophil infiltration in the bronchial mucosa before and after the late asthmatic reaction. Eur Respir J. 1993;6:840–7. [PubMed] [Google Scholar]
- 34.Holgate ST, Bodey KS, Janezic A, Frew AJ, Kaplan AP, Teran LM. Release of RANTES, MIP-1α, and MCP-1 into asthmatic airways following endobronchial allergen challenge. Am J Respir Crit Care Med. 1997;156:1377–83. doi: 10.1164/ajrccm.156.5.9610064. [DOI] [PubMed] [Google Scholar]
- 35.Lopez AF, Sanderson CJ, Gamble JR, Campbell HD, Young IG, Vadas MA. Recombinant human interleukin 5 is a selective activator of human eosinophil function. J Exp Med. 1988;167:219–24. doi: 10.1084/jem.167.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Soloperto M, Mattoso VL, Fasoli A, Mattoli S. A bronchial epithelial cell-derived factor in asthma which promotes eosinophil activation and survival as GM-CSF. Am J Physiol. 1991;260:L530–L538. doi: 10.1152/ajplung.1991.260.6.L530. [DOI] [PubMed] [Google Scholar]
- 37.Sur S, Hirohito K, Gleich GJ, Chenier TC, Hunt LW. Eosinophil recruitment is associated with IL-5, but not with RANTES, twenty-four hours after allergen challenge. J Allergy Clin Immunol. 1990;97:1272–8. doi: 10.1016/s0091-6749(96)70195-1. [DOI] [PubMed] [Google Scholar]
- 38.Bentley AM, Meng Q, Robinson DS, Hamid Q, Kay AB, Durham SR. Increases in activated T lymphocytes, eosinophils and cytokine mRNA expression for interleukin-5 and granulocyte/macrophage colony-stimulating factor in bronchial biopsies after allergen inhalation challenge in atopic asthmatics. Am J Respir Cell Mol Biol. 1993;8:35–42. doi: 10.1165/ajrcmb/8.1.35. [DOI] [PubMed] [Google Scholar]
- 39.Ohnishi T, Kita H, Weiler D, et al. IL-5 is the predominant eosinophil-active cytokine in the antigen-induced pulmonary late-phase reaction. Am Rev Respir Dis. 1993;147:901–7. doi: 10.1164/ajrccm/147.4.901. [DOI] [PubMed] [Google Scholar]
- 40.Ohnishi T, Sur S, Collins DS, Fish JE, Gleich GJ, Peters SP. Eosinophils survival activity identified as IL-5 is associated with eosinophil recruitment and degranulation and lung injury twenty-four hours after segmental antigen challenge. J Allergy Clin Immunol. 1993;92:607–15. doi: 10.1016/0091-6749(93)90085-t. [DOI] [PubMed] [Google Scholar]
- 41.Woolley KL, Adelroth E, Woolley MJ, Ellis R, Jordana M, O'Byrne PM. Effects of allergen challenge on eosinophils, eosinophil cationic protein, and granulocyte-macrophage colony-stimulating factor in mild asthma. Am J Respir Crit Care Med. 1995;151:1915–24. doi: 10.1164/ajrccm.151.6.7767540. [DOI] [PubMed] [Google Scholar]
- 42.Shaver JR, Zangrilli JG, Cho S-K, Cirelli RA, Pollice M, Hastie AT, Fish JE, Peters SP. Kinetics of the development and recovery of the lung from IgE-mediated inflammation. Am J Respir Crit Care Med. 1997;155:442–8. doi: 10.1164/ajrccm.155.2.9032176. [DOI] [PubMed] [Google Scholar]
- 43.Mori L, Kleimberg J, Mancini C, Bellini A, Marini M, Mattoli S. Bronchial epithelial cells of atopic patients with asthma lack the ability to inactivate allergens. Biochem Biophys Res Commun. 1995;217:817–24. doi: 10.1006/bbrc.1995.2845. [DOI] [PubMed] [Google Scholar]