Abstract
IL-6 is a growth factor which interferes in the apoptosis of malignant plasma cells. Here we explore its role in the spontaneous and Fas/FasL-regulated apoptosis of seven myeloma cell clones (MCC). MCC-2 and -7 were constitutively defective in Fas antigen in the presence of large membrane exposure of FasL, and showed a high rate of cell proliferation irrespective of the presence of IL-6. Cytofluorimetric analysis following propidium iodide (PI) staining revealed a minimal extent of spontaneous apoptosis, as in other IL-6-insensitive, though Fas-positive MCC, namely MCC-3 and -5. By contrast, a regular amplitude of apoptosis occurred in the remaining IL-6-dependent clones. Their propensity to cell death, as well as their FasL membrane expression, were promptly down-modulated by the cytokine, whereas no substantial effect was detected in IL-6-independent MCC. Furthermore, we investigated the quantitative secretion of FasL. Both [3-(4,5-dimethylthiazol-2-yl)-2,5diphenyl tetrazolium bromide] (MTT) cytotoxicity assay and PI staining of WC8 lymphoblasts from a Fas-transfected mouse lymphoma, incubated with supernatants from MCC, showed a variable cytocidal property, thus confirming the cellular release of FasL. However, a significant elevation of FasL secretion occurred in both Fas− MCC, whereas molecular cloning and sequencing of Fas revealed the presence of a splicing variant, namely Fas Exo4,6Del, in the cDNA from both MCC-3 and -5, which were previously demonstrated to be unresponsive to Fas stimulation. Taken together, these data provide evidence that concurrence of IL-6 insensitivity and deregulation of apoptosis in myeloma cells reflects a high malignancy grade. It is suggested that the secretion of Fas splicing variants in Fas+ plasma cells, as well as the over-production of FasL in Fas− myelomas, are differential mechanisms by which myeloma cells escape host immune surveillance.
Keywords: Fas, Fas ligand, IL-6, multiple myeloma, plasma cell
INTRODUCTION
Myeloma cells have been shown to exhibit high sensitivity to IL-6 [1], a major B cell differentiation factor which promotes their clonal expansion [2] and proliferation in bone marrow [3,4]. Although further cytokines, including IL-1α [5], tumour necrosis factor-alpha (TNF-α) [6] and IL-3 [7], can also induce in vitro a proliferative effect in synergy with IL-6 [8], the replication of malignant plasma cells in vitro is suppressed by MoAbs to either IL-6 or its cellular receptor [9,10], just as tumour progression is affected in vivo by the experimental administration of anti-IL-6 antibodies [11,12]. In addition, the progressive bone resorption typical of multiple myeloma seems largely ascribable to IL-6, whose functional levels within the bone marrow are maintained by either autocrine [13] or paracrine [14,15] secretion.
The proliferation of malignant plasma cells may also be regulated by suppression of their apoptosis. This process is essential for negative selection and homeostasis of lymphoid cells [16–18] and is influenced by three cytokines involved in B cell replication, namely IL-6, IL-10 and interferon-alpha (IFN-α), whose regulation of both spontaneous and steroid-induced apoptosis has been clearly described in some B cell tumours [19–21]. Recent investigations suggest that IFN-α2, a recombinant drug successfully used in the treatment of certain malignant haemopathies including multiple myeloma [22], is able to down-modulate rthe susceptibility of myeloma cells to Fas (Apo-1/CD95)-mediated apoptosis [23,24]. This cell activation marker is apparently over-expressed on immature B cells and several malignant plasma cell clones, though the range of its responsiveness to agonist MoAbs varies greatly in myeloma cell lines as well as in native myeloma cells [25]. Although both lack of membrane Fas expression and high Bcl-2 content in these cells have been proposed as mechanisms of resistance to Fas-induced cell death [26], the presence of a soluble inhibitory factor of Fas pathway has also been postulated, since a similar resistance is displayed by mononuclear cells from bone marrow of myeloma patients [24]. In addition, FasL, a type II glycoprotein which activates Fas either in membrane-bound manner or in soluble form [27], is variably expressed by myeloma cells. This death factor is perhaps constitutively committed to the autologous control of proliferation. However, its over-expression on malignant plasma cells is in plain discordance with their proliferative rate.
The effect of IL-6 on apoptosis of myeloma cells is not completely understood. Recent studies support its ability to modulate their apoptosis in Fas-sensitive cell lines induced by anti-Fas agonist MoAb(s) [25]. This inhibitory effect is apparently induced by a peculiar effect of IL-6 on stress-activated protein kinase (SAPK) activity which, in association with p38 mitogen-activated protein kinase (MAPK), is postulated to play an essential role in the activation of transcription factors related to Fas-regulated apoptosis [2,28].
The present study explores the effect of IL-6 on spontaneous and Fas-induced apoptosis in both IL-6-dependent and -independent myeloma cell cultures cloned from patients at different clinical stages. Our data suggest that only IL-6-dependent myeloma cells are protected by the cytokine in their spontaneous apoptosis, and that the amplitude of Fas-induced cell death parallels their sensitivity to the cytokine. Since myeloma cells can regulate their own proliferation by both autocrine and paracrine control through the Fas/FasL pathway, it is conceivable that highly malignant Fas− cells up-regulate FasL expression to escape immune suppression by cytotoxic cells, as reported in several T cell-derived neoplasms [29], solid tumours [30–32], and other myeloma cell lines [33].
MATERIALS AND METHODS
Myeloma cells and clones
Seven myeloma cell preparations were included in this study in parallel with the established U-266 myeloma cell line obtained from the American Type Culture Collection (ATCC, Rockville, MD). Mononuclear cells were obtained from the bone marrow of myeloma patients at different clinical stages according to Durie & Salmon [34] after Ficoll–Hypaque gradient centrifugation. The myeloma cells were enriched by discontinuous density gradient, including the Percoll solution at 90–30%. Plasma cells collected at both 60% and 45% gradient concentrations were enriched to > 80%.
Cell preparations were investigated by immunofluorescence analysis, whereas parallel aliquots including 1 × 106 cells were cultured in complete medium (RPMI 1640 plus 10% fetal calf serum (FCS) and 2 mml-glutamine) in the presence or absence of IL-6 500 U/ml (Genzyme, Cambridge, MA). The cultures were then maintained in a CO2 incubator for 3 additional weeks in order to establish continuous proliferation. The amplitude of myeloma cell enrichment was monitored weekly by cytofluorimetric analysis. Cultures including 90% or more plasma cells were considered suitable for the study.
Cellular analysis and measurement of apoptosis
Both fresh plasma cells and established myeloma cell clones (MCC) were phenotypically characterized cytofluorimetrically by comparing their cytoplasmic immunoglobulin (cIg) content with their membrane expression of CD38, Fas, FasL, and IL-6R. cIg was detected by treating the cells with 0.25% paraformaldehyde solution and subsequent permeabilization with cold methanol prior to their incubation with a polyclonal FITC-conjugated anti-human κ- or λ-chain antiserum (Jackson Immunoresearch, West Grove, PA). The relative gate of cIg+ cells was adopted to identify the plasma cell population. In addition, phenotype analysis included both single- and double-fluorescence techniques using the PE-conjugated MoAb to CD38 (Becton Dickinson, Mountain View, CA), the FITC MoAb IgG1 to Fas from clone UB2 (Immunotech, Marseille, France), the biotinylated IgG1κ to FasL from clone NOK-1 (Pharmingen, San Diego, CA), and the Fluorokine Kit (R&D Systems, Minneapolis, MN) to detect the IL-6R. We also measured the intracellular expression of Bcl-2 by treating the MCC with an FITC-conjugated MoAb, according to the procedure suggested by the manufacturer (Dako, Glostrup, Denmark), which included permeabilization of both cell and nuclear membranes with methanol. Cytometric analysis was completed with the Cell Quest Program in a FACScan (Becton Dickinson).
The level of Fas, FasL and IL-6R expression per MCC was also evaluated as a ratio of the mean fluorescence per antigen on MCC and their mean fluorescence at the cut-off point, at which a specific signal was considered to be positive.
The extent of apoptosis in each MCC was measured by propidium iodide (PI) cell staining [35]. In particular, 1 × 106 cells from each sample were treated with 70% ethanol at 4°C for 3 h, then incubated overnight with 100 μg PI in the presence of RNase prior to flow cytometry. The extent of the subdiploid DNA peak reflected the percentage of apoptotic cells. Fas- and doxorubicin (DOX)-induced apoptosis were also investigated. We incubated the cells with up to 1 μg/ml of agonist anti-Fas IgM from clone CH11 (Immunotech) for 20 h, whereas parallel experiments evaluated the amplitude of apoptosis after treating MCC with 0.01–10 μg/ml of DOX (Sigma, St Louis, MO) for 30 h. Parallel cell suspensions were incubated in the absence of the apoptogen drug to provide proper controls, whereas apoptosis was also estimated indirectly by performing the proliferative rate test in both apoptosis-prompted and control cultures.
Proliferation assay
3H-thymidine labelling was used to measure the proliferation of MCC in relation to the presence of IL-6 and determine the inhibition of cell proliferation in Fas- and DOX-stimulated cell preparations. Cells (1 × 105) from each MCC were incubated overnight in the presence of 3H-thymidine (7400 Bq/well) and its uptake was evaluated in a β-counter (Beckman, Palo Alto, CA), as described [36]. The average of 19 000 ct/min uptake of the U-266 cell line served as the control of myeloma cell proliferation in the presence of IL-6. Proliferation following incubation with the agonist anti-Fas IgM and with DOX was also evaluated. Its inhibition reflected apoptosis and was calculated in relation to control cellular suspensions as follows: (ct/min of Fas- or DOX-stimulated MCC/ct/min of unstimulated MCC) × 100.
Fas stimulation by anti-Fas MoAb and DOX, in presence of IL-6, was also evaluated by the bromodeoxyuridine (BrdU)/PI technique, according to the procedure suggested by the manufacturer (Becton Dickinson). Since BrdU is a thymidine analogue, it can be incorporated specifically into DNA, thus allowing the identification of cells that undergo DNA synthesis. In addition, cell proliferation and apoptosis were studied by cytofluorimetric analysis of BrdU incorporation and PI staining. Briefly, 1 × 106 cells from each MCC were incubated for 2 h in the presence of BrdU (15 μm; Sigma). Then cells were fixed with 70% cold ethanol and treated with 2 n HCl to partially denature DNA. After washing with 0.1 m Na2B4O7 to neutralize the acid, cell suspensions were incubated with FITC-conjugated MoAb to BrdU. Finally, cells were resuspended in 5 μg/ml PI solution and analysed by flow cytometer after 1 h.
Analysis of FasL secretion
The cytofluorimetric detection of FasL on MCC was also investigated in relation to its cytocidal property in the [3-(4,5-dimethylthiazol-2-yl)-2,5diphenyl tetrazolium bromide] (MTT) assay and by PI staining of target cells. The first procedure is a colorimetric method based on cleavage of yellow tetrazolium salt (MTT; Sigma) by active mitochondria to form a dark blue formazan product. It was used to measure the percentage of Fas+ killed cells with the WC8 cell line, namely a human Fas-transfected mouse T cell lymphoma, and its negative mutant WR19L as control (kindly provided by Dr S. Nagata, Osaka, Japan). The assay included a 40-h incubation of 1 × 105 target cells with each MCC culture supernatant (SN) and, in parallel, in the presence of control culture medium. Then 100 μl of MTT solution (2 mg/ml) were added to each well. After 4 h at 37°C, the dark blue formazan crystals were dissolved by adding 100 μl of dimethyl sulfoxide (DMSO; Sigma) and finally the 96-well plates were read in a spectrophotometer (Titertek Flow, Irvine, UK) at both 560 nm and 630 nm as measurement and reference wavelengths, respectively. The percentage of dead cells in each sample was calculated as: (100 − (OD of target cell + SN/OD of target cell + control medium)) × 100. Parallel suspensions of both WC8 and WR19L cells incubated with each SN were stained with PI and the relative content of subdiploid DNA peak was measured by the FACScan, as described [36].
mRNA isolation from MCC, cDNA synthesis, cloning and sequencing of Fas
mRNA was isolated from 1 × 106 cells from each MCC according to the guanidine thiocyanate-caesium chloride procedure (Invitrogen, Celbio Srl, Pero, Italy). Transcription of mRNA into first-strand cDNA was performed with the Boehringer-Mannheim (Milan, Italy) cDNA kit. Fas wild-type-specific primers were designed in relation to the described gene structure [23] as follows: 5′-ATG CTG GGC ATC TGG ACC CT-3′ (FW: pos. 195) and 5′-TCT AGA CCA AGC TTT GGA TTT C-3′ (RV: pos. 1178) as external primers, whereas 5′-GGG AAG GAG TAC ACA GAC AAA-3′ (FW: pos. 456) and 5′-GAC ACC ATT CTT TCG AAC AAA GCC-3′ (RV: pos. 935) were the internal primers. cDNA (100 ng) and 50 pmol of each primer were added to the polymerase chain reaction (PCR) mixture (Perkin Elmer Cetus, Norwalk, CT) with subsequent amplification in a thermal cycler for 35 cycles (1 min 94°C/1 min 58°C/1 min 72°C), which in our system were found to provide suitable amounts of PCR product. This was then analysed on a 2% agarose gel, cloned in the pCRTMII vector (Invitrogen) and amplified in DH5α bacterial strain (Gibco BRL, Milan, Italy) for sequencing by high-voltage electrophoresis on a 6% urea-bis-acrylamide gel with the Sequenase 2.0 kit (Amersham, Aylesbury, UK).
This analysis included the amplification of Fas cDNA from U-266 cell line as control.
Statistical analysis
Mean values of specific phenotype expression and in vitro parameters were compared by Student's t-test and, in several instances, by the Wilcoxon test as a non-parametric method.
RESULTS
Unequal expression of CD38, Fas and FasL on myeloma cells
Phenotypic analysis of freshly isolated plasma cells and MCC is shown in Table 1. cIg, IL-6R and Bcl-2 expression was essentially uniform in all samples, whereas CD38 ranged from very high percentages to ≤ 10% in MCC-2, -3 and -5. Fas expression was also minimal in MCC-2 and -7 (≤ 5%), while the distribution of FasL ranged from 10% in MCC-1 to 99% in MCC-2.
Table 1.
Patients and the phenotype of their myeloma cells.
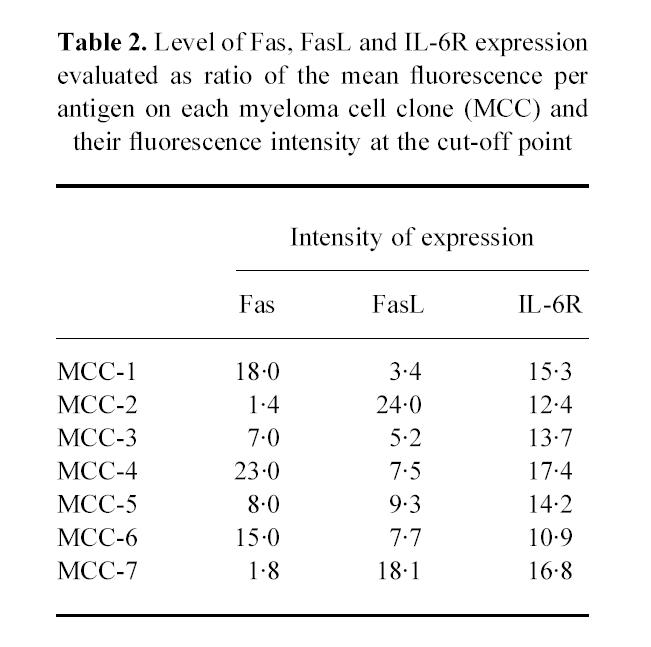
In addition, to compare the levels of Fas, FasL and IL-6R expression obtained from each MCC, a ratio of the mean fluorescence intensity (MFI) per antigen-expressing cell and the fluorescence intensity at the cut-off point was calculated. As shown in Table 2, the intensity of Fas and FasL expression was different on each MCC. In particular, MCC-1, -4 and -6 showed the highest level of Fas expression, whereas FasL was up-modulated on MCC-2 and -7. Conversely, the intensity of IL-6R staining was similar in all samples.
Table 2.
Level of Fas, FasL and IL-6R expression evaluated as ratio of the mean fluorescence per antigen on each myeloma cell clone (MCC) and their fluorescence intensity at the cut-off point
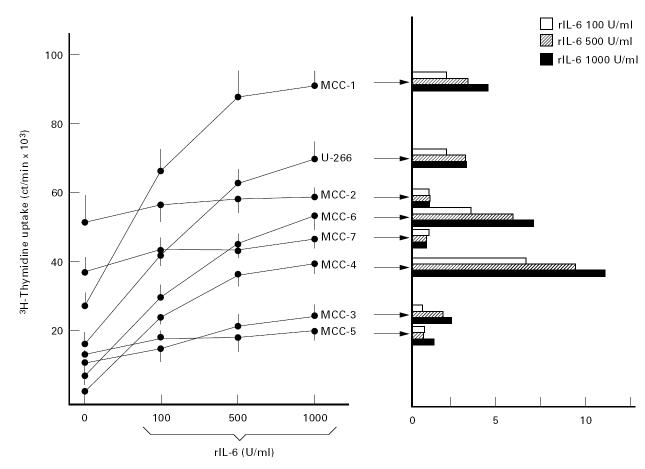
Proliferation of MCC is variably sensitive to IL-6
3H-thymidine uptake, calculated as the extent of the proliferative rate of MCC in the absence or presence of IL-6, indicated a differential sensitivity of clones to IL-6 (Fig. 1). Proliferation paralleled the progressive increase of IL-6 concentration, in particular in MCC-1, -4 and -6 whose uptake was significantly increased by 500 U/ml of the cytokine (P < 0.05 in all instances). The maximum uptake was recorded in MCC-1. Enhanced proliferation in the presence of 500 or 1000 U/ml IL-6, however, was evident in MCC-1, -4 and -6 and resembled U-266 cell growth, but not in MCC-2, -3, -5 and -7, whose uptake was virtually stable. As a result of this insensitivity, their stimulation index (SI) was below the significance limit 3.0 ± 0.37.
Fig. 1.
Proliferative assay of seven myeloma cell clones (MCC) in the presence of increasing amounts of IL-6 compared with their relative stimulation index (SI). MCC-2 and -7 showed a high extent of spontaneous proliferation which was not influenced by the presence of IL-6. A similar insensitivity was detected in MCC-3 and -5, whereas the remaining clones, including the control U-266 myeloma cell line, increased their 3H-thymidine uptake in response to IL-6, thus providing a positive SI (≥ 3.0).
Susceptibility of Fas in MCC parallels their IL-6 sensitivity
The next experiments compared the sensitivity of IL-6-dependent and independent MCC to Fas stimulation, through evaluation of their response to Fas ligation by the CH11 agonist MoAb and by increasing concentrations of DOX, whose apoptogenic potential is promoted by massive autocrine production of FasL [37]. Depressed proliferation was considered as the effect of these stimulations (Fig. 2). The expected effect of the anti-Fas MoAb was only observed in MCC-1, -4 and -6, whose inhibition was particularly evident with 0.1 μg/ml, whereas MCC-2, -3, -5 and -7, like U-266 [38], were totally insensitive (Fig. 2a). DOX produced a similar response (Fig. 2b), with the highest inhibition when MCC-1, -4 and -6 were treated with 1–10 μg/ml.
Fig. 2.
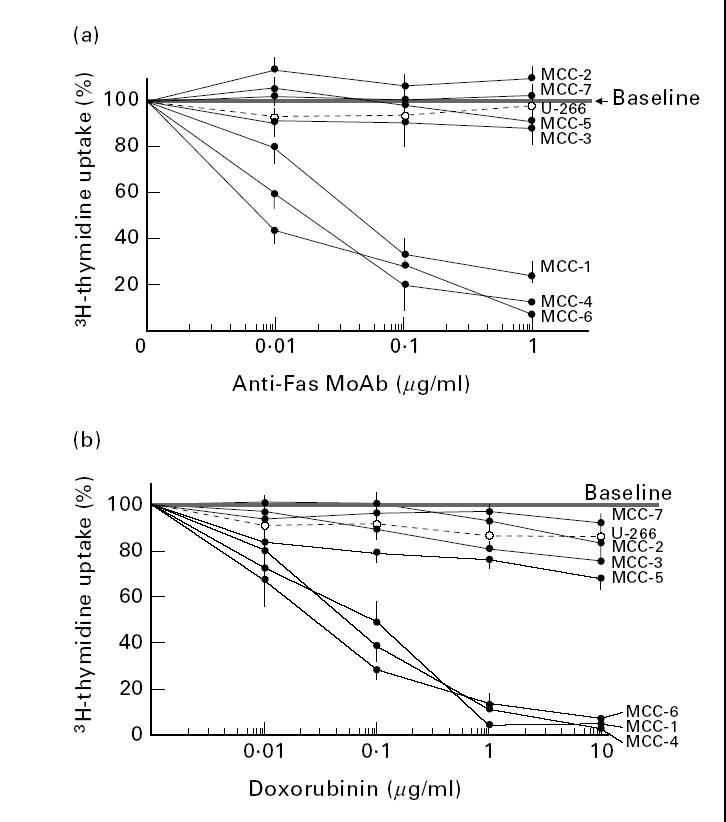
Profiles of response to Fas ligation by the relative MoAb from clone CH11 (a) and by autologous FasL induced in cultures by treatment of myeloma cell clones (MCC) with doxorubicin (b). The assay measured suppression of proliferation as the extent of Fas-induced apoptosis. In both assays MCC-1, -4 and -6 were significantly suppressed in a dose-dependent fashion, whereas MCC-2, -3, -5 and -7 were slightly affected only at the highest concentrations. The control U-266 myeloma cell line provided a similar pattern of Fas-insensitivity.
The susceptibility of MCC to Fas stimulation was also evaluated by BrdU/PI experiments. Figure 3 shows the results obtained in two representative MCC, namely the IL-6-independent MCC-2 and the strictly IL-6-dependent MCC-4 (SI = 13). The addition of anti-Fas MoAb (0.1 μg/ml) induced cell apoptosis (52.3%) and inhibited cell proliferation (4.1%) of MCC-4, whose growth was promptly increased by IL-6 (500 U/ml) (61.8%). By contrast, no effect was observed on MCC-2. Similar responses were obtained in all IL-6-dependent and -independent MCC using CH11 or DOX as apoptotic stimuli (data not shown).
Fig. 3.
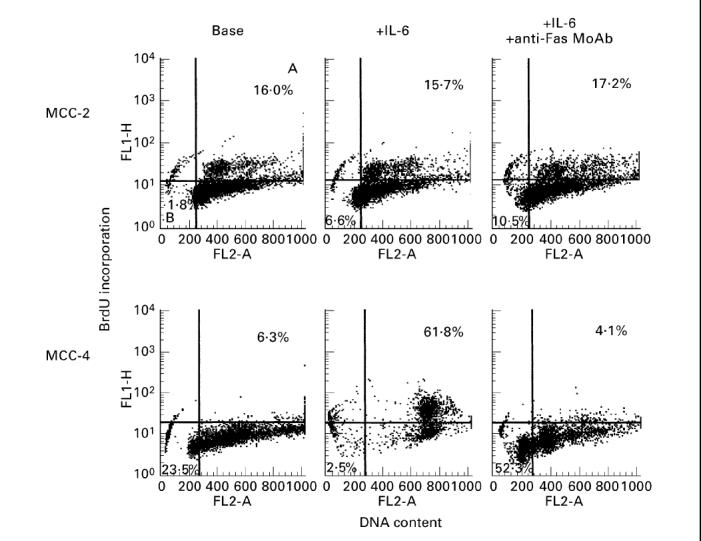
Profiles of response to Fas ligation by the relative MoAb from clone CH11 in presence of IL-6 in two representative myeloma cell clones (MCC), evaluated by bromodeoxyuridine (BrdU)/propidium iodide (PI) cell incorporation. A, Percentage of proliferating cells; B, percentage of apoptotic cells with subdiploid DNA content. The addition of anti-Fas MoAb induced cell apoptosis and inhibited cell proliferation of the IL-6-dependent MCC-4; no effect was observed on MCC-2.
IL-6 and Fas-regulated apoptosis in MCC
Since IL-6 rescues cells from dexamethasone-induced apoptosis [21], we explored its influence on both Fas- and DOX-induced apoptosis in IL-6-dependent and -independent MCC by cytofluorimetric analysis of DNA content following PI staining. The two groups of MCC displayed different response patterns (Table 3). Spontaneous apoptosis in MCC-1, -4 and -6 was significantly reduced (P < 0.05), whereas the amplitude of that induced by either the anti-Fas MoAb or DOX was influenced only slightly. The apoptotic populations of clones 2, 3, 5 and 7 were < 10%. As expected, both anti-Fas and DOX failed to increase their amplitude and the presence of IL-6 did not alter spontaneous apoptosis. It was clear that IL-6 can only rescue IL-6-sensitive myeloma cells from spontaneous apoptosis and has little effect on that induced by Fas.
Table 3.
Effect of IL-6 on spontaneous and Fas-driven apoptosis of myeloma cell clones (MCC)
FasL expression is variably down-modulated by IL-6
We also measured the variation of FasL expression in MCC treated with IL-6 by incubating 2 × 106 cells per clone with 500 U/ml IL-6 for 90 min before phenotypic analysis. Two profiles of response to IL-6 were evident: MCC-4, -5 and -6 showed an appreciable decrease of FasL expression, whereas the ligand disappeared on IL-6-treated MCC-1 and -3. Conversely, the magnitude of FasL+ population in MCC-2 and -7 remained apparently intact. The cytofluorimetric patterns of these responses are illustrated in Fig. 4.
Fig. 4.
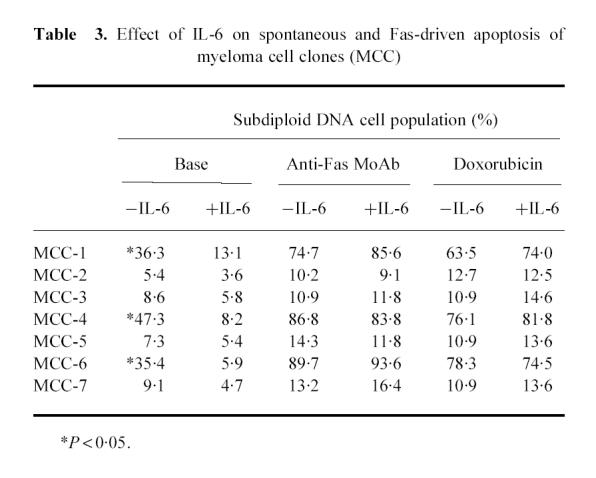
Representative cytofluorimetric patterns of variation in FasL expression after IL-6 treatment of myeloma cell clones (MCC) (right panels). IL-6-insensitive MCC-2 and -7 were not affected by IL-6 incubation, in contrast to the IL-6-sensitive MCC-4 and -6, which showed a variable down-modulation of their FasL cellular expression.
sFasL secretion is prominent in IL-6-insensitive MCC
Further analysis explored the extent of FasL secretion by MCC by measuring the cytocidal properties of their supernatants. Both WC8 and WR19L cells were used as Fas+ and Fas− cellular targets, respectively, and the assays included the MTT test.
Figure 5 illustrates the percentage of killing induced on WC8 cells after their incubation with the supernatants from MCC. Parallel experiments used the DOX-conditioned supernatants from MCC as positive controls of FasL release in culture medium. The variable cytotoxicity of these supernatants apparently reflected the constitutive expression of FasL on their clones. Indeed, although the cytocidal effect on WC8 cells of supernatants from MCC-1, -3 and -5 was arbitrarily considered as low (< 20% of killing), in contrast with moderate elevations (range 20–60% of killing) for supernatants from MCC-4 and -6, the highest extent (> 60%) was induced by those from MCC-2 and -7. As expected, cytotoxicity was comparably increased in all supernatants from cultures using DOX to stimulate both production and release of the death factor. Finally, the effect was completely absent in all instances with WR19L (data not shown), thus confirming that the cell death of WC8 cells occurred through Fas.
Fig. 5.
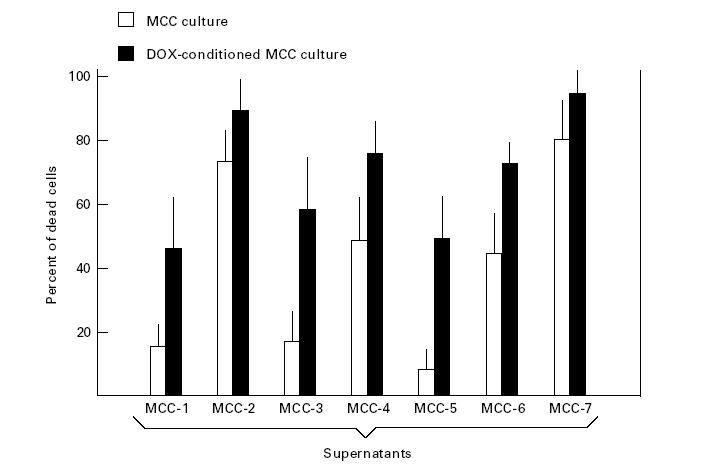
[3-(4,5-dimethylthiazol-2-yl)-2,5diphenyl tetrazolium bromide] (MTT) test measuring the cytocidal effect of supernatants from myeloma cell clones (MCC) as extent of spontaneous soluble FasL secretion. The assay used the Fas+ WC8 cells as target. A variable cytotoxicity was observed in all supernatants, though MCC-2 and -7 showed significant elevations (P < 0.05). The doxorubicin (DOX)-conditioned medium provided the positive control of FasL secretion by the corresponding cultures.
Molecular cloning of Fas cDNA revealed derangements in IL-6-insensitive MCC
Since MCC showed a variable susceptibility to apoptosis following the ligation of Fas by either the relative MoAb or its ligand in DOX-conditioned cultures, we evaluated the genetic expression of the receptor in these cell lines. The analysis included PCR amplification, cloning and sequencing of Fas cDNA from MCC. We observed a pattern of dissimilarities in PCR products. Fas cDNA from MCC-1, -4 and -6 was identical in structure to the wild-type molecule as a result of their complete homology to cDNA from U-266 cells. By contrast, in addition to the expected fragment related to the full-length cDNA of 1008 bp, a product of about 836 bp was observed, particularly in MCC-3 and -5 (Fig. 6). Sequence analysis of both products revealed their complete identity to the previously reported Fas Exo4,6Del variant of Fas [39], which is defined by the deletion of both exons 4 and 6. Sequence data were in line with the size of the amplified products and with the known Fas sequence [40], with the exception of the described polymorphisms at positions 529 (exon 4) and 700 (exon 6), which are related to the defective extracytoplasmic region and the lack of the transmembrane domain of the molecule, respectively.
Fig. 6.
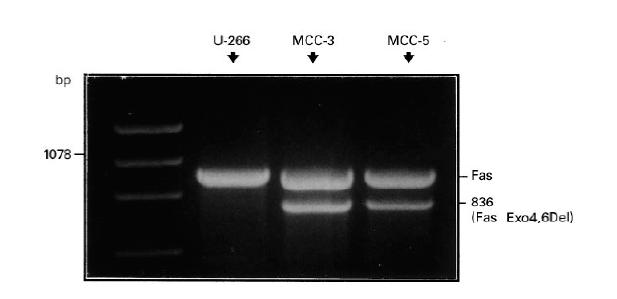
Fas cDNA from MCC-3 and -5 showing a smaller polymerase chain reaction (PCR) product with respect to the full-length Fas (wild type) detected in the control U-266 myeloma cell line.
However, a major derangement of Fas occurred in both MCC-2 and -7, since PCR amplification repeatedly demonstrated the complete absence of the expected product(s) (data not shown).
IL-6 insensitivity and apoptosis disorders as related to the extent of malignancy
Several parameters were considered to provide an arbitrary estimation of malignancy in MCC. We reasoned that since IL-6 insensitivity, defective spontaneous apoptosis, resistance to Fas ligation, FasL over-secretion, and deregulation of Fas expression were not regular events of differentiated plasma cells, their occurrence on myeloma cells could reflect malignancy. Thus, cellular derangements in MCC were arbitrarily related to the results of our functional tests. IL-6 insensitivity was attributed to MCC with the IL-6 SI < 3.0 (Fig. 1), whereas those showing no effect in their proliferative rate following treatment with agonist levels (≥ 0.1 μg/ml) of anti-Fas MoAb were considered resistant to Fas-induced apoptosis. Moreover, the defect of spontaneous apoptosis was assessed as percentage of subdiploid cell population < 13.8%, which was the lower significance limit observed in previous evaluations of spontaneous apoptosis in other in vitro established lines, including WC8 [36,41] cells (Table 3). The amplitude of FasL secretion was defined by the MTT test in relation to control experiments as high (+ +, killing > 60%), intermediate (+ −, range of killing 20–60%), or low (− −, killing < 20%). As illustrated in Table 4, the concurrency of the majority of derangements was evident in four out of seven MCC (MCC-2, -3, -5 and -7), whereas we failed to detect a similar combination in clones 1, 4 and 6. This differentiation was used to form an arbitrary distinction between high-, intermediate- or low-malignancy MCC.
Table 4.
Correlation of proliferative response to IL-6 with multiple deregulations of apoptosis in seven myeloma cell clones (MCC).
DISCUSSION
The present study focused the defective apoptosis, either spontaneous or mediated by Fas, in myeloma cells in relation to their proliferative response to IL-6, a cytokine which acts as a major growth factor for plasma cells [1,2,7,10] and rescues malignant plasma cells from apoptosis induced by dexamethasone or starvation [21]. We found that it also protects them from spontaneous apoptosis, though this effect is confined to clones whose proliferation is fully dependent on adequate levels of IL-6 in culture and does not extend to their Fas-regulated apoptosis. By contrast, resistance to Fas-related apoptosis as a result of multiple derangements of Fas pathway occurred in MCC whose proliferative rate was insensitive to IL-6. Therefore, we speculated that the high rate of IL-6-independent cell proliferation associated with relative preservation from Fas-regulated apoptosis could reflect high malignancy. In addition, the occurrence of abundant FasL secretion in several Fas− MCC argues for a cytotoxic mechanism capable of neutralizing the immune suppression, as described in other highly malignant tumours [32].
The IL-6 receptor and its signal transducer, namely gp130, are expressed on most myeloma cells. It has been emphasized that IL-6 is essential for replication of myeloma cell lines [12,13] and native MCC [19,20,24] and, although it is prevalently considered a paracrine growth factor [14], it seems likely that highly immature myeloma cells produce IL-6 as an autocrine factor of proliferation and differentiation [42]. We found that both proliferation and the SI of some in vitro established native MCC were mostly unchanged in the presence of increasing levels of IL-6, suggesting that it was not necessary for their autocrine or paracrine expansion. Since MCC-2, -3, -5 and -7, though they expressed IL-6R on their surface, showed no variations of their 3H-thymidine uptake in the presence of IL-6, we speculated that their IL-6-independent replication was related to high cellular immaturity. Their relative defect of CD38 expression could support this interpretation. Another intriguing hypothesis could be related to N-ras oncogene mutations in IL-6-independent MCC. Indeed, it has been shown [43] that introduction of an activated N-ras oncogene results in an IL-6-independent growth of the IL-6-dependent myeloma cell line ANBL, suggesting that activated mutations of ras oncogene could replace the IL-6 function and allow IL-6-independent MCC growth [44]. Further studies to verify this hypothesis are in progress in our laboratory.
A second important aspect concerns the different susceptibility of our MCC to either spontaneous or Fas-triggered apoptosis. Contrary to the variable extent of subdiploid cell population occurring in IL-6-dependent myelomas, which was down-modulated by the cytokine, the IL-6-independent clones showed a substantial defect of apoptosis. Indeed, this function was found to be suppressed both with and without the stimulation induced by Fas ligation or DOX treatment of cells. Since this drug acts through the Fas pathway by increasing the FasL mRNA expression for the autocrine induction of cell death [37], we concluded that the defective or deregulated expression of Fas in IL-6-independent clones could account for their resistance to Fas-induced apoptosis. In this context, the demonstration of Fas Exo4,6Del variant on MCC-3 and -5, as well as the complete absence of Fas cDNA on MCC-2 and -7, could represent major genetic deregulations preserving these tumour cells from their Fas-regulated control of proliferation. On the other hand, both the variability of responsiveness and the constitutive defect of Fas in malignant plasma cells have also been reported by others [25,33].
An unexpected finding of our study is the over-expression of FasL on several Fas− myelomas, including MCC-2 and -7. In contrast to the down-modulation of FasL observed in IL-6-dependent clones following IL-6 treatment, no quantitative variations of FasL expression were detected in IL-6-independent MCC. Functional tests using the supernatants from MCC-2 and -7 confirmed their large production of FasL in its soluble form, which was further increased in parallel cultures using the DOX-conditioned medium. These supernatants were highly cytocidal on WC8 cells in both MTT and PI assays. However, the secretion of FasL in such MCC was not related to the autocrine cell death because of the constitutive defect of Fas on their cell membranes. Therefore, as recently proposed for other Fas− solid tumours, including melanoma [45], astrocytoma [31] and other neoplasms of T cell lineage [29], over-expression of FasL may represent an active mechanism implicated in the tumour immune escape. This mechanism is physiologically activated in cells of the anterior chamber of the eye, in Sertoli cells of testis [46,47], and in uterus [48] to maintain a state of immune privilege in those organs by eliminating Fas+ infiltrating leucocytes. Similarly, malignant tumours counterattack the tumour-infiltrating lymphocytes by delivering their FasL to prevent host immune suppression [32]. Since we have demonstrated an identical event on a few Fas− myelomas, we believe that only highly malignant plasma cells can exert this mechanism of immune evasion, as recently described in certain myeloma cell lines [33].
Our overall results suggest that deregulated Fas apoptosis reflects a malignant phenotype of myeloma cells apparently activated to elude immune surveillance. In our myeloma clones, we identified two mechanisms of immune escape. First, production of a soluble splicing variant of Fas, as detected in MCC-3 and -5, to disable FasL of cytotoxic cells. The second mechanism occurred in Fas− myelomas (MCC-2 and -7) and resembled a recently defined function in tumour cells which present FasL to immune-competent cells. Our attribution of high- or intermediate-grade malignancy to these myelomas is indirectly supported by the rapidly progressing clinical picture in three of the four corresponding patients, whereas clones 1, 4 and 6 were evidently of low-grade malignancy, since both their spontaneous and their Fas-regulated apoptosis were apparently unaffected.
Acknowledgments
This work was supported by a grant from MURST (Ministry of University and Scientific and Technological Research), Rome, 1998. P.C. is recipient of a fellowship from the AIDS Research Project (1997) of the Italian Ministry of Health, Istituto Superiore di Sanità, Rome.
REFERENCES
- 1.Zhang X-G, Klein B, Bataille R. Interleukin-6 is a potent myeloma-cell growth factor in patients with aggressive multiple myeloma. Blood. 1989;74:11–13. [PubMed] [Google Scholar]
- 2.Klein B, Zhang X-G, Lu Z-Y, et al. Interleukin-6 in human multiple myeloma. Blood. 1995;85:863–72. [PubMed] [Google Scholar]
- 3.Banchereau J, Rousset F. Human B lymphocytes: phenotype, proliferation and differentiation. Adv Immunol. 1992;52:125–33. doi: 10.1016/s0065-2776(08)60876-7. [DOI] [PubMed] [Google Scholar]
- 4.Kishimoto T, Taga T, Akira S. Cytokine signal transduction. Cell. 1994;76:253–8. doi: 10.1016/0092-8674(94)90333-6. [DOI] [PubMed] [Google Scholar]
- 5.Kawano M, Tanaka H, Ishikawa H, et al. Interleukin-1 accelerates autocrine growth of myeloma cells through interleukin-6 in human myeloma. Blood. 1989;73:2145–8. [PubMed] [Google Scholar]
- 6.Carter A, Merchav S, Silvian-Draxler I, et al. The role of interleukin-1 and tumor necrosis factor-α in human multiple myeloma. Br J Haematol. 1990;74:424–31. doi: 10.1111/j.1365-2141.1990.tb06330.x. [DOI] [PubMed] [Google Scholar]
- 7.Bergui L, Schena M, Gaidano G, et al. Interleukin-3 and interleukin-6 synergistically promote the proliferation and differentiation of malignant plasma cell precursors in multiple myeloma. J Exp Med. 1989;170:613–20. doi: 10.1084/jem.170.2.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Merico F, Bergui L, Gregoretti MG, et al. Cytokines involved in the progression of multiple myeloma. Clin Exp Immunol. 1993;92:27–31. doi: 10.1111/j.1365-2249.1993.tb05943.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taetle R, Dos Santos B, Ohsugi Y, et al. Effect of combined antigrowth factor receptor treatment on in vitro growth of multiple myeloma. J Natl Cancer Inst. 1994;86:450–5. doi: 10.1093/jnci/86.6.450. [DOI] [PubMed] [Google Scholar]
- 10.de Hon FD, Ehlers M, Rose-John S, et al. Development of an interleukin (IL)-6 receptor antagonist that inhibits IL-6-dependent growth of human myeloma cells. J Exp Med. 1994;180:2395–400. doi: 10.1084/jem.180.6.2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klein B, Wijdenes J, Zhang K-G, et al. Murine anti-interleukin-6 monoclonal antibody therapy for a patient with plasma cell leukemia. Blood. 1991;78:1198–203. [PubMed] [Google Scholar]
- 12.Bataille R, Barlogie B, Lu ZY, et al. Biologic effects of anti-interleukin-6 murine monoclonal antibody in advanced multiple myeloma. Blood. 1995;86:685–91. [PubMed] [Google Scholar]
- 13.Kawano M, Hirano T, Matsuda T, et al. Autocrine generation and requirement of BSF-2/IL-6 for human multiple myelomas. Nature. 1988;332:83–84. doi: 10.1038/332083a0. [DOI] [PubMed] [Google Scholar]
- 14.Klein B, Zhang XG, Jourdan M, et al. Paracrine rather than autocrine regulation of myeloma-cell growth and differentiation by interleukin-6. Blood. 1989;73:517–26. [PubMed] [Google Scholar]
- 15.Caligaris-Cappio F, Bergui L, Gregoretti MG, et al. Role of bone marrow stromal cells in the growth of human multiple myeloma. Blood. 1991;77:2688–93. [PubMed] [Google Scholar]
- 16.Cohen PL, Einsenberg RA. Single gene models of systemic autoimmunity and lymphoproliferative disease. Annu Rev Immunol. 1991;9:243–70. doi: 10.1146/annurev.iy.09.040191.001331. [DOI] [PubMed] [Google Scholar]
- 17.Raff MC. Social controls on cell survival and cell death. Nature (London) 1992;356:397–9. doi: 10.1038/356397a0. [DOI] [PubMed] [Google Scholar]
- 18.Lynch DH, Ransdell F, Alderson MR. Fas and FasL in the homeostatic regulation of immune responses. Immunol Today. 1995;16:569–74. doi: 10.1016/0167-5699(95)80079-4. [DOI] [PubMed] [Google Scholar]
- 19.Levy Y, Brouet JC. Interleukin-10 prevents death of germinal center B cells by induction of the Bcl-2 protein. J Clin Invest. 1994;93:424–8. doi: 10.1172/JCI116977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Milner AE, Grand RJA, Gregory CD. Effects of interferon-α on human B cells: repression of apoptosis and prevention of cell growth are independent responses of Burkitt lymphoma lines. Int J Cancer. 1995;61:348–54. doi: 10.1002/ijc.2910610313. [DOI] [PubMed] [Google Scholar]
- 21.Lichtenstein A, Tu Y, Fady C, et al. Interleukin-6 inhibits apoptosis of malignant plasma cells. Cell Immunol. 1995;162:248–55. doi: 10.1006/cimm.1995.1076. [DOI] [PubMed] [Google Scholar]
- 22.Mandelli F, Avvisati G, Amadori S, et al. Maintenance treatment with recombinant interferon alfa-2b in patients with multiple myeloma responding to conventional induction chemotherapy. N Engl J Med. 1990;322:1430–4. doi: 10.1056/NEJM199005173222005. [DOI] [PubMed] [Google Scholar]
- 23.Itoh N, Yonehara S, Ishii A, et al. The polypeptide encoded by the cDNA of human cell surface antigen Fas can mediate apoptosis. Cell. 1991;66:233–9. doi: 10.1016/0092-8674(91)90614-5. [DOI] [PubMed] [Google Scholar]
- 24.Egle A, Villunger A, Kos M, et al. Modulation of Apo-1/Fas (CD95)-induced programmed cell death in myeloma cells by interferon-α2. Eur J Immunol. 1996;26:3119–26. doi: 10.1002/eji.1830261244. [DOI] [PubMed] [Google Scholar]
- 25.Shima Y, Nishimoto N, Ogata A, et al. Myeloma cells express Fas antigen/Apo-1 (CD95) but only some are sensitive to anti-Fas antibody resulting in apoptosis. Blood. 1995;85:757–64. [PubMed] [Google Scholar]
- 26.Tu Y, Xu F, Lin J, et al. Upregulated expression of Bcl-2 in multiple myeloma cells induced by exposure to doxorubicin, etoposide, and hydrogen peroxide. Blood. 1996;88:1805–12. [PubMed] [Google Scholar]
- 27.Tanaka M, Suda T, Takahashi T, et al. Expression of soluble form of human Fas ligand in activated lymphocytes. EMBO J. 1995;14:1129–35. doi: 10.1002/j.1460-2075.1995.tb07096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chauhan D, Kharbanda S, Ogata A, et al. Interleukin-6 inhibits Fas-induced apoptosis and stress-activated protein kinase activation in multiple myeloma cells. Blood. 1997;89:227–34. [PubMed] [Google Scholar]
- 29.Tanaka M, Suda T, Haze K, et al. Fas ligand in human serum. Nature Med. 1996;2:317–22. doi: 10.1038/nm0396-317. [DOI] [PubMed] [Google Scholar]
- 30.O'Connell J, O'Sullivan JK, Shanahan F. The Fas counterattack: Fas-mediated T cell killing by colon cancer cells expressing Fas ligand. J Exp Med. 1996;184:1075–83. doi: 10.1084/jem.184.3.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saas P, Walker PR, Hahne M, et al. Fas ligand expression by astrocytoma in vivo: maintaining immune privilege in the brain? J Clin Invest. 1997;99:1173–8. doi: 10.1172/JCI119273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Walker PR, Saas P, Dietrich PY. Role of Fas ligand (CD95L) in immune escape: the tumor cell strikes back. J Immunol. 1997;158:4521–4. [PubMed] [Google Scholar]
- 33.Villunger A, Egle A, Marschitz I, et al. Constitutive expression of Fas (APO-1/CD95) ligand on multiple myeloma cells: a potential mechanism of tumor-induced suppression of immune surveillance. Blood. 1997;90:12–20. [PubMed] [Google Scholar]
- 34.Durie BMG, Salmon SE. Multiple myeloma, macroglobulinemia and monoclonal gammopathies. In: Hoffbrand A V, Brain MC, Hirsh J, editors. Recent advances in haematology. New York Livingstone: Churchill; 1977. pp. 243–58. [Google Scholar]
- 35.Nicoletti I, Migliorati G, Pagliacci MC, et al. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J Immunol Methods. 1991;139:271–5. doi: 10.1016/0022-1759(91)90198-o. [DOI] [PubMed] [Google Scholar]
- 36.Silvestris F, Cafforio P, Frassanito MA, et al. Overexpression of Fas antigen on T cells in advanced HIV-1 infection: differential ligation constantly induces apoptosis. AIDS. 1996;10:131–41. doi: 10.1097/00002030-199602000-00002. [DOI] [PubMed] [Google Scholar]
- 37.Friesen C, Herr I, Krammer PH, et al. Involvement of the CD95 (Apo-1/Fas) receptor/ligand system in drug-induced apoptosis in leukemia cells. Nature Med. 1996;2:574–80. doi: 10.1038/nm0596-574. [DOI] [PubMed] [Google Scholar]
- 38.Westendorf JJ, Lammert JM, Jelinek DF. Expression and function of Fas (Apo-1/CD95) in patient myeloma cells and myeloma cell lines. Blood. 1995;85:3566–76. [PubMed] [Google Scholar]
- 39.Papoff G, Cascino I, Eramo A, et al. An N-terminal domain shared by Fas/APO-1 (CD95) soluble variants prevents cell death in vitro. J Immunol. 1996;156:4622–30. [PubMed] [Google Scholar]
- 40.Cheng J, Changdan L, Koopman WJ, et al. Characterization of the human fas gene: exon/intron organization and promoter region. J Immunol. 1995;154:1239–45. [PubMed] [Google Scholar]
- 41.Silvestris F, Nagata S, Cafforio P, et al. Cross-linking of Fas by antibodies to a peculiar domain of gp120, V3 loop can enhance T cell apoptosis in HIV-1 infected patients. J Exp Med. 1996;184:2287–300. doi: 10.1084/jem.184.6.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hata H, Xiao H, Petrucci MT, et al. Interleukin-6 gene expression in multiple myeloma: a characteristic of immature tumor cells. Blood. 1993;81:3357–64. [PubMed] [Google Scholar]
- 43.Billadeau D, Jelinek DF, Shah N, et al. Introduction of an activated N-ras oncogene alters the growth characteristics of the interleukin 6-dependent myeloma cell line ANBL6. Cancer Res. 1995;55:3640–6. [PubMed] [Google Scholar]
- 44.Hallek M, Bergsagel PL, Anderson KC. Multiple myeloma: increasing evidence for a multistep transformation process. Blood. 1998;91:3–21. [PMC free article] [PubMed] [Google Scholar]
- 45.Hahne M, Rimoldi D, Schröter M, et al. Melanoma cell expression of Fas (Apo-1/CD95) ligand: implications for tumor immune escape. Science. 1996;274:1363–5. doi: 10.1126/science.274.5291.1363. [DOI] [PubMed] [Google Scholar]
- 46.Griffith TS, Brunner T, Fletcher SM, et al. Fas ligand-induced apoptosis as a mechanism of immune privilege. Science. 1995;270:1189–91. doi: 10.1126/science.270.5239.1189. [DOI] [PubMed] [Google Scholar]
- 47.Bellgrau D, Gold D, Selawry H, et al. A role for CD95 ligand in preventing graft rejection. Nature (London) 1995;377:630–2. doi: 10.1038/377630a0. [DOI] [PubMed] [Google Scholar]
- 48.Hunt JS, Vassmer D, Ferguson TA, et al. Fas ligand is positioned in mouse uterus and placenta to prevent trafficking of activated leucocytes between the mother and the conceptus. J Immunol. 1997;158:4122–8. [PubMed] [Google Scholar]