Abstract
The role of isolated components obtained by gel filtration chromatography of Ascaris suum body extract (Asc) on the modulation of the immune response to ovalbumin (OvA) was evaluated and correlated with the immunogenic properties of such components. We showed that high (PI), but not low (PIII), molecular weight components have the ability to inhibit OvA-induced immediate and DTH reactions, lymph node (LN) cell proliferation, cytokine (IL-2, interferon-gamma (IFN-γ), IL-4 and IL-10) and antibody (IgG1, IgG2a, IgM and IgE) production in mice concomitantly immunized with OvA and these high mol. wt components. The pattern of cytokines synthesized in response to PI or PIII was totally different: the former induced more IL-4 and IL-10 and the latter more IL-2 and IFN-γ. The levels of Asc-specific IgG1 antibodies were higher in mice immunized with OvA plus PI and IgG2a anti-Asc antibodies predominated in those immunized with PIII. IgE antibody production, however, was low in the former group of mice. These results indicate that the high mol. wt components present in the body extract from the helminth A. suum are responsible for its suppressive effect upon Th1- and Th2-dependent immune responses to an unrelated antigen. The suppression of the Th1-dependent parameters could be related to high-level expression of IL-4 and IL-10 induced by such components.
Keywords: Ascaris suum, immunosuppression, cytokine, antibody isotype
INTRODUCTION
Ascaris suum, like many other helminths, can modulate the host's immune response, leading to enhancement or suppression of antibody production and/or cell-mediated immunity to unrelated antigens [1]. Comparative studies of populations with varying degrees of helminthic infection have indicated that these parasites cause a non-specific polyclonal stimulation of IgE synthesis, which can enhance allergic reactivity [2,3] or, when it is excessive, can be suppressive [4–7]. Under this latter circumstance, there is saturation of mast cell Fcε receptors and suppression of specific IgE antibody synthesis to environmental allergens [8]. Ascaris suum body extracts, pseudo-coelomic fluid, metabolic products or purified major allergen also display enhancing or suppressive effects on immune-specific responses to other antigens, but these effects are not correlated with polyclonal stimulation of IgE synthesis [9–13]. The mechanisms responsible for the immunoregulatory activity of helminths and the cytokines that play an important role in these mechanisms have been investigated in Nippostrongylus brasiliensis [14,15] and Schistosoma mansoni [16] infections, but are almost unknown in the case of A. suum.
We have been studying a murine model in which a suppressive activity of an A. suum body extract (Asc), mediated by heat-stable proteins, was first demonstrated on IgE and IgG antibody responses to ovalbumin (OvA) [11,17,18]. Suppression of the OvA-specific cell-mediated response by Asc was later characterized by showing that injection of high doses of the whole extract led to a dramatic decrease of DTH reactions, cell proliferation and cytokine synthesis after OvA restimulation [12,19].
The cytokine profile synthesized in response to Asc also varied depending on the amount of injected helminth extract. IL-4 and IL-10 were mainly produced in response to high doses, whereas IL-2 and interferon-gamma (IFN-γ) were enhanced at low doses of Asc. These findings indicate that high doses of A. suum extract impaired crucial T cell functions for cell-mediated and humoral immune responses to other antigens through the induction of a predominantly Th2-like response [19].
High and low mol. wt components (530 and 29 kD, respectively) obtained from Asc by gel filtration chromatography differed in their ability to induce or modulate an IgE antibody response in mice simultaneously immunized with OvA: fractions eluted in the first peak (PI) suppressed anti-OA IgE antibody production, while those eluted in the third peak (PIII) did not, but induced a significant anti-Asc IgE response [20].
The present study compares the effects of PI and PIII on cellular and humoral immune responses to OvA, as measured by hypersensitivity reactions, proliferative responses, cytokine and antibody synthesis. In addition, the cytokine and isotype profile induced in response to these Asc components is also analysed and correlated with their effects. These results will lead to a better understanding of the influence of immunoregulatory mechanisms on allergic as well as other diseases in helminth-infected populations.
MATERIALS AND METHODS
Animals
Eight to nine-week-old male DBA/2 mice were obtained from a colony at the Instituto de Ciências Biomédicas (Universidade de São Paulo, São Paulo, Brazil). Outbred Wistar rats were bred and maintained in our animal house facilities and used at 8–12 weeks of age for skin tests.
Antigens and antibodies
OvA grade II and V were obtained from Sigma Chemical Co. (St Louis, MO). Adult A. suum body extract was prepared as described earlier [11]. In summary, live worms obtained from pig intestines were washed with saline and mixed with an equal volume of borate-buffered saline (BBS) pH 8.0. After being homogenized in an Ultra-Turrax apparatus and centrifuged at 10 000 rev/min for 1 h, the precipitate was resuspended in 400 ml of BBS and stirred overnight at 4°C. This suspension was centrifuged again and the supernatant dialysed against distilled water overnight in the cold. Finally a clear supernatant obtained after centrifugation at 10 000 rev/min for 2 h was aliquoted and lyophilized. Biotinylated OvA and Asc were obtained according to a modification of the technique for immunoglobulins by Nutman [21]. Biotinylated isotype-specific goat anti-mouse antibodies and unlabelled rat anti-mouse IgE antibody were purchased from Southern Biotechnology Associates Inc. (Birmingham, AL). Monoclonal antibodies and recombinant cytokine standards for cytokines assays were kindly provided by Dr I. A. Abrahamsohn (Institute of Biomedical Sciences/USP, São Paulo, Brazil).
Gel filtration
The parasite extract (15 mg/2 ml) was loaded onto a C16/100 column of Sephacryl S-200 (Pharmacia, Uppsala, Sweden), equilibrated with 0.05 m BBS pH 8.0 and eluted at 40.0 ml/h. Fractions of 1.6 ml were collected and protein elution was estimated spectrophotometrically at 280 nm (Uvicorn II 8.300 LKB). Three peaks were obtained as previously reported [20]. Fractions corresponding to peak I (PI) and peak III (PIII) were pooled separately and concentrated to the original volume. Concentration was performed with Centriprep 100 and 10 (Amicon, Beverly, MA) concentrators (100 and 10 cut-off point, respectively). The protein banding pattern of Asc, PI and PIII obtained by SDS–PAGE and immunoblotting has been previously published [20]. Three bands were identified in PI contrasting with several bands displayed by PIII.
Immunization and hypersensitivity reactions
Groups of four to six mice were injected subcutaneously with 50 μg of OvA emulsified in 0.1 ml Freund's complete adjuvant (FCA) on both sides of the base of the tail. In some groups, 70 μl of Asc (containing 500 μg of protein) or the same volume of PI (containing 125 μg) or PIII (containing 200 μg of protein) were added to OvA before emulsification. Hypersensitivity reactions were elicited 8 days after immunization by injection of 30 μl of 2% aggregated OvA (1 h, 70°C) in the left hind footpad and the same volume of saline in the right. Footpad swelling was monitored for the next 24 h using a Mitutoyo pocket thickness gage (0.01–10 mm) and expressed as the increase in thickness relative to the saline-injected paw. Normal animals injected with aggregated OvA were always included as test controls for non-specific swelling. The results were expressed by the arithmetic mean ± s.e.m. of each group. A two-way analysis of variance followed by multiple comparisons using the Tukey method [22] was employed to compare control with immunized groups as well as the differently immunized groups.
Proliferation assay
Nine days after immunization, cell suspensions from inguinal and periaortic lymph nodes (LN) were prepared in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10 mm HEPES, 0.05 mm 2-mercaptoethanol, sodium pyruvate, non-essential amino acids, penicillin/streptomycin and 1% heat-inactivated normal mouse serum (DMEM-S). LN cell proliferation was measured by incorporation of 3H-thymidine (2.0 Ci/mmol; Amersham Int., Aylesbury, UK). Triplicate preparations of cells (5 × 105/well) were incubated with OvA or Asc at 100 μg protein/ml or PI or PIII at 50 μg/ml for 96 h. Tritiated thymidine (0.5 μCi/well) was added 18 h before cell harvesting and incorporated 3H-TdR determined by liquid scintillation spectrometry. The data were presented as the mean ct/min incorporated by the triplicate cultures ± s.d. and as stimulation index (SI), which represents the ct/min of 3H-thymidine incorporated by stimulated LN cells divided by ct/min incorporated by unstimulated cells.
Cytokine assays
For in vitro cytokine measurements, LN cells were cultured in 24-well tissue culture plates at a final concentration of 10 or 6 × 106 cells/ml in DMEM-S. The cells were stimulated with OvA (500 μg/ml), PI (250 μg/ml) or PIII (250 μg/ml). The antigen concentrations were previously established for maximal cytokine release. Supernatant fluids were harvested after 24 h (cultures of 10 × 106 cells) or 72 h (cultures of 6 × 106 cells) and assayed for cytokine content. All the cytokines were measured by specific two-site sandwich ELISA, using the following MoAbs: for IFN-γ, XMG 1.2 and biotinylated AN 18; for IL-2, JES6-IA12 and biotinylated JES6-5H4; for IL-4, BVD-1D11 and biotinylated BVD6-24G2 and for IL-10, C252-2A5 and biotinylated SXC-1. Binding of biotinylated MoAbs was detected using streptavidin–biotinylated horseradish peroxidase complex (Amersham) and 2-2′-azino-bis(3-ethylbenz-thiazoline-6-sulphonic acid) substrate solution (Sigma) in 0.1 m citrate buffer containing hydrogen peroxide. The sensitivity of IL-4 assays was increased by using the ELAST ELISA amplification system purchased from DuPont NEN (Wilmington, DE). Samples were quantified by comparison with standard curves of recombinant mouse cytokines. The results were expressed as the arithmetic mean of duplicate cultures ± s.d.
Titration of antibody isotypes by ELISA
Plasma obtained 9 days after immunization were tested for IgG1, IgG2a and IgM antibodies using antigen-coated 96-well plates and biotinylated goat anti-mouse monospecific antisera. The reactions were developed with streptavidin–peroxidase conjugate (Jackson ImmunoResearch Labs Inc., West Grove, PA), OPD and H2O2 and the plates were read at 450 nm on an automated ELISA reader (Dynatech, Chantilly, VA; MR 5000). IgE antibodies were titrated by reverse (IgE-capture) ELISA, using rat anti-mouse IgE MoAb-coated plates and biotinylated antigens [19]. Titration curves were carried out for all the samples. The results were expressed as the mean of the sample optical densities (OD) from each group in an appropriate dilution (within the linear part of the curve) for each isotype and antigen (OvA or Asc) + s.e.m. Analysis of variance followed by multiple comparisons using the Tukey method [22] was employed to compare the antibody response of the several groups. The IgE antibody response was also measured by passive cutaneous anaphylaxis (PCA) as previously described [19]. PCA reactions were performed in rats, using a sensitization period of 18–24 h and OvA or Asc in Evan's blue solution for challenge. The PCA titre was expressed as the reciprocal of the highest dilution that gave a lesion of > 5 mm in diameter in triplicate tests.
RESULTS
Effect of peak I and peak III fractions on OvA-specific hypersensitivity reactions
OvA-specific hypersensitivity reactions were elicited in mice immunized with OvA alone or with OvA plus the whole extract, PI or PIII components and challenged with aggregated OvA in the footpad after 8 days. The kinetics of immediate hypersensitivity and DTH reactions are shown in Fig. 1. OvA-immunized mice developed an immediate reaction in the footpad at 6 h after challenge and a delayed-type reaction after 24 h. Immediate and DTH reactions to OvA were suppressed in mice immunized with OvA + Asc and in mice immunized with OvA + high mol. wt fraction PI of the extract. In contrast to PI, the low mol. wt fraction PIII did not suppress OvA-specific immediate or DTH reactions.
Fig. 1.
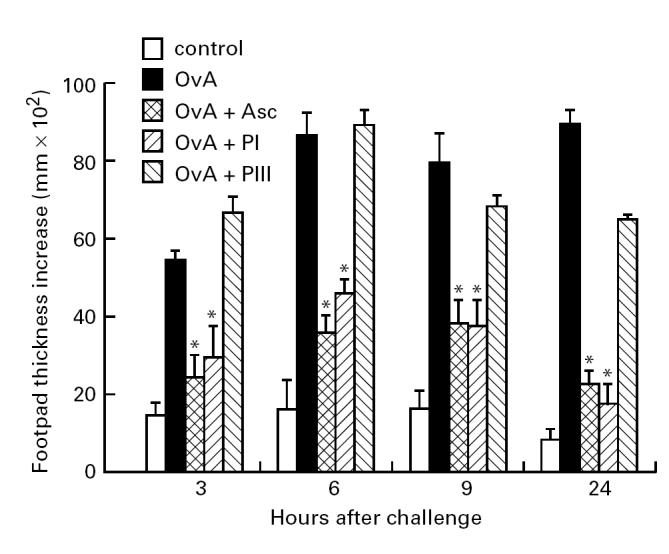
Hypersensitivity reactions in DBA/2 mice immunized with ovalbumin (OvA), OvA + Ascaris suum body extract (Asc), OvA + PI or OvA + PIII in Freund's complete adjuvant (FCA) and challenged 8 days later with aggregated OvA in the footpad. Normal mice (control) were also challenged in the same way for non-specific swelling. *P < 0.05 compared with OvA-immunized group.
Regarding antibody production, simultaneous immunization of mice with OvA + Asc or with OvA + PI resulted in a significant reduction of anti-OvA antibody levels of IgG1, the main isotype produced. IgG2a, IgM and IgE antibodies were also diminished in these mice compared with those immunized only with OvA or OvA + PIII (Fig. 2). Reduction of IgE antibody production was further confirmed by PCA quantification: titres of 5 and 10 were obtained in the former groups compared with 160 and 80 in the latter groups, respectively (data not shown).
Fig. 2.
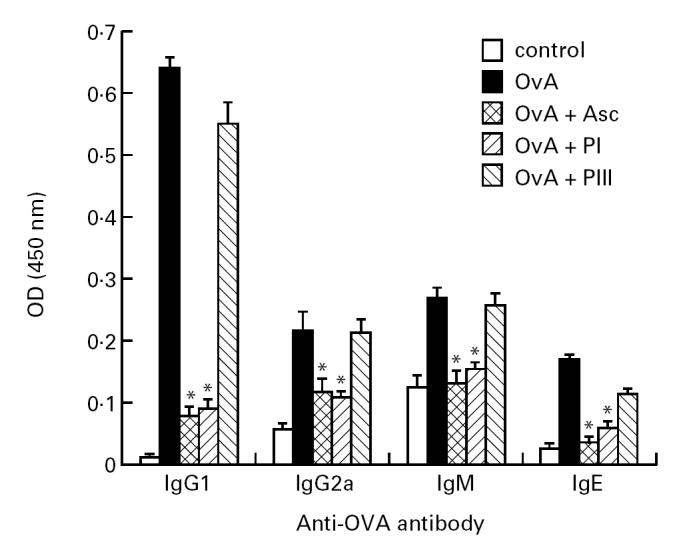
Antibody isotypes produced against ovalbumin (OvA) by OvA, OvA + Ascaris suum body extract (Asc), OvA + PI or OvA + PIII-immunized or normal DBA/2 mice. Isotype levels in immune plasma obtained after 9 days of immunization or normal plasma (control) were titrated by ELISA using monospecific antisera. The results are expressed as the mean of five to six samples/group (+ s.e.m.), in 1:512 (IgG1) or 1:8 (IgG2a, IgM and IgE) dilution. *P < 0.05 compared with OvA-immunized group. OD, Optical density.
When the pattern of anti-Asc antibody isotypes was analysed by ELISA (Fig. 3), IgG1 antibodies were mostly detected in the plasma of OvA + PI-immunized mice, while IgG2a antibody levels were elevated in OvA + PIII-immunized mice. IgE antibodies were induced in higher amounts by PIII, as confirmed by PCA titration (Fig. 3, upper right).
Fig. 3.
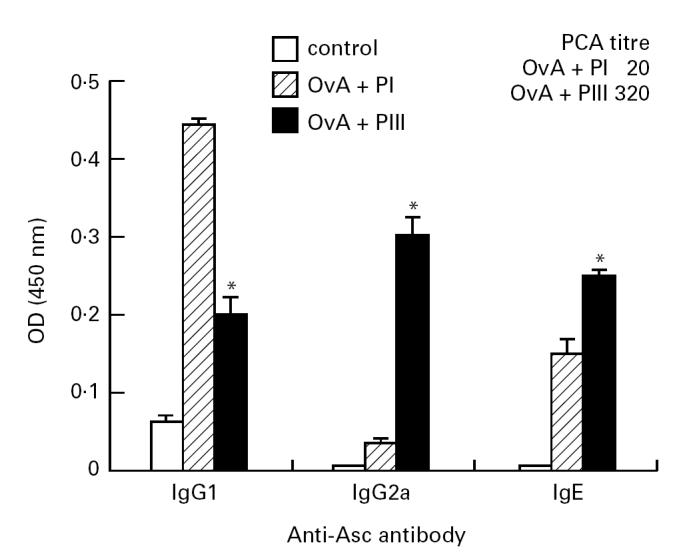
Antibody isotypes produced against Ascaris suum body extract (Asc) by ovalbumin (OvA) + PI or OvA + PIII-immunized or normal DBA/2 mice. Isotype levels in immune plasma obtained after 9 days of immunization or normal plasma (control) were titrated by ELISA using monospecific antisera. The results are expressed as the mean of five to six samples/group (+ s.e.m.), in 1:128 (IgG1), 1:256 (IgG2a), or 1:16 (IgE) dilution. Upper right: IgE antibody titres obtained by passive cutaneous anaphylactic (PCA) reaction in pools of plasma from OvA + PI- or OvA + PIII-immunized mice. *P < 0.05 compared with PI-immunized group. OD, Optical density.
Lymphoproliferative responses to OvA are suppressed by Asc or PI components
Effect of PI and PIII on cytokine profile and antibody production in response to OvA
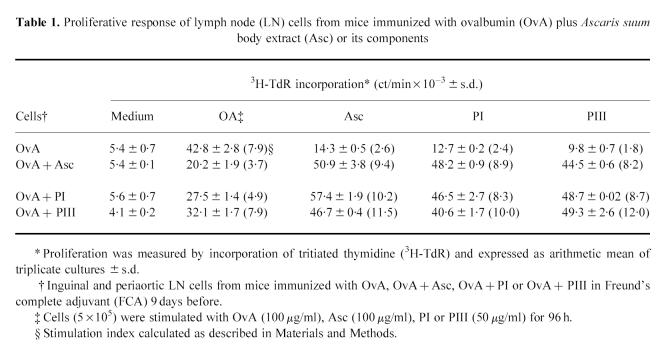
Nine days after immunization, proliferation of LN cells from the four groups of mice was measured after in vitro OvA restimulation for 96 h. The results presented in Table 1 show that simultaneous immunization of mice with OvA + PI or OvA + Asc prevented LN cells from responding in vitro to OvA. In contrast, the proliferative response of LN cells from OvA + PIII-immunized mice was similar to OvA-immunized mice, when analysed as SI. The difference in ct/min between these two groups was also statistically not significant. When the cultures were restimulated in vitro with either Asc, PI or PIII, a significant proliferation was obtained in LN cells from mice immunized with OvA plus one of these antigens compared with the non-specific response obtained in cells from mice injected only with OvA.
Table 1.
Proliferative response of lymph node (LN) cells from mice immunized with ovalbumin (OvA) plus Ascaris suum body extract (Asc) or its components
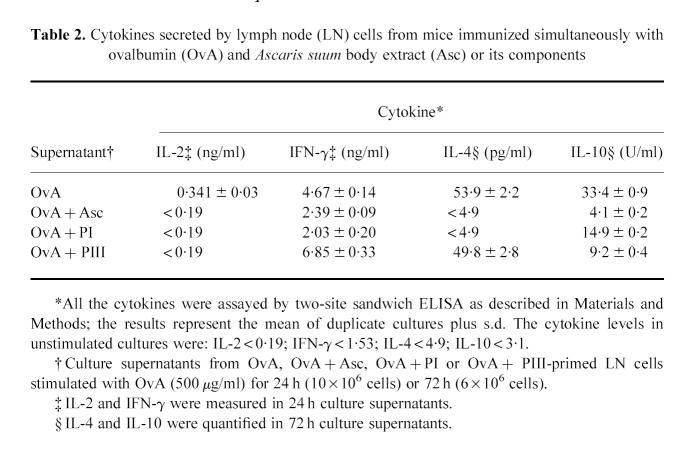
LN cells obtained from mice immunized with OvA or OvA + Asc or its components 9 days before were then cultured with OvA and the supernatants collected for the quantification of secreted cytokines. Cultures from OvA + Asc or OvA + PI-immunized mice exhibited reduced IL-2, IFN-γ, IL-4 and IL-10 secretion when compared with those from OvA-immunized mice (Table 2). In contrast, cultures from OvA + PIII cells showed no decrease at all in IFN-γ or IL-4 and only IL-2 and IL-10 were impaired.
Table 2.
Cytokines secreted by lymph node (LN) cells from mice immunized simultaneously with ovalbumin (OvA) and Ascaris suum body extract (Asc) or its components
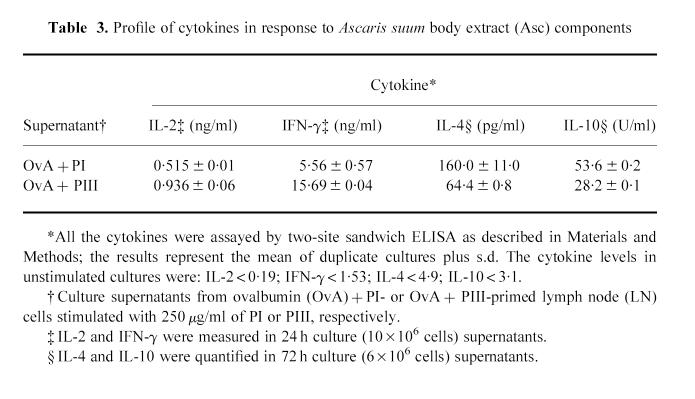
Cytokines were also measured in supernatants of LN cell cultures from OvA + PI or OvA + PIII-immunized mice after in vitro restimulation with PI or PIII, respectively. Table 3 shows that the levels of IL-4 and IL-10 were higher in response to PI, whereas PIII induced more IL-2 and IFN-γ.
Table 3.
Profile of cytokines in response to Ascaris suum body extract (Asc) components
Profile of cytokines and antibody isotypes in response to PI or PIII
DISCUSSION
Previous studies have described the suppressive effect of a whole body extract from A. suum on humoral and cell-mediated immune responses to an unrelated antigen [11,17,19]. Here we report the role of isolated components obtained by gel filtration chromatography of the whole extract on the modulation of the immune response to OvA and the immunogenic properties of such components.
For this, we simulated the immunization conditions with the whole extract using PI and PIII components in concentrations comparable to those found in the unfractionated extract to immunize DBA/2 mice. We also included a group of mice that received whole extract with OvA.
We showed that the suppressive effect was limited to PI components that inhibited both humoral and cell-mediated immune responses. These results confirm and extend those of our previous report [20] that described the ability of PI, but not PIII, components from Asc to modulate the anti-OvA antibody response of the IgE isotype. In addition, we demonstrated that a four times less amount of PI displays the same immunosuppressive activity as the whole extract. This activity was apparently restricted to the OvA-specific immune response, since reactivity against Ascaris antigens in general was obtained. Asc, PI and PIII are antigenically quite complex, as demonstrated by immunoblotting [20], and probably more immunogenic. However, we have observed in some parameters like synthesis of IFN-γ and Asc-specific IgG1 and IgE antibody production that PI has a suppressive effect on PIII-specific immune response too (unpublished results and [20]).
To rule out that suppression could be due to MHC class II-dependent preferential presentation of PI antigens compared with OvA, we also performed the same experiments in another mouse strain, A/Sn, and obtained similar effects of Asc or its components on the OvA-specific immune response (data not shown).
It must be pointed out that the overall pattern of cytokines synthesized by either strain in response to PI or PIII components was quite similar: the former induced more IL-4 and IL-10 and the latter more IFN-γ and IL-2. The Asc-specific antibody isotypes produced by both strains upon immunization confirmed the known influence of these cytokines on isotype selection [23], since higher levels of IgG1 antibodies were obtained in OvA + PI- and of IgG2a antibodies in OvA + PIII-immunized mice. The high-level expression of IL-4, however, was not associated with high IgE antibody production in OvA + PI-immunized mice. It is well known that class switching to IgE in mice is strictly dependent on IL-4 [24–26], although recently IL-4-independent switch has been reported in a retroviral infection [27]. An explanation for our results could be that PIII components induced other Th2-type cytokines that synergized with IL-4 to promote more IgE antibody production. In fact, we have recently observed that IL-5, IL-9 and IL-13 are secreted in higher amounts by LN cells from PIII-immunized mice than from PI-immunized ones (data not published).
Among the Th2 cell products, IL-10 is known to inhibit DTH reactions elicited by secondary antigenic challenge or by local transfer of Th1 clones in mice, and this effect can be synergized by IL-4 [28,29]. Therefore, these cytokines that are produced in large amounts in response to Asc and PI could be down-regulating the effector stages of OvA-specific DTH reactivity. Immunization with Asc or PI not only suppressed the DTH reactions, but also diminished in vitro proliferative response and cytokine secretion (IL-2, IFN-γ, IL-4 and IL-10) by OvA + Asc or OvA + PI-primed LN cells when restimulated with OvA. Furthermore, OvA-specific antibody production of different isotypes (IgG1, IgG2a, IgM and IgE) was largely affected by the suppressive mechanism in mice concomitantly immunized with OvA and Asc or PI, but not with PIII. These results indicate therefore that both Th1- and Th2-dependent parameters of the OvA-specific immune response were impaired.
In this regard, we have recently observed that in vivo neutralization of IL-4 and IL-10 in mice by combined MoAb treatment before immunization with OvA + Asc restored the immediate hypersensitivity reactions and IgG2a antibody production in these animals. This effect was associated with enhanced IFN-γ secretion by OvA + Asc-primed LN cells after in vitro restimulation with OvA and a shift towards the Th1 cytokine phenotype when these same cells were restimulated with Asc [30]. Such results rule out the possibility that the cytokine profiles of the animals exposed to the extract or its components might be totally coincidental to the effects seen. They also reinforce that the high levels of IL-4 and IL-10 induced by Asc and PI could be mediating the suppression of most of the Th1-dependent parameters of the immune response to OvA. Since this effect reduced the frequency of OvA-responding cells in OvA + Asc-immunized mice at least three-fold compared with OvA-immunized mice [31], the release of IL-2 and IFN-γ induced by OvA was also suppressed, although PI-specific cells produced these cytokines by themselves.
Altogether, the results we obtained in this model using the Ascaris extract are original and differ from those reported in other helminth models. In S. mansoni infection that induces a predominant Th2 response after egg deposition, it was demonstrated that IL-10 produced by the Th2 cells down-regulate only Th1 cytokine secretion, mainly IFN-γ, to sperm whale myoglobin and HIV glycoprotein, skewing the response towards Th2 [32,33]. Mice infected with the microfilariae Brugia malayi or immunized with the parasite extract develop a pronounced Th2 response [34] that can modulate the Th1 response to a mycobacterial antigen (PPD), increasing IL-4 and IL-5 secretion. This effect is mediated by an IL-4-, and not an IL-10-dependent mechanism [35].
In A. suum infection, the data presented here and elsewhere [20] indicate that immunoregulatory mechanisms activated by high mol. wt components suppress Th1- and Th2-dependent immune responses in general, including the one to the worm's low mol. wt antigens. These immunoregulatory mechanisms could be responsible for the suppression of specific IgE synthesis to environmental allergens and to Ascaris antigens in heavily helminth-infected children [8], in addition to the effect of high polyclonal IgE levels found in their plasma. In conclusion, we would like to suggest that the expression of allergic reactivity in helminth-infected populations can be modulated not only by polyclonal stimulation of IgE synthesis, but also by an antigen-specific immunosuppressive mechanism.
Acknowledgments
This work was supported by grants from FAPESP and CNPq. E.L.F.-M. was in receipt of a fellowship from FAPESP (92/3630-7). The technical assistance of Mr Ademir Veras da Silva and Mr Ulisses Rodrigues da Silva was highly appreciated. We wish also to thank Dr Ises A. Abrahamsohn and Dr Gustavo P. Amarante-Mendes for critical review of the manuscript.
REFERENCES
- 1.Barriga OO. Immunomodulation by nematodes: a review. Vet Parasit. 1984;14:299–320. doi: 10.1016/0304-4017(84)90098-0. [DOI] [PubMed] [Google Scholar]
- 2.Joubert JR, Van Schalkwyk DJ, Turner KJ. Ascaris lumbricoides and the human immunogenic response: enhanced IgE-mediated reactivity to common inhaled allergens. S Afr Med J. 1980;57:409–12. [PubMed] [Google Scholar]
- 3.Lynch NR, Di Prisco-Fuenmayor MC. High allergic reactivity in a tropical environment. Clin Allergy. 1984;14:233–40. doi: 10.1111/j.1365-2222.1984.tb02202.x. [DOI] [PubMed] [Google Scholar]
- 4.Turner KJ, Feddma L, Quinn EH. Non-specific potentiation of IgE by parasitic infections in man. Int Arch Allergy Appl Immunol. 1979;58:232–6. doi: 10.1159/000232197. [DOI] [PubMed] [Google Scholar]
- 5.Kaplan JE, Larrick JW, Yost JA. Hyper-immunoglobulinemia E in the Waorani, an isolated Amerindian population. Am J Trop Med Hyg. 1980;29:1012–7. doi: 10.4269/ajtmh.1980.29.1012. [DOI] [PubMed] [Google Scholar]
- 6.Lynch NR, Lopez RI, Isturiz G, et al. Allergic reactivity and helminthic infection in Amerindians of the Amazon basin. Int Arch Allergy Appl Immunol. 1983;72:369–72. doi: 10.1159/000234899. [DOI] [PubMed] [Google Scholar]
- 7.Lynch NR, López RI, Di Prisco-Fuenmayor MC, et al. Allergic reactivity and socioeconomic level in a tropical environment. Clin Allergy. 1987;17:199–207. doi: 10.1111/j.1365-2222.1987.tb02004.x. [DOI] [PubMed] [Google Scholar]
- 8.Lynch NR, Hagel I, Perez M, et al. Effect of antihelminthic treatment on the allergic reactivity of children in a tropical slum. J Allergy Clin Immunol. 1993;92:404–11. doi: 10.1016/0091-6749(93)90119-z. [DOI] [PubMed] [Google Scholar]
- 9.Komatsu T, Nishimura T, Sano R, et al. Ascaris suum: suppression of the reaginic and hemagglutinating antibody responses in the mouse by crude extract and maintenance fluid. Exp Parasitol. 1979;47:158–68. doi: 10.1016/0014-4894(79)90069-9. [DOI] [PubMed] [Google Scholar]
- 10.Stromberg EE. Potentiation of the reaginic (IgE) antibody response to ovalbumin in the guinea pig with a soluble metabolic product from Ascaris suum. J Immunol. 1980;125:833–6. [PubMed] [Google Scholar]
- 11.Macedo MS, Mota I. Antigenic competition in IgE antibody production: I. Establishment of parameters involved in primary and secondary responses. Immunology. 1980;40:701–8. [PMC free article] [PubMed] [Google Scholar]
- 12.Macedo MS, Barbuto JAM. Murine delayed-type hypersensitivity is suppressed by Ascaris suum extract. Brazilian J Med Biol Res. 1988;21:523–5. [PubMed] [Google Scholar]
- 13.Lee TDG, McGibbon A. Potentiation of IgE responses to third-party antigens mediated by Ascaris suum soluble products. Int Arch Allergy Immunol. 1993;102:185–90. doi: 10.1159/000236570. [DOI] [PubMed] [Google Scholar]
- 14.Ishizaka K. Regulation of the IgE antibody response. Int Arch Allergy Appl Immunol. 1989;88:8–13. doi: 10.1159/000234739. [DOI] [PubMed] [Google Scholar]
- 15.Urban JF JR, Madden KB, Svetic A, et al. The importance of Th2 cytokines in protective immunity to nematodes. Immunol Rev. 1992;127:205–20. doi: 10.1111/j.1600-065x.1992.tb01415.x. [DOI] [PubMed] [Google Scholar]
- 16.Sher A, Gazzinelli RT, Oswald IP, et al. Role of T-cell derived cytokines in the downregulation of immune responses in parasitic and retroviral infection. Immunol Rev. 1992;127:183–204. doi: 10.1111/j.1600-065x.1992.tb01414.x. [DOI] [PubMed] [Google Scholar]
- 17.Soares MFM, Macedo MS, Mota I. Suppressive effect of Ascaris suum extract on IgE and IgG antibody responses in mice. Brazilian J Med Biol Res. 1987;20:203–11. [PubMed] [Google Scholar]
- 18.Soares MFM, Oliveira EB, Mota I, et al. Suppression of IgE antibody production by Ascaris suum extract: characterization of suppressive components. Brazilian J Med Biol Res. 1988;21:527–9. [PubMed] [Google Scholar]
- 19.Ferreira AP, Faquim ES, Abrahamsohn IA, et al. Immunization with Ascaris suum extract impairs T cell functions in mice. Cell Immunol. 1995;162:202–10. doi: 10.1006/cimm.1995.1070. [DOI] [PubMed] [Google Scholar]
- 20.Soares MFM, Mota I, Macedo MS. Isolation of Ascaris suum components which suppress IgE antibody responses. Int Arch Allergy Immunol. 1992;97:37–43. doi: 10.1159/000236093. [DOI] [PubMed] [Google Scholar]
- 21.Nutman TB. Measurement of polyclonal immunoglobulin synthesis using ELISA. In: Colligan JE, Margulies AM, Shevach EM, Strober W, editors. Current protocols in immunology. New York: John Wiley and Sons; 1991. pp. 7.12.1–6. [DOI] [PubMed] [Google Scholar]
- 22.Zar JH. Biostatistical analysis. New York: Printice-Hall; 1984. [Google Scholar]
- 23.Finkelman FD, Holmes J, Katona IM, et al. Lymphokine control of in vivo immunoglobulin isotype selection. Ann Rev Immunol. 1990;8:303–30. doi: 10.1146/annurev.iy.08.040190.001511. [DOI] [PubMed] [Google Scholar]
- 24.Finkelman FD, Katona IM, Urban JF JR, et al. IL-4 is required to generate and sustain in vivo IgE responses. J Immunol. 1988;141:2335–41. [PubMed] [Google Scholar]
- 25.Kuhn R, Rajewski K, Muller W. Generation and analysis of interleukin-4 deficient mice. Science. 1991;254:707–10. doi: 10.1126/science.1948049. [DOI] [PubMed] [Google Scholar]
- 26.Coffman RL, Lebman DA, Rothman P. Mechanism and regulation of immunoglobulin isotype switching. Adv Immunol. 1993;54:229–70. doi: 10.1016/s0065-2776(08)60536-2. [DOI] [PubMed] [Google Scholar]
- 27.Morawetz RA, Gabriele L, Rizzo LV, et al. Interleukin (IL)-4-independent immunoglobulin class switch to immunoglobulin (Ig)E in the mouse. J Exp Med. 1996;184:1651–61. doi: 10.1084/jem.184.5.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Powrie F, Menon S, Coffman RL. Interleukin-4 and interleukin-10 synergize to inhibit cell-mediated immunity in vivo. Eur J Immunol. 1993;23:2223–9. doi: 10.1002/eji.1830230926. [DOI] [PubMed] [Google Scholar]
- 29.Li L, Elliott JF, Mosmann TR. IL-10 inhibits cytokine production, vascular leakage, and swelling during T helper 1 cell-induced delayed-type hypersensitivity. J Immunol. 1994;153:3967–78. [PubMed] [Google Scholar]
- 30.Macedo MS, Faquim-Mauro EL, Ferreira AP, et al. Immunomodulation induced by Ascaris suum extract in mice: effect of anti-IL-4 and anti-IL-10 antibodies. Scand J Immunol. 1998;47:10–18. doi: 10.1046/j.1365-3083.1998.00251.x. [DOI] [PubMed] [Google Scholar]
- 31.Ferreira AP. Institute of Biomedical Sciences, University of São Paulo; 1995. PhD thesis. [Google Scholar]
- 32.Kullberg MC, Pearce EJ, Hieny SE, et al. Infection with Schistosoma mansoni alters Th1/Th2 cytokine responses to a non-parasite antigen. J Immunol. 1992;148:3264–70. [PubMed] [Google Scholar]
- 33.Actor JK, Shirai M, Kullberg MC, et al. Helminth infection results in decreased virus-specific CD8+ cytotoxic T-cell and Th1 cytokine responses as well as delayed virus clearance. Proc Nat Acad Sci USA. 1993;90:948–52. doi: 10.1073/pnas.90.3.948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pearlman E, Kroeze WK, Hazlett FE, et al. Brugia malayi: acquired resistance to microfilariae in Balb/c mice correlates with local Th2 responses. Exp Parasitol. 1993;76:200–8. doi: 10.1006/expr.1993.1023. [DOI] [PubMed] [Google Scholar]
- 35.Pearlman E, Kazura JW, Hazlett FE, et al. Modulation of murine cytokine responses to mycobacterial antigens by helminth-induced T helper 2 cell responses. J Immunol. 1993;151:4857–64. [PubMed] [Google Scholar]