Abstract
The aim of this study was to further assess the role of pooled human immunoglobulin (PHIG) on cytokine production from PBMC stimulated with a bacterial superantigen. Human PBMC were cultured with Streptococcus pyrogenic exotoxin A (SPE-A) with or without PHIG and several proinflammatory cytokine levels of culture supernatants were measured. Serum cytokine levels of KD patients before and after PHIG therapy were also examined. PHIG greatly reduced the production of IL-12, interferon-gamma (IFN-γ) and other cytokines from SPE-A-stimulated PBMC, while exogenous IL-12, but neither IL-1 nor tumour necrosis factor-alpha (TNF-α), restored IFN-γ production inhibited by PHIG. Although PHIG partially adsorbed SPE-A, its inhibitory effect on cytokine production was not played by anti-SPE-A antibody. Although purified CD4+ T cells cultured with human HLA-DR-transfected mouse L cells and SPE-A could not effectively produce IFN-γ, they produced large amounts of IFN-γ if exogenous IL-12 was introduced. KD patients in the acute phase had higher levels of serum IFN-γ than did controls and patients with bacterial infection. Although IL-12 levels of children with or without KD were not significantly different, IL-12 levels of children were much higher than those of adults. However, serum levels of IL-12 of KD patients were transiently but significantly decreased by PHIG therapy and IFN-γ amounts subsequently reverted to basal levels thereafter. These findings indicate that PHIG inhibits IL-12 production of SPE-A-activated monocytes and thereby decreases IFN-γ synthesis by T cells and suggest that inhibition of IL-12 and IFN-γ production is an important part of the mechanisms underlying PHIG therapy on KD.
Keywords: Streptococcus pyrogenic exotoxin A, pooled human immunoglobulin, Kawasaki disease, IL-12, interferon-gamma
INTRODUCTION
Intravenous administration of pooled human immunoglobulin (PHIG) is reportedly effective against not only severe bacterial and viral infections [1] but also certain diseases such as idiopathic thrombocytopenic purpura [2], some autoimmune diseases [3,4] and KD [5,6]. However, the mechanism underlying its effect has not been fully elucidated. It was reported that PHIG inhibits bacterial superantigen-induced production of proinflammatory cytokines, such as interferon-gamma (IFN-γ) and tumour necrosis factor-beta (TNF-β) [7,8] from PBMC in vitro. On the other hand, IL-12 is a recently reported cytokine [9–12] which activates natural killer (NK) cells and T cells and is considered to be critical against bacterial infections. IL-12 is produced by phagocytic cells and antigen-presenting cells (APC) [11,12], such as monocytes, macrophages and dendritic cells when these cells are activated by bacterial components, including lipopolysaccharide (LPS) [11–14]. IL-12 is also known as an inducer of IFN-γ, IL-2 and others [12,15,16]. Particularly, IL-12 is an essential and potent inducer of IFN-γ from NK cells, T cells and NK-type T cells [12,17,18] and is also critically involved in anti-tumour immunity [15,16,18,19].
KD is an acute multisystem vasculitis which primarily affects young children under 6 years of age [20]. Approximately 20% of KD patients develop coronary artery abnormalities and some of them suffer from myocardial ischaemia and even myocardial infarction [21,22]. Although the aetiology is still unknown, the possibility that bacteria, their components and bacterial superantigens of staphylococcus or streptococcus, are involved in this disease has been considered [23–25]. Bacterial superantigen-induced toxic shock syndrome mimics KD in some aspects and some patients with infective enteritis due to Yersinia pseudotuberculosis meet the diagnostic criteria of KD [26]. No specific virus has been identified in KD patients; instead, marked granulocytosis, which is not usually seen in viral infections, is observed in KD. Among therapies tested, i.v. injection of PHIG is empirically effective for KD and improving coronary artery complications [5,6]. However, the mechanism of this treatment remains largely unknown. In the present study, we demonstrate that PHIG inhibits IL-12 production from monocytes and suppresses IFN-γ production by T cells stimulated with Streptococcus pyrogenic exotoxin A (SPE-A). In addition, we show that PHIG therapy decreases serum IL-12 levels in patients in the acute phase of KD and seems to initiate reduction of serum IFN-γ.
MATERIALS AND METHODS
Reagents
SPE-A was purified from culture filtrate of Streptococcus pyogenes strain NY-5 [27]. PHIG (Venoglobulin IH, lot no. B025VH) was provided by the Green Cross Corp. (Osaka, Japan). Human recombinant IL-12 (p70) (1.7 × 107 U/mg) was kindly provided by Hoffman-LaRoche (Nutely, NJ), and recombinant IL-1α and TNF-α were purchased from R&D Systems (Minneapolis, MN).
Cell culture
PBMC were obtained from six healthy human adult volunteers by Ficoll–Paque gradient centrifugation and suspended in RPMI 1640 containing 10% fetal bovine serum (FBS). SPE-A (100 ng/ml)-stimulated PBMC (1 × 106/ml) were cultured for 9 days at 37°C and 5% CO2, with or without PHIG (5 mg/ml), and cell culture supernatants were harvested at each 24 h and were subjected to ELISA. In some experiments, SPE-A-stimulated PBMC were incubated with PHIG and/or IL-12 (20 U, 1000 pg/ml) for 144 h. Cell culture supernatants were harvested each 48 h.
High performance liquid chromatography analysis of SPE-A after incubation with PHIG
SPE-A (100 ng/ml) was incubated at 37°C and 5% CO2 with or without 5 mg/ml PHIG for 2 h and then the decrease of the SPE-A peak areas by PHIG was analysed on a gel permeation high performance liquid chromatography (HPLC; Shimazu, Japan) using a G4000PWXL column (Touso, Japan) and a UV spectrophotometric detector (SPD-6 A; Shimazu).
Isolation of CD4+ T cells and culture with human HLA-DR-expressing murine L cells
CD4+ HLA-DR− cells were isolated by cell sorting using EPICS ELITE (Coulter, Hialeah, FL). The HLA-DR-transfected murine fibroblast cell line (L cells) was kindly provided by Dr R. I. Lechler (Department of Immunology, Royal Medical School, Hammersmith Hospital, London, UK) [28]. CD4+ T cells (1 × 106) and 2 × 105 mitomicin C-treated (100 μg/ml, 30 min) L cells were cultured with SPE-A with or without exogenous IL-12.
Cytokine assays
Cytokine levels were determined by sandwich ELISA using a Pelkine Compact IFN-γ ELISA kit, a Pelican Compact human TNF-α ELISA kit (Central Laboratory of the Netherlands Red Cross Blood Transfusion Service, Amsterdam, The Netherlands), a Quantikine IL-1α immunoassay kit, Quantikine TNF-β immunoassay kit (R&D Systems), and a Cytoscreen human IL-12 (p40 and p70) immunoassay kit (Biosource, Camarillo, CA), human IL-12 p70 (Genzyme, Cambridge, MA), respectively.
RNA isolation and cDNA synthesis and polymerase chain reaction
Total RNA was isolated from MNC by the acid guanidinium phenol chloroform method. RNA (5 μg) was converted to cDNA using Moloney Murine Leukaemia virus reverse transcriptase (Gibco BRL, Grand Island, NY) and random primer (Takara Shuzo Co., Kyoto, Japan) at 42°C for 1 h in a reaction mixture.
cDNA (2 μl) was added to reaction mixture containing 10 mm Tris pH 8.3, 3 mm MgCl2, 0.2 mm dNTP, 2.5 U Taq DNA polymerase (Toyobo Co., Osaka, Japan), and 0.5 μm of each primer. For amplification of the desired cDNA, the following gene-specific primers were used: IL-12 p35 sense 5′-CTT CAC CAC TCC CAA AAC CTG-3′, antisense 5′-AGC TCG TCA CTC TGT CAA TAG-3′ [29]; IL-12 p40 sense 5′-CAT TCG CTC CTG CTG CTT CAC-3′, antisense 5′-TAC TCC TTG TTG TCC CCT CTG-3′ [30]; β-actin sense 5′-CGT GAC ATC AAA GAG AAG CTG TG-3′, antisense 5′-GCT CAG GAG GAG CAA TGA TCT TGA-3′. Polymerase chain reaction (PCR) was carried out in an automatic DNA thermal cycler (ASTEC, Tokyo, Japan). The cycle programme was set to denature at 94°C for 1 min, to anneal at 60°C for 1 min, and to extend at 72°C for 1.5 min for a total of 32 cycles. Electrophoresis of the PCR products was performed on 2% agarose gels and the PCR fragments were visualized by ethidium bromide staining under UV illumination.
Serum levels of IL-12, IFN-γ and TNF-α in patients with KD
To determine the effect of PHIG therapy, serum levels of IL-12, IFN-γ and TNF-α in patients with KD (n = 28, 3.2 ± 2.0 years old) in the acute phase and in the convalescent phase after PHIG therapy were compared with those of patients with bacterial infections (n = 13, 4.7 ± 3.9 years old), age-matched healthy controls (n = 10, 2.5 ± 1.8 years old) and healthy adult volunteers (n = 8). Four KD patients received 2 g/kg of PHIG once while the others received 400 mg/kg of PHIG for 5 days. Blood samples were collected before PHIG therapy, within 2 days after PHIG therapy, and within 10 days after therapy when all patients were already afebrile. Patients with bacterial infection included three with streptococcal tonsillitis, two with Escherichia coli urinary tract infections, two with Haemophilus influenzae cellulitis, one with Salmonella colitis, and five with septicaemia (two H. influenzae, one E. coli, one Staphylococcus aureus, one Streptococcus viridans). Informed consent was obtained from parents of the children.
Statistical analysis
Statistically significant differences were assessed using Mann–Whitney U-test. Differences were considered significant if P < 0.05.
RESULTS
PHIG inhibits IFN-γ and IL-12 production by PBMC stimulated with SPE-A
In confirmation of earlier reports [7,8], PHIG significantly inhibited IFN-γ production by PBMC stimulated with SPE-A (Fig. 1). PHIG also greatly inhibited IL-12 production (Fig. 1). Production of both cytokines reached a plateau after 4–6 days of culture. Detected IL-12 was mainly p40 chain, because low levels of IL-12 p70 heterodimer (10–20 pg/ml) were detected by ELISA at only 48 h after SPE-A stimulation (data not shown). However, PHIG inhibited IL-12 p35 and p40 mRNA expression of PBMC stimulated by SPE-A (Fig. 2) (depending upon individuals, weak p35 mRNA expression was sometimes detectable in unstimulated PBMC). PHIG exerted a substantial inhibitory effect if added after culture started (data not shown).
Fig. 1.
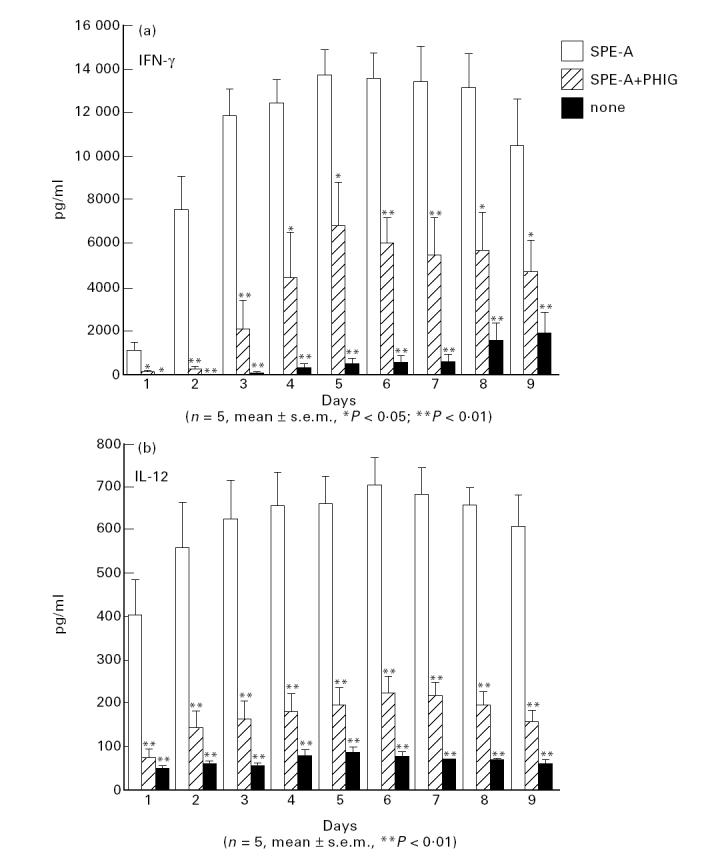
Time course of IFN-γ and IL-12 production by PBMC stimulated with Streptococcus pyrogenic exotoxin A (SPE-A) and the inhibitory effect of pooled human immunoglobulin (PHIG).
Fig. 2.
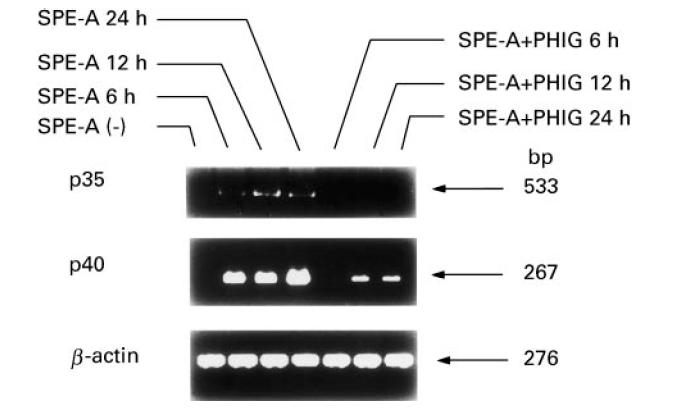
Inhibition by pooled human immunoglobulin (PHIG) of IL-12 p35 and p40 mRNA expression of PBMC stimulated with Streptococcus pyrogenic exotoxin A (SPE-A). PBMC were stimulated with SPE-A with or without PHIG for indicated periods and IL-12 p35 and p40 mRNA expression was examined by reverse transcriptase-polymerase chain reaction (RT-PCR).
Partial adsorption of SPE-A by PHIG
HPLC analysis revealed that 2 h incubation of SPE-A with PHIG reduced the area of the SPE-A peaks (retention time 15 min) from 292 274 ± 27 697 counts to 190 253 ± 26 358 counts (n = 3) (Fig. 3), whereas the areas of PHIG peaks increased from 1879 723 ± 7899 counts to 1996 568 ± 62 619 counts. Therefore, it was demonstrated that while we could not rule out the contribution of the non-specific binding, PHIG adsorbed 34.9% of SPE-A. However, the amounts of IFN-γ and IL-12 produced by PBMC stimulated with even 10 ng/ml SPE-A were not significantly different from those produced by PBMC stimulated with 100 ng/ml SPE-A (data not shown). Thus, although PHIG may contain SPE-A-neutralizing antibody, the inhibitory effect of PHIG on cytokine productions can not be explained by SPE-A-neutralizing antibody.
Fig. 3.
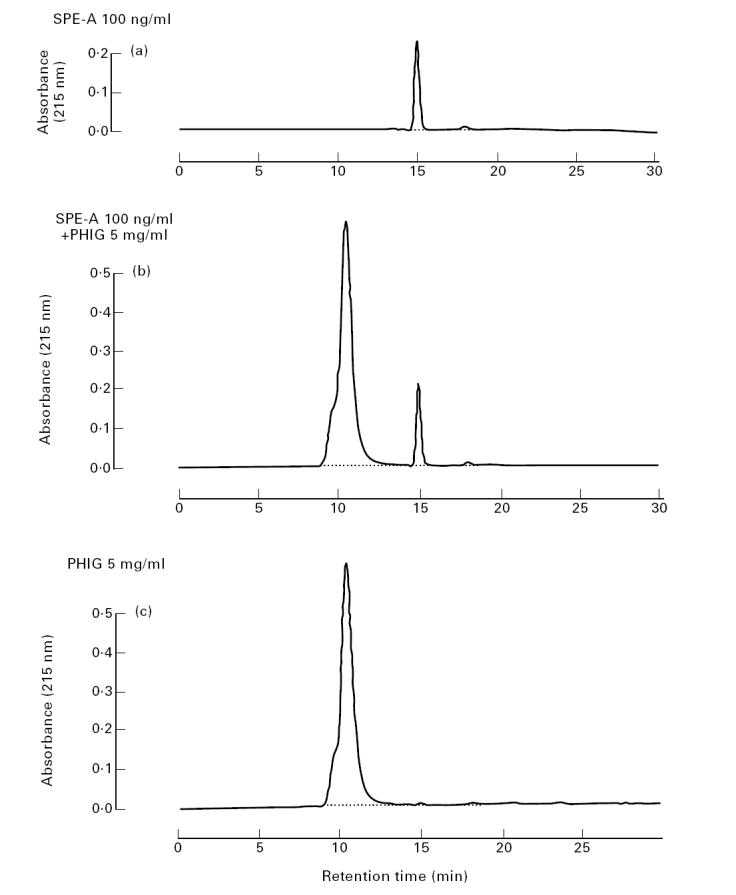
High performance liquid chromatography (HPLC) analysis of pooled human immunoglobulin (PHIG)-treated Streptococcus pyrogenic exotoxin A (SPE-A). SPE-A (100 ng/ml) was incubated with or without 5 mg/ml PHIG for 2 h. Then, buffer-diluted SPE-A (a), SPE-A + PHIG (b) and PHIG (c) were subjected to a gel permeation HPLC and areas of SPE-A and PHIG peaks were compared.
Exogenous IL-12 restores PHIG-inhibited IFN-γ production of PBMC stimulated with SPE-A but does not affect suppressed production of IL-1α, TNF-α, IL-1β, and PBMC proliferation
Although PHIG inhibits PBMC production of IFN-γ, the addition of exogenous IL-12 to the culture resulted in a significant recovery of IFN-γ production (Fig. 4). Similar up-regulatory effects of IL-12 were obtained if SPE-A and IL-12 were introduced to the culture after PBMC had been treated with PHIG for 2 h (not shown). Neither exogenous IL-1α nor TNF-α affected IFN-γ production (data not shown). PHIG also inhibited the production of IL-1α, TNF-α and TNF-β, but exogenous IL-12 did not significantly up-regulate the production of these cytokines (Fig. 4). IL-12 alone did not induce production of substantial amounts of IFN-γ (Fig. 4), indicating that IL-12-induced recovery of PHIG-inhibited IFN-γ production requires T cell–SPE-A interaction. Exogenous IL-12 significantly up-regulated IFN-γ production in a dose-dependent manner, but the effect reached a plateau at 500 pg/ml of IL-12 (not shown). Exogenous IL-12 did not restore the decreased SPE-A-stimulated PBMC proliferation (40% reduction) by PHIG.
Fig. 4.
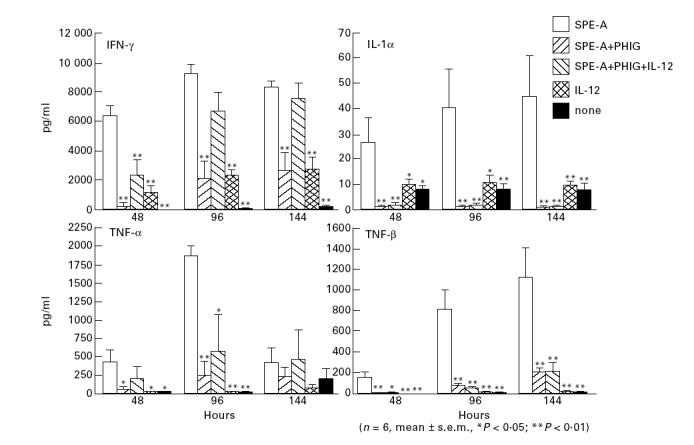
The effect of exogenous IL-12 on pooled human immunoglobulin (PHIG)-reduced cytokine synthesis of PBMC stimulated with Streptococcus pyrogenic exotoxin A (SPE-A).
IL-12 is required for IFN-γ production from CD4+ T cells stimulated with SPE-A in the presence of L cells
Since it is known that bacterial superantigens bind HLA-DR antigens of APC in an MHC-unrestricted manner and stimulate a large population of certain Vβ T cells [31], purified HLA-DR− CD4+ cells were cultured with mitomicin C-treated human DR molecule-transfected murine L cells. Although SPE-A alone could induce only small amounts of IFN-γ (Fig. 5), the addition of exogenous IL-12 with SPE-A induced the production of large amounts of IFN-γ, while IL-12 alone was not so effective (Fig. 5). These findings support that while MHC class II molecules of accessory cells present SPE-A to T cells or their T cell receptors, IL-12 is required for effective production of IFN-γ from T cells.
Fig. 5.
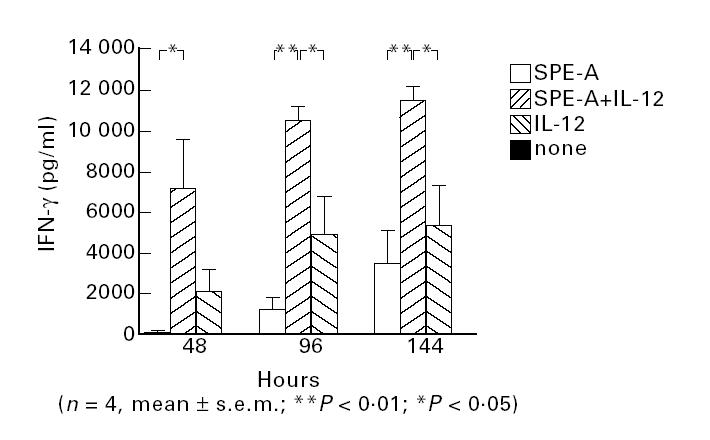
Requirement of exogenous IL-12 for effective IFN-γ production from CD4+ T cells cultured with L cells and stimulated with Streptococcus pyrogenic exotoxin A (SPE-A). HLA-DR− CD4+ T cells were purified by sorting and cultured with mitomicin C-treated L cells and SPE-A, and culture supernatants at indicated periods were subjected to ELISA.
Intravenous PHIG therapy in patients with KD decreases serum IL-12 and IFN-γ levels
Since bacterial superantigens were reportedly a candidate for the cause of KD and bacterial superantigens are potent inducers of IL-12, we examined serum IL-12, TNF-α and IFN-γ levels in KD patients. Patients in the acute phase of KD had significantly higher levels of serum IFN-γ (but not IL-12 or TNF-α) than age-matched patients with bacterial infection (P < 0.05) and controls (Fig. 6a). Interestingly, children had remarkably higher serum IL-12 levels than adults, whereas IFN-γ and TNF-α levels were similar (Fig. 6a). The IL-12 detected in sera was IL-12 p40; IL-12 p70 was not detected.
Fig. 6.
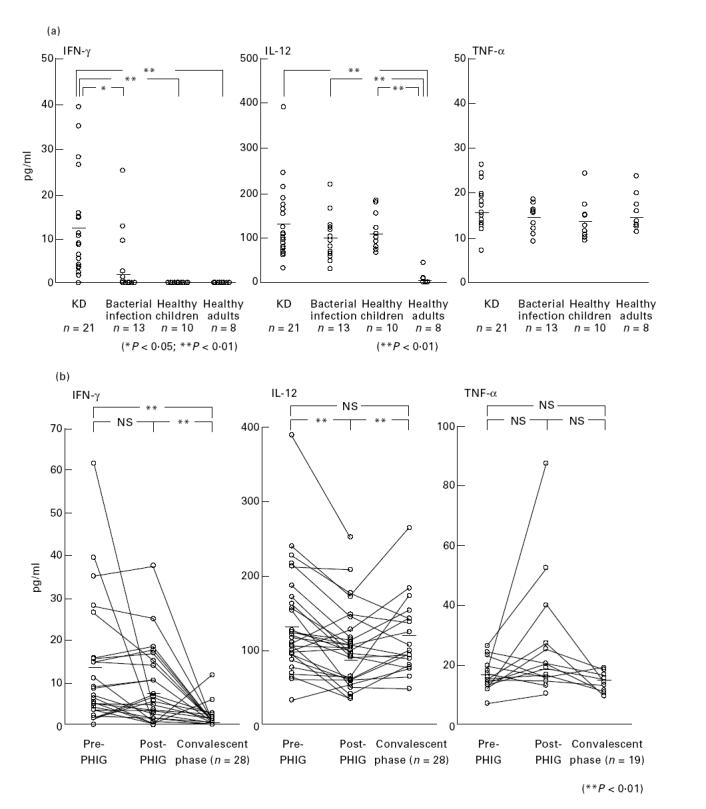
(a) Serum cytokine levels in patients with acute phase of KD, age-matched patients with bacterial infection, age-matched healthy children, and healthy adults. (b) The effect of pooled human immunoglobulin (PHIG) therapy on serum cytokine levels of KD patients. Serum cytokine levels of KD patients were compared at the acute phase, just after PHIG therapy, and at the convalescent phase. NS, Not significant.
PHIG therapy transiently but significantly decreased serum IL-12 in patients with KD (133.3 ± 16.7 versus 95.8 ± 12.4; P < 0.01) and IFN-γ levels started to decrease and reached basal levels within 10 days after PHIG therapy (12.6 ± 2.7 versus 1.5 ± 0.6; P < 0.01) (Fig. 6b). While PHIG therapy did not significantly affect TNF-α levels in KD patients, apparent increases of TNF-α levels immediately after PHIG therapy were observed in three patients (Fig. 6b).
DISCUSSION
In the present study, we demonstrated that PHIG inhibits IL-12 production from monocytes stimulated with SPE-A and thereby inhibits IFN-γ production by T cells. IFN-γ production recovered (if not fully) after introduction of exogenous IL-12 (but not IL-1α nor TNF-α) to the culture. Exogenous IL-12 did not affect the PHIG-induced reduced production of TNF-α, IL-1α and TNF-β. The inhibitory effect of PHIG on IL-12 and IFN-γ production was not due to SPE-A neutralizing antibody contained in PHIG. Serum IFN-γ levels were significantly elevated in febrile KD patients compared with patients with bacterial infection or controls, and i.v. PHIG therapy reduced serum IL-12 levels and seemed to trigger a decrease in serum IFN-γ.
IL-12 produced by phagocytic cells [11,12] stimulated with bacteria and bacterial components activates NK cells and T cells to produce IFN-γ. In a positive feedback loop, IFN-γ then further stimulates monocytes to secrete monokines [32] and augments other functions, probably including phagocytosis and antigen-presenting capacity. Thus, IL-12 and IFN-γ are essential cytokines against bacterial infection and induce T helper type 1 immune responses. IL-12 is also known to be an anti-tumour cytokine; it activates NK cells, conventional T cells [9–12,15,16] and T cells with NK cell markers [18,19,29,30,33,34] and endows potent anti-tumour activities to these cells in both human and mice. Another important function of IL-12 is its anti-parasitic effect; it is required for mouse resistance against Toxoplasma gondii and Leishmania major [35–37]. On the other hand, the role of IL-12 in viral infections is controversial; it aggravates viral infections in certain conditions [38].
It was reported that PHIG inhibits in vitro activation of PBMC by bacterial components or their factors; it inhibits proliferation and cytokine production of PBMC stimulated with LPS or certain bacterial superantigens [7,8,39]. The production of various monokines (IL-1, TNF-α) and other cytokines (IFN-γ, TNF-β) from PBMC stimulated with bacterial superantigens or LPS is reportedly blocked by PHIG in vitro [7]. In addition to these cytokines, we demonstrate here that PHIG inhibits IL-12 production of PBMC stimulated with a bacterial superantigen and may therefore reduce IFN-γ production by T cells. Although biologically active IL-12 p70 levels in culture supernatant of SPE-A-stimulated PBMC were much lower than IL-12 p40 (biologically inactive) levels, others also reported that significant IL-12 p70 levels were detected in culture supernatants of bacteria-stimulated monocytes only when monocytes had been pretreated with IFN-γ [40]. Trinchieri also reported that the levels of p70 heterodimer produced by IL-12-producing cell lines were even 50-fold lower than those of p40 chain [12,41]. Nevertheless, it is accepted that IL-12 p40 production correlates well with p70 production [42].
PHIG reportedly blocks the SPE-A binding of monocytes and/or presentation of SPE-A by monocyte MHC class II molecules to T cells [31]. Nevertheless, it can not solely explain the inhibitory effect of PHIG, because exogenous IL-12 indeed recovered IFN-γ production of T cells suppressed by PHIG in the presence of SPE-A while IL-12 could not induce an effective IFN-γ production in the absence of SPE-A. It is possible that PHIG may block costimulatory molecules of monocytes and T cells which are required for effective presentation of SPE-A or activation of these cells. It should be noted that since PHIG also decreased SPE-A-induced IL-10 production and staphylococcal enterotoxin B-induced production of IL-12 and IFN-γ (our unpublished data), it is suggested that PHIG broadly inhibits production of both proinflammatory and anti-inflammatory cytokines induced by bacterial superantigens. The finding that excess amounts of exogenous IL-12 p70 (500 pg/ml) were required to substantially recover IFN-γ production inhibited by PHIG could be explained by the possibility that PHIG inhibits not only the production of proinflammatory cytokines other than IL-12 (including IFN-γ-inducing factor, IL-18 and IL-15) but also antigen-presenting capacity of monocytes to T cells.
Some recent reports suggest that staphylococcus and streptococcus or their superantigens may be involved in KD [23–25]. These superantigen-producing bacteria were detected in rectums or throats of KD patients but not in control patients [25]. Further, bacteria superantigen-induced toxic shock syndrome or toxic shock-like syndromes mimic KD in some features [25], and several bacterial superantigen-specific Vβ T cells reportedly expand in patients with KD [23].
The finding that PHIG therapy transiently but significantly decreases serum IL-12 in patients with KD is consistent with the in vitro effect on cytokine production. However, it is of note that although IFN-γ levels were elevated only in the acute-phase KD patients, IL-12 levels were not significantly different among children with or without KD and much higher than those of adults. Matsubara et al. [43] also reported that IFN-γ levels are elevated in the acute phase of KD. Around 6 months after birth, maternally derived antibodies in children decrease and children may have to compensate for the reduction of antibodies. In other words, infants may be more susceptible to microbes than adults. Further, around 6 months after birth the process of weaning begins and the introduction of new foods introduces many microbes into the intestine and changes intestinal bacterial flora. Therefore, elevated serum IL-12 levels in infants could be a reasonable response to activate innate cellular immunity. However, IFN-γ levels may be suppressed by certain mechanisms in normal infants to avoid unnecessary immune reactions. In fact, daily injections of IL-12 into mice elevates serum IFN-γ only for the initial 48 h; IFN-γ amounts return to basal levels thereafter despite the continuance of IL-12 injections [12,16]. In KD patients, this balance may be broken [42] and an elevation of IFN-γ is induced. Masked bacterial infections or their components may trigger for this imbalance. PHIG may reverse the imbalance by a reduction of IL-12 (even if transient) and subsequent inhibition of IFN-γ synthesis.
According to previous reports [44,45] that PHIG contains staphylococcal superantigen-neutralizing antibodies, it is suggested here that PHIG may also include a substantial amount of anti-SPE-A antibody. There is still controversy regarding the exact cause of KD (bacterial superantigens, conventional bacterial antigens or other antigens) [23–26,46], and we do not conclude that bacterial superantigens are the cause of KD. However, the results of the present study suggest that while antigen- and cytokine-neutralizing antibodies in PHIG may be partly responsible for the effect of PHIG on KD, the inhibitory effect of PHIG on IL-12 production and other proinflammatory cytokine production can be one of the important mechanisms of the therapeutic effect of PHIG.
REFERENCES
- 1.The National Institute of Child Health and Human Development Intravenous Immunoglobulin Study Group. Intravenous immunoglobulin for the prevention of bacterial infections in children with symptomatic human immunodeficiency virus infection. N Engl J Med. 1991;325:73–80. doi: 10.1056/NEJM199107113250201. [DOI] [PubMed] [Google Scholar]
- 2.Imbach P, Barandun S, d'Apuzzo V, et al. High-dose intravenous gammaglobulin for idiopathic thrombocytopenic purpura in childhood. Lancet. 1981;i:1228–30. doi: 10.1016/s0140-6736(81)92400-4. [DOI] [PubMed] [Google Scholar]
- 3.Flores G, Cunningham-Rundles C, Newland AC, et al. Efficacy of intravenous immunoglobulin in the treatment of autoimmune hemolytic anemia. Am J Hematol. 1993;44:237–42. doi: 10.1002/ajh.2830440404. [DOI] [PubMed] [Google Scholar]
- 4.Dwyer JM. Drug therapy: manipulating the immune system with immuneglobulin. N Engl J Med. 1992;326:107–16. doi: 10.1056/NEJM199201093260206. [DOI] [PubMed] [Google Scholar]
- 5.Furusho K, Nakano H, Shinomiya K, et al. High-dose intravenous gamma-globulin for Kawasaki disease. Lancet. 1984;ii:1055–7. doi: 10.1016/s0140-6736(84)91504-6. [DOI] [PubMed] [Google Scholar]
- 6.Newburger JW, Takahashi M, Burns JC, et al. The treatment of Kawasaki syndrome with intravenous immunoglobulin. N Engl J Med. 1986;315:341–7. doi: 10.1056/NEJM198608073150601. [DOI] [PubMed] [Google Scholar]
- 7.Abe Y, Horiuchi A, Miyake M, et al. Anti-cytokine nature of natural human immunoglobulin: one possible mechanism of the clinical effect of intravenous immunoglobulin therapy. Immunol Rev. 1994;139:5–19. doi: 10.1111/j.1600-065x.1994.tb00854.x. [DOI] [PubMed] [Google Scholar]
- 8.Skansen-Saphir U, Andersson J, Bjork L, et al. Lymphokine production induced by streptococcus pyrogenic exotoxin-A is selectively down-regulated by pooled human IgG. Eur J Immunol. 1994;24:916–22. doi: 10.1002/eji.1830240420. [DOI] [PubMed] [Google Scholar]
- 9.Wolf SF, Temple PA, Kobayashi M, et al. Cloning of cDNA for natural killer cell stimulatory factor, a heterodimeric cytokine with multiple biologic effects on T and natural killer cells. J Immunol. 1991;146:3074–81. [PubMed] [Google Scholar]
- 10.Schoenhaut DS, Chua O, Wolitzky AG, et al. Cloning and expression of murine IL-12. J Immunol. 1992;148:3433–40. [PubMed] [Google Scholar]
- 11.Brunda MJ. Interleukin-12. J Leuk Biol. 1994;55:280–8. doi: 10.1002/jlb.55.2.280. [DOI] [PubMed] [Google Scholar]
- 12.Trinchieri G. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu Rev Immunol. 1995;13:251–76. doi: 10.1146/annurev.iy.13.040195.001343. [DOI] [PubMed] [Google Scholar]
- 13.Ozman L, Pericin M, Hakimi J, et al. Interleukin 12, interferon γ, and tumor necrosis factor α are the key cytokines of the generalized Shwartzman reaction. J Exp Med. 1994;180:907–15. doi: 10.1084/jem.180.3.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wysocka M, Kubin M, Vieira LQ, et al. Interleukin-12 is required for interferon-γ production and lethality in lipopolysaccharide-induced shock in mice. Eur J Immunol. 1995;25:672–6. doi: 10.1002/eji.1830250307. [DOI] [PubMed] [Google Scholar]
- 15.Nastala CL, Edington HD, McKinney TG, et al. Recombinant IL-12 administration induces tumor regression in association with IFN-γ production. J Immunol. 1994;153:1697–706. [PubMed] [Google Scholar]
- 16.Gately MK, Warrier RR, Honasoge S, et al. Administration of recombinant IL-12 to normal mice enhances the cytolytic lymphocyte activity and induces the production of interferon-gamma in vivo. Int Immunol. 1994;6:157–67. doi: 10.1093/intimm/6.1.157. [DOI] [PubMed] [Google Scholar]
- 17.Magram J, Connaughton SE, Warrier RR, et al. IL-12-deficient mice are defective in IFN-γ production and type 1 cytokine response. Immunity. 1996;4:471–81. doi: 10.1016/s1074-7613(00)80413-6. [DOI] [PubMed] [Google Scholar]
- 18.Ogasawara K, Takeda K, Hashimoto W, et al. Involvement of NK1+ T cells and their IFN-γ production in the generalized Shwartzman reaction. J Immunol. 1998;160:3522–7. [PubMed] [Google Scholar]
- 19.Hashimoto W, Takeda K, Anzai R, et al. Cytotoxic NK1.1 Ag+αβ T cells with intermediate TCR induced in the liver of mice by IL-12. J Immunol. 1995;154:4333–40. [PubMed] [Google Scholar]
- 20.Kawasaki T, Kosaki F, Okawa S, et al. New infantile acute febrile mucocutaneous lymph node syndrome prevailing in Japan. Pediatrics. 1974;54:271–6. [PubMed] [Google Scholar]
- 21.Kato H, Ichinose E, Yoshioka F, et al. Fate of coronary aneurysms in Kawasaki disease: serial coronary angiography and long-term follow-up study. Am J Cardiol. 1982;49:1758–66. doi: 10.1016/0002-9149(82)90256-9. [DOI] [PubMed] [Google Scholar]
- 22.Suzuki A, Kamiya T, Kuwahara N, et al. Coronary arterial lesions of Kawasaki disease: cardiac catheterization findings of 1100 cases. Pediatr Cardiol. 1986;7:3–9. doi: 10.1007/BF02315475. [DOI] [PubMed] [Google Scholar]
- 23.Abe J, Kotzin BL, Jujo K, et al. Selective expansion of T cells expressing T cell receptor variable regions Vβ2 and Vβ8 in Kawasaki disease. Proc Natl Acad Sci USA. 1992;89:4066–70. doi: 10.1073/pnas.89.9.4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Curtis N, Zheng R, Lamb JR, et al. Evidence for a superantigen mediated process in Kawasaki disease. Arc Dis Child. 1995;72:308–11. doi: 10.1136/adc.72.4.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leung DYM, Meissner HC, Fulton DR, et al. Toxic shock syndrome toxin-secreting Staphylococcus aureus in Kawasaki syndrome. Lancet. 1993;342:1385–8. doi: 10.1016/0140-6736(93)92752-f. [DOI] [PubMed] [Google Scholar]
- 26.Sato K, Ouchi K, Taki M. Yersinia pseudotuberculosis infection on children, resembling Izumi fever and Kawasaki disease. Pediatr Infect Dis. 1983;2:123–6. doi: 10.1097/00006454-198303000-00011. [DOI] [PubMed] [Google Scholar]
- 27.Kamezawa Y, Nakahara T. Purification and characterization of streptococcal erythrogenic toxin type A produced by Streptococcus pyogenes strain NY-5 cultured in the synthetic medium NCTC-135: comparison with the dialyzed medium (TP-medium)-derived toxin. Microbiol Immunol. 1989;33:183–94. doi: 10.1111/j.1348-0421.1989.tb01512.x. [DOI] [PubMed] [Google Scholar]
- 28.Warrens AN, Heaton T, Sidhu S, et al. Transfected murine cells expressing HLA class II can be used to generate alloreactive human T cells. J Immunol Methods. 1994;169:25–33. doi: 10.1016/0022-1759(94)90121-x. [DOI] [PubMed] [Google Scholar]
- 29.Takahashi M, Ogasawara K, Takeda K, et al. LPS induces NK1.1+αβ T cells with potent cytotoxicity in the liver of mice via production of IL-12 from Kupffer cells. J Immunol. 1996;156:2436–42. [PubMed] [Google Scholar]
- 30.Seki S, Hashimoto W, Ogasawara K, et al. Antimetastatic effect of NK1+ T cells on experimental haematogenous tumor metastases in the liver and lungs of mice. Immunology. 1997;92:561–6. doi: 10.1046/j.1365-2567.1997.00383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gascogine NR, Ames KT. Direct binding of secreted T-cell receptor beta chain to superantigen associated with class II histocompatibility complex protein. Proc Natl Acad Sci USA. 1991;88:613–6. doi: 10.1073/pnas.88.2.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ma X, Chow JM, Gri G, et al. The interleukin 12 p40 gene promoter is primed by interferon γ in monocytic cells. J Exp Med. 1996;183:147–57. doi: 10.1084/jem.183.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takeda K, Seki S, Ogasawara K, et al. Liver NK1.1+ CD4+αβ T cells activated by IL-12 as a major effector in inhibition of experimental tumor metastasis. J Immunol. 1996;156:3366–73. [PubMed] [Google Scholar]
- 34.Satoh M, Seki S, Hashimoto W, et al. Cytotoxic γδ or αβ T cells with a natural killer cell marker, CD56, induced from human peripheral blood lymphocytes by a combination of IL-12 and IL-2. J Immunol. 1996;157:3886–92. [PubMed] [Google Scholar]
- 35.Heinzel FP, Schoenhaut DS, Rerko RM, et al. Recombinant interleukin 12 cures mice infected with Leishmania major. J Exp Med. 1993;177:1505–9. doi: 10.1084/jem.177.5.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sypek JP, Chung CL, Mayor SEH, et al. Resolution of cutaneous leishmaniasis: interleukin 12 initiates a protective T helper type 1 immune response. J Exp Med. 1993;177:1797–802. doi: 10.1084/jem.177.6.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khan IA, Matsuura T, Kasper LH. Interleukin-12 enhances murine survival against acute toxoplasmosis. Infect Immun. 1994;62:1639–42. doi: 10.1128/iai.62.5.1639-1642.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Orange JS, Wolf SF, Biron CA. Effect of IL-12 on the response and susceptibility to experimental viral infections. J Immunol. 1994;152:1253–64. [PubMed] [Google Scholar]
- 39.Anderson UG, Bjork L, Skansen-Saphir U, et al. Down-regulation of cytokine production and interleukin-2 receptor expression by pooled human IgG. Immunology. 1993;79:211–6. [PMC free article] [PubMed] [Google Scholar]
- 40.Matsumoto H, Suzuki K, Tsuyuguchi K, et al. Interleukin-12 gene expression in human monocyte-derived macrophages stimulated with Mycobacterium bovis BCG. Cytokine regulation and effect of NK cells. Infect Immun. 1997;65:4405–10. doi: 10.1128/iai.65.11.4405-4410.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Trinchieri G. Interleukin-12: a cytokine produced by antigen-presenting cells with immunoregulatory functions in the generation of T-helper cells type 1 and cytotoxic lymphocytes. Blood. 1994;84:4008–27. [PubMed] [Google Scholar]
- 42.Leung DYM, Burns JC, Newburger JW, et al. Reversal of lymphocyte activation in Kawasaki syndrome by intravenous gammaglobulin. J Clin Invest. 1987;79:468–72. doi: 10.1172/JCI112835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Matsubara T, Furukawa S, Yabuta K. Serum levels of tumor necrosis factor, interleukin 2 receptor, and interferon-gamma in Kawasaki disease involved coronary-artery lesions. Clin Immunol Immunopathol. 1990;56:29–36. doi: 10.1016/0090-1229(90)90166-n. [DOI] [PubMed] [Google Scholar]
- 44.Takei S, Arora YK, Walker SM. Intravenous immunoglobulin contains specific antibodies inhibitory to activation of T cells by staphylococcal toxin superantigens. J Clin Invest. 1993;91:602–7. doi: 10.1172/JCI116240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Leung DYM. Kawasaki syndrome: immunomodulatory benefit and potential toxin neutralization by intravenous immune globulin. Clin Exp Immunol. 1996;104(Suppl. 1):9–54. [PubMed] [Google Scholar]
- 46.Pietra BA, De Inocencio J, Giannini EH, et al. TCR V beta family repertoire and T cell activation markers in Kawasaki disease. J Immunol. 1994;153:1881–8. [PubMed] [Google Scholar]


