Abstract
MHC class II molecules present to CD4+ T cells protein fragments which mostly derive from the extracellular and from the endosomal compartments. Determinants of cytosolic proteins are, however, also displayed by MHC class II molecules following pathways which are still not yet fully characterized. Here we describe the isolation of DRB1*1103-restricted T cell clones specific for the measles virus (MV) nucleoprotein peptide 185–199 (N185). Experiments were then conducted to delineate how this determinant is assembled with DR molecules. In vitro binding analyses indicated that complexes between the N185 peptide and DRB1*1103 protein are optimally constituted at pH 4–4.5. In cellular experiments it was observed that chloroquine, leupeptin and emetine, which are classical inhibitors of presentation of MHC class II-restricted antigens, when added during infection of B cells with MV, prevent presentation of the N185 determinant. In addition, it was found that the N185 determinant is efficiently presented when the nucleoprotein is exogenously provided to B cells, either by blocking MV fusion with the peptide FFG or by the use of purified nucleoprotein. In contrast, it was observed that nucleoprotein recombinant vaccinia virus (vv-N)-infected B cells weakly stimulated N185-specific T cells, indicating that the restricted localization of the nucleoprotein in the cytosol resulted in a poor presentation of the N185 determinant. Taken together, these findings suggest that it is prior to delivery of the nucleoprotein into the cytosol that the N185 determinant is efficiently assembled with newly synthesized DR molecules in the acidic environment of the endosomal compartment.
Keywords: antigen processing and presentation, human T cells, measles virus, MHC class II, peptide binding
INTRODUCTION
The origin of the antigen fragments recognized by CD4+ T cells is dictated by the biosynthesis route of MHC class II molecules. Shortly after synthesis, MHC class II αβ heterodimers constitute large complexes with a third polypeptide, the invariant chain, which has the dual function of preventing premature peptide loading of MHC class II molecules and targeting MHC class II molecules to a specialized endosomal compartment [1–11]. There the invariant chain undergoes a gradual proteolytic degradation resulting in the release of MHC class II molecules whose peptide binding site remains occupied by invariant chain fragments. Their dissociation is catalysed by the MHC class II-like protein HLA-DM and the peptide-free MHC class II molecules constitute stable complexes with peptides generated during proteolysis of polypeptides in the endosomal and lysosomal compartments [12,13]. These complexes are finally delivered to the plasma membrane for inspection by T cells [14]. It is expected from this sequence of events that MHC class II molecules are mostly loaded with peptides originating from proteins of the extracellular and endosomal compartments. Sequencing of peptides extracted from MHC class II preparations indeed confirmed this assumption [15]. Intriguingly, some peptides constitutively displayed by MHC class II molecules derive from cytosolic proteins [15,16]. In addition, isolation of CD4+ T cells specific for cytosolic antigen was reported in several instances [17,18]. How such determinants are loaded onto MHC class II molecules is still largely unknown. One possibility is that these proteins, together with portions of the cytosol, are engulfed by endoplasmic reticulum-derived membranes, a process termed autophagy. The resulting double-layered vesicles then fuse with lysosomes, leading to degradation of the ingested cytosolic components [19]. Some cytosolic proteins can be also translocated as such or as large fragments into lysosomes, a process mediated by the heat shock protein (hsp)70 [20]. Formally, one may also hypothesize that peptides, produced from cytosolic proteins by the action of the proteasome, or other proteolytic machineries of the cytosol, could be transported into the endoplasmic reticulum through the transporters associated with antigen processing for binding by newly assembled MHC class II molecules. Alternatively, determinants of cytosolic proteins from infectious agents could be captured by MHC class II molecules upon intersection with the endosomal compartment, i.e. before delivery into the cytosol.
Over recent years many studies have been conducted to unravel the processing pathways followed by determinants borne by cytosolic polypeptides. Interestingly, it was established that MHC class II molecules can capture cytosol-derived determinants in a pre-Golgi compartment [21–24]. However, alternative processing requirements were observed in other experimental systems [25–31]. Still, how determinants are transported from the cytoplasm to the endosomal compartment for loading on MHC class II molecules remains to be elucidated.
Here we develop a method which allowed us to isolate DRB1*1103-restricted T cell clones specific for the measles virus (MV) N185 peptide. This simple procedure is applicable to the isolation of T cells of most antigen specificities. Then in vitro and in vivo studies are reported which indicate that the N185 determinant is bound by newly synthesized DR molecules in an acidic endosomal compartment. This conclusion supports the notion that following MV infection, the MHC class II molecules bind the N185 determinant prior to its delivery into the cytosol when it is still localized in the endosomal compartment.
MATERIALS AND METHODS
Cell lines and viruses
Tissue culture and virus propagation were performed as previously described [32]. Recombinant vaccinia viruses carrying the MV fusion protein (vv-F) and the nucleoprotein (vv-N) genes were kind gifts from Dr F. Wild (INSERM U404, Institut Pasteur de Lyon, France).
The nucleoprotein-specific (N-specific) T cell clones N5 and N2 were isolated from peripheral blood mononuclear cells (PBMC) of a healthy donor. PBMC (1.4 × 105) in 200 μl RPMI medium supplemented with 5% human serum (HS) were restimulated with 2 μg cyanogen bromide (CNBr)-digested nucleoprotein. Then the procedure previously reported was followed [32].
Nucleoprotein purification
Crude preparations of nucleoprotein were isolated as described [33]. HeLa cells infected with vv-N for 24 h were collected, washed once with PBS and resuspended in 1 ml 0.1 m HEPES and 0.05 m NH4Cl, pH 8.0, supplemented with 1 mm dithiothreitol and 0.25% Nonidet P40. After 30 min on ice, the lysate was centrifuged for 15 min at 800 g and 4°C. The supernatant was then layered on a 3-ml cushion of 30% glycerol in 0.1 m HEPES and 0.05 m NH4Cl pH 8.0 and spun for 90 min at 75 000 g and 4°C (SW55TI rotor; Beckman Instruments, Palo Alto, CA). Fractions (1 ml) were collected and the pellet was resuspended in 500 μl PBS. Protein samples were separated by 10% SDS–PAGE and then analysed by Western blotting as described [34] using 1 μg/ml purified anti-N MoAb BNP173 [35].
Peptides and peptide–DR binding assay
Samples of nucleoprotein were reduced and alkylated in urea and then digested with CNBr as described [36]. Protein and peptide samples were analysed by coomassie staining of 10% SDS–PAGE.
Peptides were synthesized and biotinylated as reported [37]. Sequences were PDTAADSELRRWIKY (N185) and GDLLGILESRGIKAR (MV fusion protein peptide 254–268: F254).
Binding between DR and biotinylated peptides was measured as described [37].
T cell proliferation assay
T cell proliferation assays were performed essentially as reported [32]. B cells were pretreated for 3 h at 37°C with 50 μm chloroquine, 400 μm leupeptin, 4 μm emetine, 400 μm Z-D-FFG peptide (Bachem, Bubendorf, Switzerland) or 1% normal AB HS before addition of MV. Ultraviolet-treated MV (UV-MV) was prepared as described [32].
RESULTS AND DISCUSSION
Isolation of MV nucleoprotein
In preliminary experiments, vv-N-infected B cells were used to restimulate PBMC and to screen panels of MV-specific T cell clones. Since following this approach we repeatedly failed to isolate N-specific T cell clones, we developed an alternative strategy using nucleoprotein peptides to restimulate PBMC. A procedure to isolate preparations of nucleoprotein was therefore first established. Taking advantage of the capacity of the nucleoprotein to self-assemble into nucleocapsids even in the absence of other MV components, nucleocapsid preparations were purified from lysates of vv-N-infected HeLa cells. The presence of nucleoprotein in the different fractions was assessed by Western blotting analysis. The nucleoprotein was strongly detected by the anti-N MoAb BNP173 in the nucleus fraction as a band of 63 kD. While the nucleoprotein was barely detectable in the supernatant fractions, a large amount was found in the pellet (Fig. 1a). Coomassie-stained SDS–PAGE indicated that this material contained a prominent component of 63 kD corresponding to the nucleoprotein (Fig. 1b).
Fig. 1.
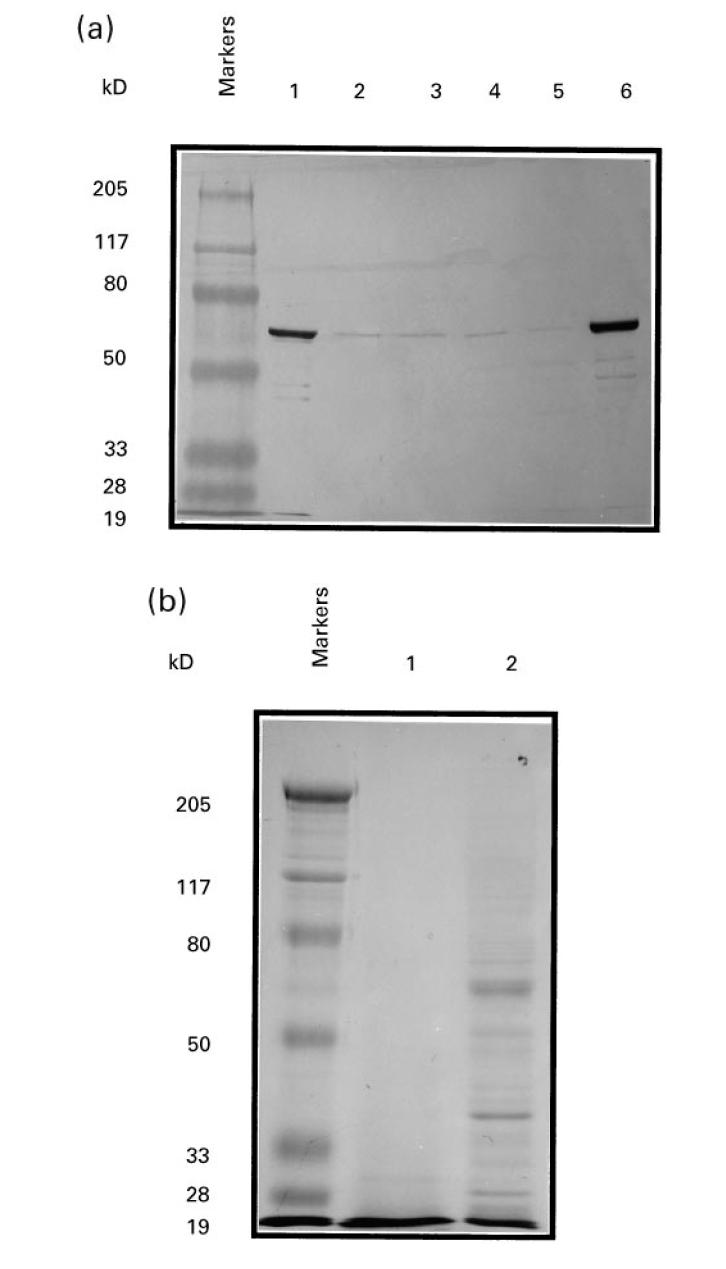
Preparation of measles virus (MV) nucleoprotein and cyanogen bromide (CNBr)-digested nucleoprotein. (a) Post-nuclear lysate of recombinant vaccinia virus containing the nucleoprotein gene (vv-N)-infected HeLa cells was fractionated by centrifugation through a glycerol cushion. Aliquots of the fractions (3 μg) were separated by 10% SDS–PAGE and analysed by Western blotting using the anti-N MoAb BNP173. Lane 1, pellet from high speed centrifugation of post-nuclear vv-N-infected HeLa cell lysate; lanes 2–5, bottom to top 1-ml fractions of supernatant from high-speed centrifugation of post-nuclear vv-N-infected HeLa cell lysate; lane 6, pellet from low-speed centrifugation of vv-N-infected HeLa cell lysate. (b) Reduced and alkylated nucleoprotein material was digested with CNBr, separated by 10% SDS–PAGE and stained with coomassie blue. Lane 1, CNBr-digested nucleoprotein (20 μg); lane 2, nucleoprotein (5 μg).
Nucleoprotein peptides were then generated by CNBr digestion of reduced and alkylated nucleoprotein material. Using this procedure, the nucleoprotein was cleaved into 15 fragments ranging from 4 up to 120 residues. Coomassie-stained SDS–PAGE of CNBr-digested nucleoprotein showed that only material with apparent molecular mass < 30 kD was detected, indicating that the nucleoprotein samples were effectively fragmented (Fig. 1b).
Isolation of N185-specific, DRB1*1103-restricted T cell clones
When panels of MV-specific T cell clones were generated by restimulation of PBMC with MV, only fusion protein-specific T cells were isolated [32]. To favour growth of N-specific T cells relative to those specific for other MV proteins, PBMC were restimulated with CNBr-digested nucleoprotein. It was postulated that a more vigorous restimulation of N-specific T cells might be obtained with nucleoprotein peptides than with intact nucleoprotein which requires processing by antigen-presenting cells (APC) prior to presentation to T cells. Subsequently, T cell clones were isolated from lines specific for MV-infected autologous B cells. These clones were then tested for their capacity to proliferate upon stimulation with various APC preparations. Results obtained with one typical T cell clone from a panel of 10 independently isolated T cell clones are presented in Fig. 2a. B cells infected with MV or vv-N, or supplied with CNBr-digested nucleoprotein, induced proliferation of N5 T cells, whereas uninfected or vv-F-infected B cells were not stimulatory. These observations indicated that the N5 T cell clone was specific for the MV nucleoprotein. The procedure for the isolation of N-specific T cell clones could be certainly applied to many other antigens for which it has been difficult to isolate specific T cells. An attractive aspect of this simple method is that it does not require pure preparations of antigen. This procedure was indeed successfully followed for the isolation of MV fusion protein-specific T cells using a CNBr digest of partially purified recombinant fusion protein expressed in Escherichia coli (S.D., unpublished results). In addition, since in this method T cells are restimulated with peptides which do not require processing, it is believed that under these circumstances the sets of T cell clones isolated are not biased by the processing events of whole proteins, but rather reflect the population of T cells present after natural encounter with antigen.
Fig. 2.
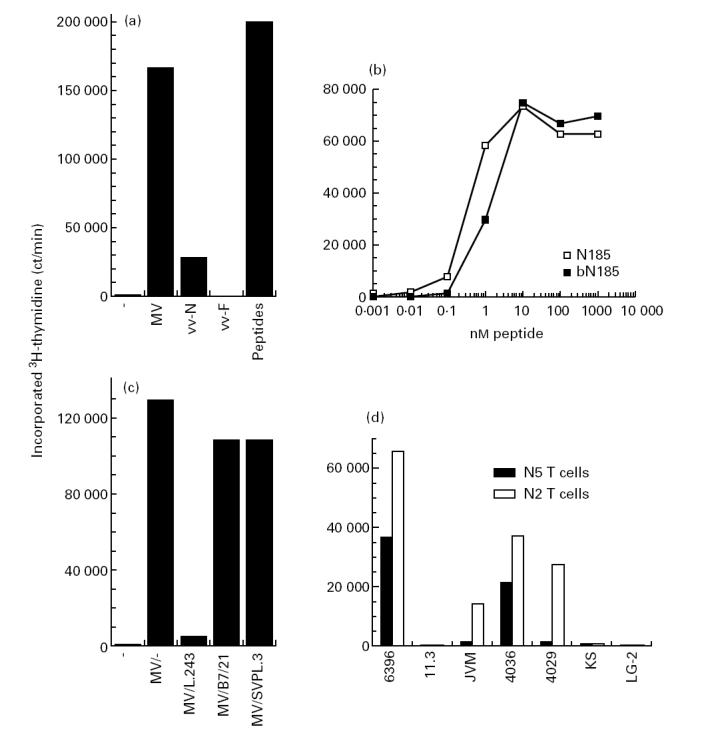
Isolation of N185 peptide-specific, DRB1*1103-restricted T cell clones. T cells (3 × 104) were added to γ-irradiated B cells (2 × 104) with the indicated antigen. After 2 days, 1 μCi 3H-thymidine was added for the last 18 h of culture and incorporated radioactivity was determined. (a) Proliferation of N5 T cells was measured following stimulation by uninfected 6396 B cells (−), measles virus (MV)-infected 6396 B cells (MV), recombinant vaccinia virus containing the nucleoprotein gene (vv-N)-infected 6396 B cells (vv-N), recombinant vaccinia virus carrying the MV fusion protein (vv-F)-infected 6396 B cells (vv-F) and uninfected 6396 B cells in the presence of 1 μg cyanogen bromide (CNBr)-digested nucleoprotein (peptides). (b) Proliferation of N5 T cells was measured following stimulation by 6396 B cells and graded concentrations of N185 or bN185 peptide. (c) Proliferation of N5 T cells was measured following stimulation by uninfected 6396 B cells (−) or MV-infected 6396 B cells in the absence (MV/-) or the presence of anti-DR MoAb L.243 (MV/L.243), anti-DP MoAb B7/21 (MV/B7/21) or anti-DQ MoAb SVPL.3 (MV/SVPL.3). (d) Proliferation of N5 and N2 T cells was measured following stimulation by various MV-infected B cell lines (6396: DR1,DRw11; 11.3: DRB1*1101,DR3; JVM: DRB1*1102 homozygous; 4036: DRB1*1103,DR4; 4029: DRB1*1102,DRB1*1104; KS: DRB1*1104,DR6; and LG-2: DRB1*0101 homozygous). Values are means of duplicates. Variations were < 15% of the mean.
To localize precisely the region which contains the determinant recognized by the N5 T cells, 140 synthetic peptides of 15 residues covering the whole nucleoprotein sequence were tested for their capacity to induce T cell proliferation [35]. The N peptides 185–199 and 189–203 were the only ones found to induce proliferation of the N5 T cells. Titration showed that the N185 peptide is 100-fold more active than the N peptide 189–203 (data not shown). Then the stimulatory capacities of the unmodified N185 peptide and of the biotinylated N185 peptide (bN185) were compared. Half-maximal N5 T cell proliferation was obtained with 0.4 and 1.3 nm of N185 and bN185 peptide, respectively (Fig. 2b). Albeit the antigenicity of the bN185 peptide is decreased relative to its unmodified counterpart, we assumed that the bN185 peptide constitutes a suitable N185 peptide homologue for MHC class II binding studies (see below).
The MHC class II restriction element utilized by the N185 determinant was then defined by the use of MoAbs directed against the different MHC class II isotypes. The anti-DR MoAb L.243 completely abrogated N5 T cell proliferation induced by MV-infected B cells, while the anti-DP MoAb B7/21 and anti-DQ MoAb SVPL.3 did not prevent proliferation (Fig. 2c). These observations indicate that the N185 determinant is presented to N5 T cells by DR molecules. Since HLA typing established that the N5 T cell clone was isolated from a DR1,w11 donor, B cell lines expressing either DRB1*0101 or various DRw11 subtypes were tested for their capacity to present the N185 determinant to T cell clones. Among the different MV-infected B cell lines analysed, the N5 T cell clone was only stimulated by the autologous B cell line 6396 (DRB1*0101,DRw11) and the B cell line 4036 (DRB1*1103,DR4), while it was not by the B cell lines JVM (DRB1*1102 homozygous), 4029 (DRB1*1102,DRB1*1104), 11.3 (DRB1*1101,DR3), KS (DRB1*1104,DR6) and LG-2 (DRB1*0101 homozygous). Surprisingly, the independently isolated N185-specific T cell clone N2 was, in contrast, also stimulated by the B cell lines JVM (DRB1*1102 homozygous) and 4029 (DRB1*1102,DRB1*1104) (Fig. 2d). Since the T cell clones studied distinguished between different DRw11 subtypes, it ruled out that DRw52 molecules, which are co-expressed with DRw11 molecules, could be the restriction element [38]. This conclusion is supported by the observation that DRw52-transfected L cells failed to present the N185 peptide to these two T cell clones ([36] and data not shown). Together, these results establish that both DRB1*1102 and DRB1*1103 molecules bind and present to T cells the N185 determinant.
The DRB1*1103 and DRB1*1102 alleles differ from the two more frequent DRB1*1101 and DRB1*1104 alleles only in position 71, where an arginine is changed for a glutamic acid [39]. Analysis of the crystal structure of the DR1 protein indicated that this residue is critically involved in peptide binding [40]. Because of this dramatic modification in the P4 pocket, it is expected that the DRB1*1101 and DRB1*1104 alleles do not bind, or at best poorly, the N185 determinant.
Optimal binding between the N185 peptide and DRB1*1103 protein at acidic pH
To outline the MHC class II processing requirements of the N185 determinant, we undertook to characterize the interaction between this determinant and DRB1*1103 molecules. A capture binding assay was first developed in which complexes between bN185 peptide and DR protein are captured in wells coated with anti-DR MoAb and subsequently revealed with streptavidin–peroxidase conjugate. Since no DRB1*1103 homozygous B cell lines were available, the autologous B cell line 6396 was used as a source of DRB1*1103 protein. These preparations, however, contained both DRB1*0101 and DRB1*1103 molecules. Thus, it was first established that the N185 peptide binds only to DRB1*1103 and not to DRB1*0101 molecules. DR preparations from 6396 cells (DRB1*0101 + DRB1*1103) and from LG-2 cells (DRB1*0101) were incubated with graded concentrations of bN185 or bF254 peptide. It was observed that binding of the bN185 peptide was > 100-fold higher with DRB1*0101 + DRB1*1103 than with DRB1*0101 protein alone (Fig. 3a). Binding analysis using the DRB1*0101-restricted F254 peptide showed that both preparations had a similar capacity to bind this peptide, indicating that the DRB1*0101 molecules in these two preparations were active (Fig. 3b). Together, these results show that the N185 peptide binds to DRB1*1103 and to a negligible extent to DRB1*0101 protein.
Fig. 3.
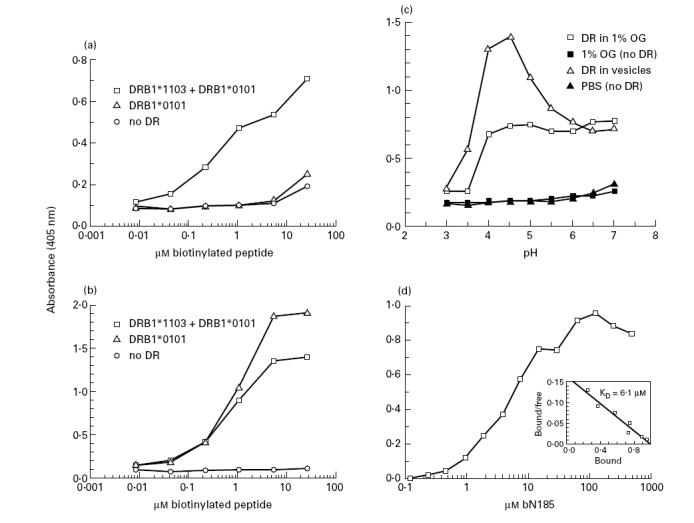
Optimal binding between the N185 and DRB1*1103 protein at acidic pH. Biotinylated peptide and DR samples (10 nm) were incubated under various conditions for 2 days at 37°C. Biotinylated peptide–DR complexes were then captured and revealed in wells of microtitre plates. (a,b) Increasing concentrations of bN185 and bF254 peptide were incubated with DR material in 1% n-octyl β-d-glucopyranoside isolated from 6396 B cells (DRB1*1103 + DRB1*0101) or LG-2 B cells (DRB1*0101) or without DR (no DR) at pH 4.5. (a) bN185 peptide, (b) bF254 peptide, (c) bN185 peptide (16 μm) were incubated with or without purified DRB1*1103 samples either solubilized in 1% n-octyl β-d-glucopyranoside or in lipid vesicles in 100 mm citrate-phosphate buffer at the indicated pH. (d) Increasing concentrations of bN185 peptide were incubated at pH 4.5 with DRB1*1103 samples reconstituted in lipid vesicles. The KD value for the bN185–DRB1*1103 complexes was calculated by Scatchard analysis (d, insert). Values are means of duplicates. Variations were < 15% of the mean. Results are representative of at least three independent experiments.
It is now well established that for many determinants, binding to MHC class II molecules takes place in the acidic environment of a specialized endosomal compartment [7–11]. To establish whether this is also the case for the N185 determinant, we investigated what were the optimal pH conditions of binding between the N185 peptide and DRB1*1103 molecules. Since several studies showed that peptide binding is influenced by the pH conditions only when MHC class II molecules are inserted in lipid membranes, binding analyses were conducted in detergent and in lipid vesicles [37,41]. It was observed that the DRB1*1103 protein solubilized in detergent binds the N185 peptide to a similar extent over a broad range of pH, from pH 4 to 7. By contrast, the DRB1*1103 protein inserted in lipid vesicles markedly binds more efficiently the N185 peptide at pH 4–4.5 than at higher pH values. In both instances, binding was abrogated at pH < 4 (Fig. 3c). The observation that in vitro N185 peptide binding to DRB1*1103 was favoured at acidic pH supports the hypothesis that the naturally processed N185 determinant is bound by DRB1*1103 molecules in the acidic milieu of specialized endosomes [7–11].
The affinity constant (KD) of the interaction between the N185 peptide and DRB1*1103 protein was then evaluated under optimal acidic binding conditions. The binding kinetic between the N185 peptide and DRB1*1103 molecules was first established to determine the incubation time required to reach equilibrium. It was found that > 90% binding obtained after 96 h was already reached in 48 h, indicating that after 48 h incubation, the interaction between the N185 peptide and the DRB1*1103 molecules was close to equilibrium (data not shown). A constant amount of DRB1*1103 protein in lipid vesicles was incubated with increasing concentrations of bN185 peptide for 48 h at pH 4.5. Maximal binding was obtained with 50 μm bN185 peptide (Fig. 3d). By Scatchard analysis, it was estimated that the KD of the bN185–DRB1*1103 complexes is 6.1 μm (insert of Fig. 3d). This KD is within the range of values reported for other DR-restricted determinants [37].
Endosomal processing of the N185 determinant
By the use of different antigen preparations and inhibitors interfering with various cellular functions, the processing requirements of the N185 determinant were further characterized. Before addition of MV, B cells were pretreated for 3 h with the inhibitors to ensure that the N185 determinant was captured only by DR molecules synthesized after addition of the inhibitors. Following overnight infection, B cells were fixed with glutaraldehyde to prevent any additional processing events after removal of the inhibitors. Finally, graded numbers of B cells were tested for their capacity to induce T cell proliferation. The results presented are those obtained with a number of MV-infected or antigen-pulsed B cells cultured in the absence of inhibitors and inducing half-maximal T cell proliferation. Following this procedure, it was observed that the lysosomotropic agent chloroquine largely inhibited, although not completely, proliferation of the N5 T cells and that the protease inhibitor leupeptin and the protein synthesis inhibitor emetine completely abrogated proliferation of these T cells (Fig. 4). At this point, it could not be distinguished whether emetine prevented presentation of the N185 determinant by inhibition of DR protein or nucleoprotein synthesis. To discriminate between these two possibilities, the antigenicity of UV-MV was evaluated. First, it was shown that addition of UV-MV to Vero cells did not cause cell fusion or cytopathic effects, indicating that UV treatment abrogated MV infectivity and therefore prevented de novo viral protein synthesis (data not shown). Then, it was found that UV-MV was as potent as live MV in inducing T cell proliferation (Fig. 4). This observation ruled out that de novo synthesis of nucleoprotein was required for presentation of the N185 determinant and, consequently, implied that the N185 determinant is captured by newly synthesized DR molecules. Together, these results strongly indicate that processing of the N185 determinant or binding to DR molecules, or both events, take place in the endosomal compartment.
Fig. 4.
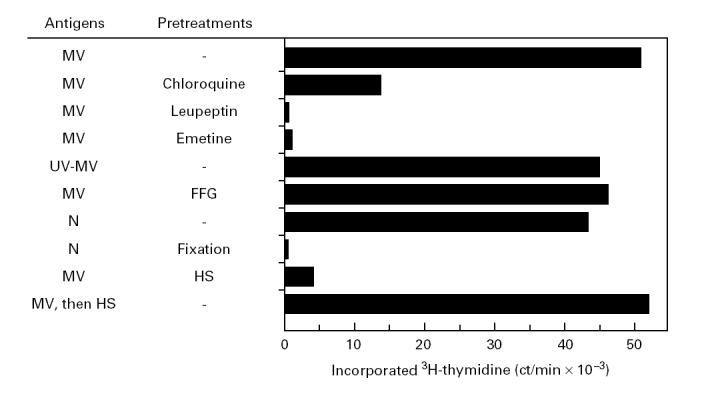
Assembly of the N185–DRB1*1103 complexes in the endosomal compartment. 6396 B cells were pretreated for 3 h with the indicated inhibitors before infection with measles virus (MV) or incubation in the presence of UV-MV or nucleoprotein (5 μg/ml). One day later, the B cells were glutaraldehyde-fixed and γ-irradiated. Then, N5 T cells (3 × 104) were cultured with the different preparations of B cells (2 × 104). After 2 days, 1 μCi 3H-thymidine was added for the last 18 h of culture and incorporated radioactivity was determined. Values are means of duplicates. Variations were < 15% of the mean.
It was then tested whether the N185 determinant was processed and presented even when delivery of nucleoprotein into the cytosol was prevented. The virus fusion inhibitor peptide Z-D-FFG was used to block introduction of nucleocapsids into the cytosol. In preliminary experiments, it was determined that treatment of Vero cells with 400 μm Z-D-FFG peptide caused complete inhibition of cell fusion and cytopathic effects induced by MV infection (data not shown). B cells infected with MV in the presence of Z-D-FFG peptide were as efficient stimulators as untreated B cells (Fig. 4). This experiment showed that efficient presentation of the N185 determinant does not necessitate transport of the nucleoprotein into the cytoplasm. This notion was further supported by the observation that B cells pulsed with purified nucleoprotein were potent stimulators of the N5 T cells. By contrast, glutaraldehyde-fixed B cells pulsed with the same antigen preparation failed to elicit N5 T cell proliferation (Fig. 4). This latter result excluded the possibility that T cell stimulation was due to nucleoprotein fragments generated extracellularly.
When MV or purified nucleoprotein is provided to APC, the N185 determinant intersects the endosomal compartment. In contrast, in vv-N-infected B cells the nucleoprotein is confined to the cytoplasm. In this situation a poor presentation of the N185 determinant is observed compared with MV-infected or nucleoprotein-pulsed B cells (see Fig. 2a). This result is in support of our conclusion that presentation of the N185 determinant is efficiently achieved following an endosomal pathway which does not involve cytosolic steps. Besides, it suggests that determinants can nevertheless be transferred from the cytoplasm to the endosomal compartment. How this transport mechanism operates is still to be established.
Finally, the role of receptor-mediated uptake of MV in the presentation of the N185 determinant was evaluated. HS, which contains MV neutralizing antibodies, was used to impair CD46-mediated uptake of MV by B cells [42]. The presence of HS during MV infection of B cells prevented presentation of the N185 determinant (Fig. 4). Presentation of the fusion protein determinants 254–268 and 314–328 was also abrogated (data not shown). By contrast, addition of HS after infection did not affect presentation of the MV determinants, excluding the possibility of direct inhibitory effects of HS on the B or the T cells (Fig. 4). Altogether, these observations suggest that presentation of the N185 determinant requires the efficient CD46-mediated uptake of MV without necessitating entry of nucleoprotein into the cytosol. Similarly to membrane-bound immunoglobulins, the CD46 protein concentrates MV particles and delivers them into an endosomal compartment where they undergo processing.
The phenomenon of antigen processing and presentation by MHC class II molecules to CD4+ T cells has been largely deciphered over the past 20 years. In spite of intensive investigations, it still remains to be clarified how processing and presentation of determinants from cytosolic proteins is controlled [17,18,21–31]. The present study describes processing features of a determinant from the MV nucleoprotein. We found that assembly of complexes between the N185 determinant and DR molecules takes place in the endosomal compartment before transport of the nucleoprotein into the cytosol. In some situations, the antigens are, however, strictly confined to the cytoplasm. In several of these cases, presentation of determinants from these antigens was found to be poor or even not to occur (see Fig. 2a and [29–31]). Moreover, sequencing of peptides extracted from class II preparations revealed that determinants from cytosolic proteins are the exceptions [15,16]. These results would suggest that MHC class II molecules fail to be supplied with a large array of cytosolic peptides. A crucial aspect of future work in this domain will consist in delineating the cellular mechanisms involved in the transport of determinants from the cytoplasm to the endosomal compartment.
Acknowledgments
This work was supported by a grant from the Swiss National Fund for Scientific Research (no. 3100-040311).
REFERENCES
- 1.Bakke O, Dobberstein B. MHC class II-associated invariant chain contains a sorting signal for endosomal compartments. Cell. 1990;63:707–16. doi: 10.1016/0092-8674(90)90137-4. [DOI] [PubMed] [Google Scholar]
- 2.Lotteau V, Teyton L, Peleraux A, Nilsson T, Karlsson L, Schmid SL, Quaranta V, Peterson PA. Intracellular transport of class II MHC molecules directed by invariant chain. Nature. 1990;348:600–5. doi: 10.1038/348600a0. [DOI] [PubMed] [Google Scholar]
- 3.Neefjes JJ, Stollorz V, Peters PJ, Geuze HJ, Ploegh HL. The biosynthetic pathway of MHC class II but not class I molecules intersects the endocytic route. Cell. 1990;61:171–83. doi: 10.1016/0092-8674(90)90224-3. [DOI] [PubMed] [Google Scholar]
- 4.Roche PA, Cresswell P. Invariant chain association with HLA- DR molecules inhibits immunogenic peptide binding. Nature. 1990;345:615–8. doi: 10.1038/345615a0. [DOI] [PubMed] [Google Scholar]
- 5.Peters PJ, Neefjes JJ, Oorschot V, Ploegh HL, Geuze HJ. Segregation of MHC class II molecules from MHC class I molecules in the Golgi complex for transport to lysosomal compartments. Nature. 1991;349:669–76. doi: 10.1038/349669a0. [DOI] [PubMed] [Google Scholar]
- 6.Roche PA, Marks MS, Cresswell P. Formation of a nine-subunit complex by HLA class II glycoproteins and the invariant chain. Nature. 1991;354:392–4. doi: 10.1038/354392a0. [DOI] [PubMed] [Google Scholar]
- 7.Amigorena S, Drake JR, Webster P, Mellman I. Transient accumulation of new class II MHC molecules in a novel endocytic compartment in B lymphocytes. Nature. 1994;369:113–20. doi: 10.1038/369113a0. [DOI] [PubMed] [Google Scholar]
- 8.Tulp A, Verwoerd D, Dobberstein B, Ploegh HL, Pieters J. Isolation and characterization of the intracellular MHC class II compartment. Nature. 1994;369:120–6. doi: 10.1038/369120a0. [DOI] [PubMed] [Google Scholar]
- 9.West MA, Lucocq JM, Watts C. Antigen processing and class II MHC peptide-loading compartments in human B-lymphoblastoid cells. Nature. 1994;369:147–51. doi: 10.1038/369147a0. [DOI] [PubMed] [Google Scholar]
- 10.Qiu Y, Xu X, Wandinger-Ness A, Dalke DP, Pierce SK. Separation of subcellular compartments containing distinct functional forms of MHC class II. J Cell Biol. 1994;125:595–605. doi: 10.1083/jcb.125.3.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rudensky AY, Maric M, Eastman S, Shoemaker L, DeRoos PC, Blum JS. Intracellular assembly and transport of endogenous peptide–MHC class II complexes. Immunity. 1994;1:585–94. doi: 10.1016/1074-7613(94)90048-5. [DOI] [PubMed] [Google Scholar]
- 12.Denzin LK, Robbins NF, Carboy-Newcomb C, Cresswell P. Assembly and intracellular transport of HLA-DM and correction of the class II antigen-processing defect in T2 cells. Immunity. 1994;1:595–606. doi: 10.1016/1074-7613(94)90049-3. [DOI] [PubMed] [Google Scholar]
- 13.Weber DA, Evavold BD, Jensen PE. Enhanced dissociation of HLA-DR-bound peptides in the presence of HLA-DM. Science. 1996;274:618–20. doi: 10.1126/science.274.5287.618. [DOI] [PubMed] [Google Scholar]
- 14.Wubbolts R, Fernandez-Borja M, Oomen L, et al. Direct vesicular transport of MHC class II molecules from lysosomal structures to the cell surface. J Cell Biol. 1996;135:611–22. doi: 10.1083/jcb.135.3.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chicz RM, Urban RG, Gorga JC, Vignali DA, Lane WS, Strominger JL. Specificity and promiscuity among naturally processed peptides bound to HLA-DR alleles. J Exp Med. 1993;178:27–47. doi: 10.1084/jem.178.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nelson CA, Roof RW, McCourt DW, Unanue ER. Identification of the naturally processed form of hen egg white lysozyme bound to the murine major histocompatibility complex class II molecule I-Ak. Proc Natl Acad Sci USA. 1992;89:7380–3. doi: 10.1073/pnas.89.16.7380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jacobson S, Sékaly RP, Jacobson CL, McFarland HF, Long EO. HLA class II-restricted presentation of cytoplasmic measles virus antigens to cytotoxic T cells. J Virol. 1989;63:1756–62. doi: 10.1128/jvi.63.4.1756-1762.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van Binnendijk RS, Poelen MCM, De Vries P, Voorma HO, Osterhaus Adme, Uytdehaag Fgcm. Measles virus-specific human T cell clones. Characterization of specificity and function of CD4+ helper/cytotoxic and CD8+ cytotoxic T cell clones. J Immunol. 1989;142:2847–54. [PubMed] [Google Scholar]
- 19.Dunn WA. Autophagy and related mechanisms of lysosome- mediated protein degradation. Trends Cell Biol. 1994;4:139–43. doi: 10.1016/0962-8924(94)90069-8. [DOI] [PubMed] [Google Scholar]
- 20.Terlecky SR. Hsp70s and lysosomal proteolysis. Experientia. 1994;50:1021–5. doi: 10.1007/BF01923456. [DOI] [PubMed] [Google Scholar]
- 21.Nuchtern JG, Biddison WE, Klausner RD. Class II MHC molecules can use the endogenous pathway of antigen presentation. Nature. 1990;343:74–76. doi: 10.1038/343074a0. [DOI] [PubMed] [Google Scholar]
- 22.Adorini L, Ullrich SJ, Appella E, Fuchs S. Inhibition by brefeldin A of presentation of exogenous protein antigens to MHC class II-restricted T cells. Nature. 1990;346:63–66. doi: 10.1038/346063a0. [DOI] [PubMed] [Google Scholar]
- 23.Brooks A, Hartley S, Kjer-Nielsen L, Perera J, Goodnow CC, Basten A, McCluskey J. Class II-restricted presentation of an endogenously derived immunodominant T-cell determinant of hen egg lysozyme. Proc Natl Acad Sci USA. 1991;88:3290–4. doi: 10.1073/pnas.88.8.3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Malnati MS, Marti M, LaVaute T, Jaraquemada D, Biddison W, DeMars R, Long EO. Processing pathways for presentation of cytosolic II-restricted T cells. Nature. 1992;357:702–4. doi: 10.1038/357702a0. [DOI] [PubMed] [Google Scholar]
- 25.Jin Y, Shih WK, Berkower I. Human T cell response to the surface antigen of hepatitis B virus (HBsAg). Endosomal and nonendosomal processing pathways are accessible to both endogenous and exogenous antigen. J Exp Med. 1988;168:293–306. doi: 10.1084/jem.168.1.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jaraquemada D, Marti M, Long EO. An endogenous processing pathway in vaccinia virus-infected cells for presentation of cytoplasmic antigens to class II-restricted T cells. J Exp Med. 1990;172:947–54. doi: 10.1084/jem.172.3.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hackett CJ, Yewdell JW, Bennink JR, Wysocka M. Class II MHC-restricted T cell determinants processed from either endosomes or the cytosol show similar requirements for host protein transport but different kinetics of presentation. J Immunol. 1991;146:2944–51. [PubMed] [Google Scholar]
- 28.Weiss S, Bogen B. MHC class II-restricted presentation of intracellular antigen. Cell. 1991;64:767–76. doi: 10.1016/0092-8674(91)90506-t. [DOI] [PubMed] [Google Scholar]
- 29.Brooks AG, McCluskey J. Class II-restricted presentation of a hen egg lysozyme determinant derived from endogenous antigen sequestered in the cytoplasm or endoplasmic reticulum of the antigen presenting cells. J Immunol. 1993;150:3690–7. [PubMed] [Google Scholar]
- 30.Cardoso AI, Beauverger P, Gerlier D, Wild TF, Rabourdin-Combe C. Formaldehyde inactivation of measles virus abolishes CD46-dependent presentation of nucleoprotein to murine class I-restricted CTLs but not to class II-restricted helper T cells. Virology. 1995;212:255–8. doi: 10.1006/viro.1995.1479. [DOI] [PubMed] [Google Scholar]
- 31.Oxenius A, Bachmann MF, Ashton-Rickardt PG, Tonegawa S, Zinkernagel RM, Hengartner H. Presentation of endogenous viral proteins in association with major histocompatibility complex class II: on the role of intracellular compartmentalization, invariant chain and the TAP transporter system. Eur J Immunol. 1995;25:3402–11. doi: 10.1002/eji.1830251230. [DOI] [PubMed] [Google Scholar]
- 32.Demotz S, Péléraux A. Processing of DR1-restricted determinants from the fusion protein of measles virus following two distinct pathways. Mol Immunol. 1996;33:387–97. doi: 10.1016/0161-5890(95)00148-4. [DOI] [PubMed] [Google Scholar]
- 33.Bankamp B, Horikami SM, Thompson PD, Huber M, Billeter M, Moyer SA. Domains of the measles virus N protein required for binding to P protein and self-assembly. Virology. 1996;216:272–7. doi: 10.1006/viro.1996.0060. [DOI] [PubMed] [Google Scholar]
- 34.Demotz S, Danieli C. Release of DR molecules from complexes with invariant chain through the formation of a C-terminal 25 kDa invariant chain fragment. Mol Immunol. 1993;30:1623–32. doi: 10.1016/0161-5890(93)90435-e. [DOI] [PubMed] [Google Scholar]
- 35.Fournier P, Ammerlaan W, Ziegler D, et al. Differential activation of T cells by antibody-modulated processing of the flanking sequences of class II-restricted peptides. Int Immunol. 1996;8:1441–51. doi: 10.1093/intimm/8.9.1441. [DOI] [PubMed] [Google Scholar]
- 36.Demotz S, Lanzavecchia A, Eisel U, Niemann H, Widmann C, Corradin G. Delineation of several DR-restricted tetanus toxin T cell epitopes. J Immunol. 1989;142:394–402. [PubMed] [Google Scholar]
- 37.Robadey C, Ammerlaan W, Muller CP, Cloutier I, Sékaly RP, Haefliger JA, Demotz S. The processing routes determined by negatively charged residues in DR1-restricted T cell determinants. J Immunol. 1997;159:3238–46. [PubMed] [Google Scholar]
- 38.Tiercy JM, Gorski J, Jeannet M, Mach B. Identification and distribution of three serologically undetected alleles of HLA-DR by oligonucleotide DNA typing analysis. Proc Natl Acad Sci USA. 1988;85:198–202. doi: 10.1073/pnas.85.1.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Steimle V, Hinkkanen A, Schlesier M, Epplen JT. A novel HLA-DR beta I sequence from the DRw11 haplotype. Immunogenetics. 1988;28:208–10. doi: 10.1007/BF00375861. [DOI] [PubMed] [Google Scholar]
- 40.Stern LJ, Brown JH, Jardetzky TS, Gorga JC, Urban RG, Strominger JL, Wiley DC. Crystal structure of the human class II MHC protein HLA-DR1 complexed with an influenza virus peptide. Nature. 1994;368:215–21. doi: 10.1038/368215a0. [DOI] [PubMed] [Google Scholar]
- 41.Sherman MA, Runnels HA, Moore JC, Stern LJ, Jensen PE. Membrane interactions influence the peptide binding behavior of DR1. J Exp Med. 1994;179:229–34. doi: 10.1084/jem.179.1.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gerlier D, Trescol-Biemont MC, Varior-Krishnan G, Naniche D, Fugier-Vivier I, Rabourdin-Combe C. Efficient major histocompatibility complex class II-restricted presentation of measles virus relies on hemagglutinin-mediated targeting to its cellular receptor human CD46 expressed by murine B cells. J Exp Med. 1994;179:353–8. doi: 10.1084/jem.179.1.353. [DOI] [PMC free article] [PubMed] [Google Scholar]


