Abstract
Anti-gliadin antibodies can be found in the serum of patients with overt and subclinical coeliac disease, but also in that of some controls. The aim of the present study was to identify the linear epitopes of the α-gliadin molecule to which the humoral response is directed. Therefore, the IgG and IgA antibody reactivity against an overlapping set of synthetic peptides covering the entire sequence of α-gliadin was measured in the sera from patients with coeliac disease, from controls with elevated titres of anti-gliadin antibodies and from healthy children using an ELISA technique. The antibodies mainly recognize peptides derived from the N-terminal region of α-gliadin, containing the motif QPFXXQXPY. Reactivity was also detected against two other synthetic peptides, which do not contain this motif and represent a sequence encoded further to the C-terminal region of α-gliadin. Anti-gliadin antibodies in sera from patients with coeliac disease and from controls recognize the same linear epitopes. Thus, serological investigation of the specificity of these antibodies using a peptide ELISA does not allow discrimination between patients and controls.
Keywords: coeliac disease, anti-α-gliadin antibodies, synthetic peptides, ELISA
INTRODUCTION
Coeliac disease (CoD) may be caused by a defect in oral tolerance to gliadins, components of the dietary protein gluten [1]. Gliadins, part of the alcohol-soluble protein fraction of wheat, can be separated into four major subfractions called α, β, γ and ω according to their electrophoretic mobility [2]. Based on amino acid sequence data, gliadins are classified as ω-type gliadins, α-type gliadins (containing α- and β-gliadins), and γ-type gliadins [3,4]. Evidence has been obtained by different groups that proteins and peptides derived from α-type gliadins are implicated in the pathogenesis of CoD [4]. Peptides derived from α-gliadin, which induce mucosal damage in in vitro cultures of small bowel biopsy specimens, are mostly located at the N-terminal region of the α-gliadin molecule [5–8].
CoD patients generally have high serum AGA titres [9–11]. However, several studies have described the presence of AGA in healthy individuals [12], in other gastrointestinal diseases [13], in patients with associated diseases like rheumatoid arthritis, diabetes mellitus or Down's syndrome [14–16], and in asymptomatic relatives of CoD patients. Although these apparently non-coeliac individuals could be silent or latent CoD patients [17–24], AGA are not informative for the diagnosis of CoD because of their limited specificity and sensitivity. Determination of IgA anti-endomysium antibodies is currently being used for the screening of (subclinical) CoD with high specificity [25–28].
However, the regions of α-gliadin that are involved in B cell reactivity might be discriminative between CoD patients and healthy individuals. Therefore, we investigated whether AGA from CoD patients and healthy individuals could be directed against different linear epitopes of α-gliadin.
PATIENTS AND METHODS
Patients with coeliac disease
Serum was obtained from 29 children with CoD, diagnosed by intestinal biopsies according to the criteria of the European Society for Paediatric Gastroenterology and Nutrition [29,30]. Their mean age at the time of the first small intestinal biopsy was 5.6 years (range 1–16 years; 15 girls). Serum samples were collected during the diagnostic procedure. From 12 CoD patients (mean age 5.7 years, range 1–16 years; seven girls) samples were obtained while on a gluten-containing diet (five of them following gluten challenge for at least 3 months), as well as after at least 3 months on a gluten-free diet (mean 18.6 months). Serum from the other CoD patients was obtained when either on a gluten-containing diet (n = 9) or on a gluten-free diet (n = 8). Sera of three patients on a gluten-containing diet were not analysed for IgA anti-α-gliadin antibodies (IgA-AGA), because of lack of serum.
Control individuals (group I)
Serum was obtained from 24 control children (mean age 5.5 years, range 1–16 years; nine girls) suspected to suffer from CoD and having high serum titres of IgG- and/or IgA-AGA, but who showed an absence of anti-endomysium antibodies and in whom a gluten-sensitive enteropathy was excluded by virtue of a normal small intestinal biopsy. These children are first-degree relatives of CoD patients (n = 3) or fall mainly into disease categories known to be associated with a general dysregulation of the immune system (i.e. Down's syndrome (n = 13) [15]) or with a disturbance of mucosal immunity (i.e. cow's milk allergy (n = 2), lactose malabsorption (n = 1)). The remaining ones suffered from disorders different from CoD, like failure to thrive, chronic diarrhoea, chronic obstipation or chronic abdominal pain.
Control individuals (group II)
A second control group was formed by 11 age-matched, healthy, bone marrow transplantation (BMT) donors without any signs of disorders affecting the respiratory or gastrointestinal tract (mean age 5.7 years, range 1–16 years; six girls). The sera from these children were proven to be negative for anti-endomysium antibodies.
Gliadin ELISA
Microtitre plates (96-well polystyrene; Costar, Cambridge, MA) were coated overnight at 4°C with 100 μl gliadin ([31]; 100 μg/ml) in 70% (v/v) ethanol. All incubations were followed by several washing steps with PBS containing 0.05% (v/v) Tween-20 (PBS–T). Serial two-fold dilutions of sera in PBS–T were applied (ranging from 1:100 to 1:1600), followed by an incubation for 2 h at 37°C. The plates were incubated for 1 h at 37°C with peroxidase-conjugated goat anti-human IgG (Sigma, St Louis, MO; 1:30 000 in PBS–T) or peroxidase-conjugated goat anti-human IgA (Sigma, 1:30 000 in PBS–T). The substrate (3,3′,5,5′-tetramethylbenzidine (Sigma; 0.1 mg/ml) and 0.015% (v/v) hydrogen peroxide in 0.1 m sodium acetate/citric acid buffer pH 4.0) was added and incubation was performed for 30 min (IgG) or 60 min (IgA) at 37°C. Colour development was stopped by the addition of 0.8 m sulphuric acid. Absorbance was measured at 450 nm using an automatic ELISA plate reader (Titertek Multiskan plus; ICN, Costa Mesa, CA). The sera were analysed in duplicate in a single experiment, which enables direct comparison of absorbance measurements.
Synthetic peptides
Synthetic peptides were made on an Abimed 422 synthesizer (Abimed, Langenfeld, Germany) using routine Fmoc chemistry. For the purpose of biotinylation a spacer was introduced by coupling with Nα-fluorenylmethoxycarbonyl-6-aminohexanoic acid. Biotinylation was performed by coupling (3 h) with preactivated biotin (30 min preincubation of 60 μmol biotin, 60 μmol 1-benzotriazoloxy-tris-(N-pyrrolidino)-phosphonium hexafluorophosphate and 120 μmol N-methylmorpholine in 250 μl N-methyl-pyrrolidone). All the biotinylated peptides were dissolved at a concentration of 500 μg/ml in PBS containing 6‰ (v/v) dimethylsulfoxide and stored at −70°C until use.
Peptide set 1. Forty-eight 21mer peptides overlapping by 15 amino acids (aa) covering almost the complete sequence of the α-gliadin molecule from wheat, Triticum aestivum, were synthesized (peptide set 1; with numbers 1 to 48). The amino acid sequence of A-gliadin as determined by Kasarda et al. [32] was used. Deletions in this sequence (e) were completed according to the amino acid sequence derived from the nucleotide sequence of the cDNA restriction fragment pGliA-42 (c). In addition, the applied sequence also included the 20 amino acid residues of the signal peptide [32]. Furthermore, a glutamine residue was inserted between amino acid positions 69 and 70 (position numbering corresponding to sequence c in [32]), based on the amino acid sequence derived from the nucleotide sequence of the same cDNA restriction fragment as mentioned above [33].
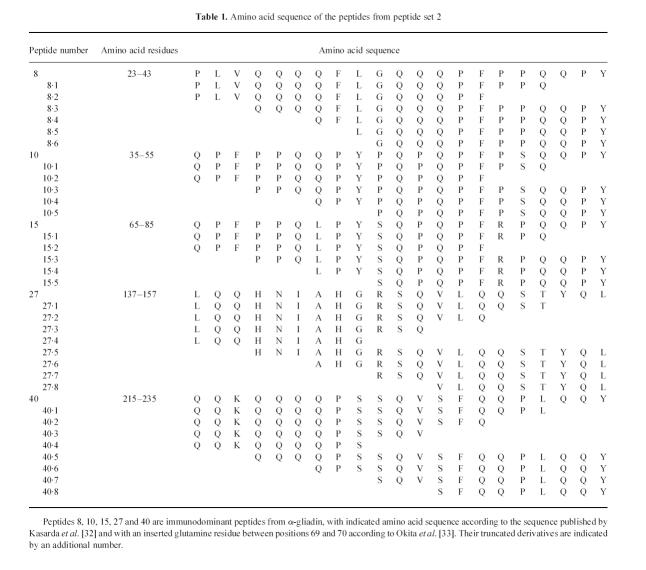
Peptide set 2. Another set of 32 N- and C-terminal truncated peptides (smallest 9mer, longest 18mer) was synthesized based on the sequence of five immunodominant peptides (peptides: 8, 10, 15, 27 and 40). The five immunodominant peptides from set 1 and these 32 truncated peptides formed peptide set 2 (see Table 1).
Table 1.
Amino acid sequence of the peptides from peptide set 2
Peptide ELISA
Microtitre plates (96-well; Costar) were coated overnight at 4°C with streptavidin (Sigma; 4 μg/ml in PBS). All incubations were followed by several washing steps with PBS–T. Non-specific protein binding was blocked by the addition of PBS–T containing 1% (w/v) bovine serum albumin (BSA; Sigma) (PBS–T–BSA) and incubated for 1 h at room temperature. The biotinylated peptides (1 μg/ml in PBS–T–BSA) were coupled to the wells during a 1-h incubation at room temperature. The sera were diluted in PBS–T–BSA, for the measurement of antibodies of the IgG class 1:500 and for the IgA class 1:200, and incubated for 2 h at room temperature. Subsequently, the plates were incubated with peroxidase-conjugated goat anti-human IgG (Sigma) or peroxidase-conjugated goat anti-human IgA (Sigma; 1:30 000 in PBS–T–BSA). The plates were developed and measured as described above. Controls for each sample included a conjugate control, a serum control (no peptide was coated) and a positive control, in which a biotinylated goat anti-human immunoglobulin (Sigma; 1:70 000) instead of the biotinylated peptide was coupled to the streptavidin. Sera were considered to react with a peptide when the optical density (OD 450) was higher than twice the OD of the serum control.
RESULTS
IgG and IgA antibody reactivity against gliadin
The humoral activity against the whole gliadin molecule in sera of the 12 CoD patients while on a gluten-containing diet and while on a gluten-free diet, respectively, and in sera of the 22 age-matched controls (11 controls from group I and all controls from group II) is presented in Fig. 1. As expected, children with CoD on a gluten-containing diet and controls from group I, selected because of their high AGA titres, showed a much higher mean level of AGA than the children from control group II (BMT donors). The mean AGA level of the children with CoD diminished after a gluten-free diet and was comparable to the mean level of the children from control group II.
Fig. 1.
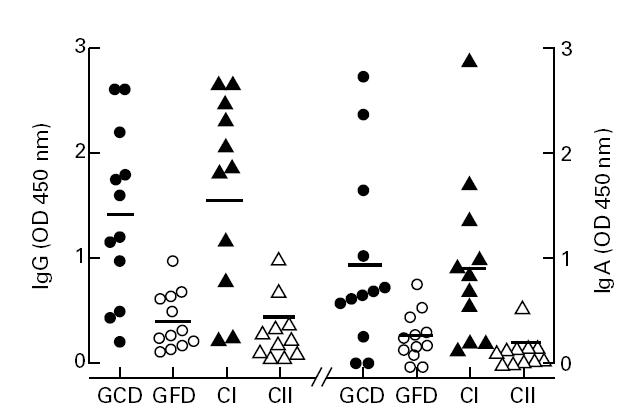
IgG and IgA against gliadin in the sera of 12 children with coeliac disease (CoD) while on a gluten-containing diet (GCD), while on a gluten-free diet (GFD), and in sera of controls (group I, CI; n = 11; group II, CII; n = 11). The OD 450 at serum dilution 1:1600 for IgG and 1:100 for IgA is presented. The geometric mean value for each group is indicated by the horizontal line.
IgG and IgA antibody reactivity against synthetic peptides of α-gliadin
Peptide set 1
The reactivity against synthetic peptides of α-gliadin of IgG and IgA antibodies in sera from CoD patients while on a gluten-containing diet or, if this serum was not available, on a gluten-free diet and from the control individuals of group I and II is shown in Fig. 2 (Fig. 2a–c, IgG; Fig. 2d–f, IgA). Data are presented as the percentage of sera considered to react with a peptide, according to the criteria formulated in Patients and Methods.
Fig. 2.
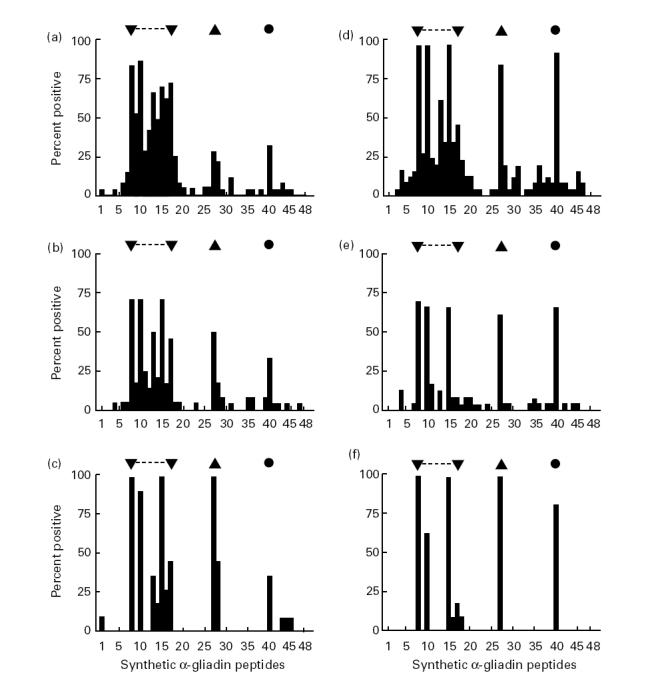
Percentage of sera with antibody reactivity against synthetic peptides of α-gliadin (peptide set 1). Sera were analysed at a dilution of 1:500 for antibodies of the IgG class and 1:200 for antibodies of the IgA class. Reactivity to a distinct peptide was considered to be present according to the criteria formulated in Patients and Methods. Results are shown for IgG (a–c) and IgA (d–f) class antibodies in the sera of children with coeliac disease (CoD) while on a gluten-containing diet or, if sera were not available at that time, while on a gluten-free diet (a, n = 29 sera; d, n = 26 sera) and for control children from groups I (b, n = 24 sera; e, n = 24 sera) and II (c, n = 11 sera; f, n = 11 sera). ▾, ▴, •, The most commonly recognized region and peptides, respectively.
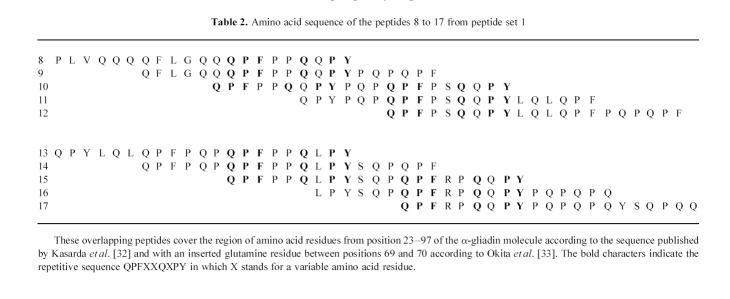
Sera of children with CoD and of individuals from control groups I and II contained IgG and IgA antibodies directed against the same region(s) of the α-gliadin molecule, mainly confined to the region of amino acid residues 23–97 covered by peptides 8 to 17 (Fig. 2). The amino acid sequence of these immunodominant peptides contains a common repetitive motif of nine amino acids, i.e. QPFXXQXPY, in which X stands for a variable residue (Table 2). The IgA-AGA were mainly restricted to three of these peptides: nos 8, 10 and 15 sharing this repetitive motif at the C-terminus (Fig. 2d–f). Two other peptides, 27 and 40, were also recognized by IgA-AGA and to a lesser extent by IgG-AGA. These peptides neither contain the motif QPFXXQXPY nor share a common sequence with each other. Although the AGA levels of patients with CoD on a gluten-free diet were lower than on a gluten-containing diet, the pattern of reactivity against the synthetic peptides of α-gliadin was the same (results not shown).
Table 2.
Amino acid sequence of the peptides 8 to 17 from peptide set 1
Peptide set 2
This peptide set consisted of N- and C-terminal truncated forms of the five immunodominant peptides from set 1 (8, 10, 15, 27 and 40) and was synthesized to determine the minimal linear B cell epitopes. IgA-AGA against these peptides in the sera from 10 coeliac children consuming gluten and from 11 age-matched controls of group II were measured (Fig. 3). The results indicated that the reactivity of IgA against the immunodominant peptides arising from peptide set 1 strongly diminished or even disappeared completely when the C-terminal part, consisting of -QPY (peptides 8, 10 and 15), -YQL (peptide 27) and -QQY (peptide 40), was eliminated. In contrast, N-terminal truncations affected the binding of IgA less dramatically. An identical pattern of modulation of the reactivity by truncation of the distinct peptides was obtained for IgG-AGA (data not shown).
Fig. 3.
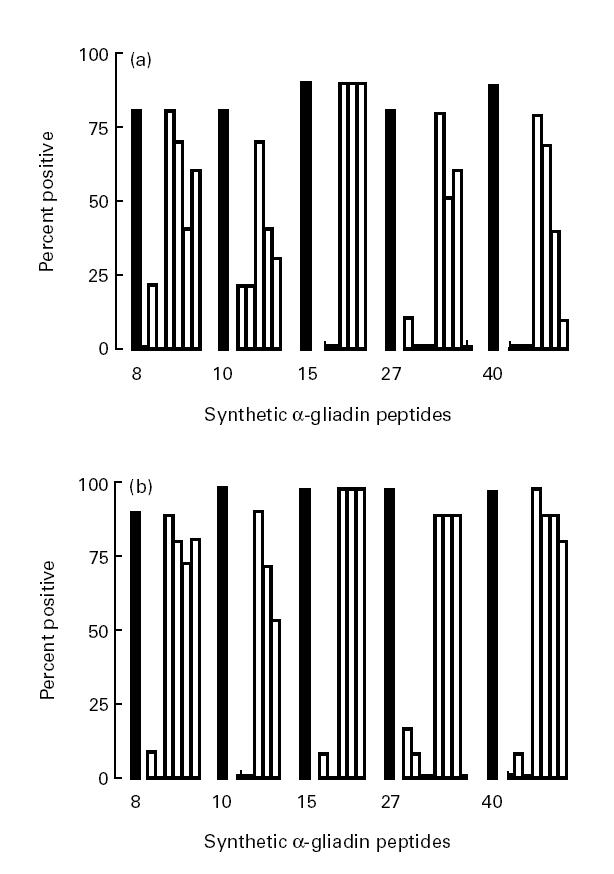
Percentage of sera with IgA against synthetic peptides of α-gliadin (peptide set 2). Sera were analysed at a dilution of 1:200 and IgA reactivity to a distinct peptide was considered to be present according to the criteria formulated in Patients and Methods. Results are shown for children with coeliac disease (CoD) while on a gluten-containing diet (a, n = 10) and for the individuals of group II (b, n = 11). ▪, Responses to the five immunodominant peptides emerging from peptide set 1; □, responses to their respective truncated derivatives (see also Table 1).
DISCUSSION
In this study we analysed the specificity of AGA present in sera from CoD patients and from two groups of controls (with high and normal AGA levels, groups I and II, respectively). Using different synthetic peptides of the α-gliadin molecule, we found that the AGA present in the sera from children with CoD and from both control groups were directed against the same region(s) of the α-gliadin molecule. Although the mean AGA levels in CoD patients decreased by following a gluten-free diet, the pattern of reactivity was the same during gluten consumption and after a gluten-free diet. Thus, the pattern of humoral activity against the linear epitopes of α-gliadin was not specific for coeliac patients and was not affected by their diet. This observation holds for IgG-AGA as well as for IgA-AGA.
The most commonly detected reactivity was directed against peptides 8 to 17 representing the region of the amino acid residues 23–97 of α-gliadin and containing the unique repetitive sequence QPFXXQXPY. IgA-AGA, and to a lesser extent IgG-AGA, were also present against two other synthetic peptides which are encoded by regions located further to the C-terminus of the α-gliadin molecule, i.e. peptides 27 (amino acid residues 137–157) and 40 (amino acid residues 215–235), respectively. These peptides contain neither a motif resembling QPFXXQXPY nor a shared motif. To characterize further the linear epitopes of α-gliadin eliciting a B cell response, we tried to define the minimal epitope. The C-terminus of these immunodominant peptides seems to be important in this respect, as determined by truncation of the peptides.
To our knowledge, only Lähdeaho et al. [34] have studied the humoral activity against linear epitopes of α-gliadin. Serum pools of patients with untreated CoD and controls with AGA were analysed for their reactivity against synthetic peptides of α-gliadin, using synthetic peptides of 10 amino acids in length, overlapping by five residues. They described five reactive peptides of α-gliadin (amino acid residues 41–50, 66–75, 166–175, 206–215 and 236–245; amino acid position numbering according to sequence e [32]). Two of these peptides, i.e. representing amino acid residues 41–50 and 206–215, respectively, were considered to be CoD-associated B cell epitopes. These peptides are part of our 21mer peptides 10–11 and 42–43, respectively. We found IgG- and IgA-AGA reactivity against peptide 10–11 in most sera obtained from CoD patients as well as from controls, and hardly any reactivity against peptide 42–43 among sera of CoD patients and controls. The discrepancy between our findings and those of Lähdeaho et al. [34] might be explained by the fact that we investigated sera of individual patients and controls instead of pools for their reactivity against larger peptides of α-gliadin, applying strict criteria to discriminate between a positive and a negative signal.
The detrimental effect of α-gliadin, or of peptides derived from it, on in vitro cultured small bowel biopsy specimens has been the subject of several studies. Most of the peptides inducing mucosal damage are located at the N-terminal region of the α-gliadin molecule, i.e. amino acid residues 31–43/49 [6–8]. The authors suggested that the observed effect was due to immune activation within the intestinal mucosa. However, it is not known whether these effects were specific for coeliac patients, since no biopsy specimen from controls was challenged in their study. HLA-DQ2-restricted T cell reactivity was found against a peptide (p19) spanning the amino acid region 31–49 of α-gliadin [35]. This region of amino acid residues 31–49 forms part of the region we found to be immunodominant in inducing a B cell response, indicating some overlap of T and B cell epitopes.
Several studies concerning the reactivity of the C-terminal part of α-gliadin in organ culture display conflicting results. De Ritis et al. [5] attributed reactivity to a peptide corresponding with amino acid residues 128–246 from α-gliadin, whereas Shidrawi et al. [7] and Devery et al. [36] did not find reactivity in this region. Sturgess et al. [6] and Mantzaris & Jewell [37] challenged CoD patients in vivo and reported that peptides corresponding to amino acid residues 202–220 and 206–217 (amino acid position numbering according to sequence c [32]), respectively, induced minor mucosal damage in one out of four and two out of two of their patients. We obviously detected antibody reactivity against peptides 27 (amino acid residues 137–157) and 40 (amino acid residues 215–235) from the C-terminal region (amino acid position numbering according to sequence c [32]). Peptide 27 has no amino acid sequence in common with the active peptides reported by Sturgess et al. [6] and Mantzaris & Jewell [37]. The C-terminal part of peptide 40, last three amino acid residues, is part of the active peptide described by Sturgess et al. [6].
Our study shows that synthetic 21mer peptides are suitable to detect linear B cell epitopes. We applied 21mer peptides which are expected to have some resemblance to the three-dimensional structure of the corresponding part of the natural protein. However, it can not be excluded that the conformation of natural (discontinuous) epitopes is relevant for the induction of a CoD-specific humoral immune response against α-gliadin.
Not only anti-gliadin antibodies, but also autoantibodies like anti-reticulin, anti-jejunal and anti-endomysial antibodies are found in patients with CoD [11]. Picarelli et al. [38] have described that anti-endomysial antibodies are produced in cultures of small bowel biopsy specimens from (un)treated CoD patients after addition of gliadin. This is not the case when biopsies from controls are cultured in the same conditions. These anti-endomysial antibodies were rapidly synthesized after gliadin challenge and did not show cross-reactivity with gliadin. In a recent study, tissue-transglutaminase (tTG) was defined as the antigen from the extracellular matrix which is recognized by anti-endomysial antibodies [39]. An attractive concept has been proposed by Sollid et al. [40], in which complex formation between gliadin and tTG allows interaction between gliadin-specific T cells and tTG-specific B cells, resulting in production of anti-tTG antibodies explaining the high disease specificity and sensitivity to the followed diet. They suggest that gliadin can form complexes not only with tTG, but also with other self-molecules, resulting in the production of autoantibodies.
In conclusion, anti-gliadin antibodies can be found in sera from children with CoD, irrespective of their diet, as well as in sera from age-matched control individuals. These antibodies recognize the same linear epitopes of α-gliadin. Thus, the humoral immune response against α-gliadin seems not to contain CoD-specific components. However, despite the identical specificity of AGA in CoD patients and controls, the clonal diversity and/or avidity of AGA might differ between patients and controls. These aspects require further investigation.
Acknowledgments
This project was supported by a grant from Nutricia B.V., Zoetermeer, The Netherlands. The authors gratefully thank Dr C. Vennegoor for her advice regarding the development of the peptide ELISA and Dr M. W. Schilham for critically reading the manuscript.
REFERENCES
- 1.Dicke WK, Weijers HA, van de Kamer JH. Coeliac disease II. The presence in wheat of a factor having a deleterious effect in cases of coeliac disease. Acta Paediatr. 1953;42:34–42. doi: 10.1111/j.1651-2227.1953.tb05563.x. [DOI] [PubMed] [Google Scholar]
- 2.Wieser H. Relation between gliadin structure and coeliac toxicity. Acta Paediatr. 1996;85(Suppl. 412):3–9. doi: 10.1111/j.1651-2227.1996.tb14239.x. [DOI] [PubMed] [Google Scholar]
- 3.Autran JC, Lew EJL, Nimmo CC, et al. N-terminal amino acid sequencing of prolamins from wheat and related species. Nature. 1979;282:527–9. [Google Scholar]
- 4.Shewry PR, Tatham AS, Kasarda DD. Cereal proteins and coeliac disease. In: Marsh MN, editor. Coeliac disease. Oxford: Blackwell Scientific Publications; 1992. pp. 305–48. [Google Scholar]
- 5.De Ritis G, Auricchio S, Jones HW, et al. In vitro (organ culture) studies of the toxicity of specific A-gliadin peptides in celiac disease. Gastroenterology. 1988;94:41–49. doi: 10.1016/0016-5085(88)90607-5. [DOI] [PubMed] [Google Scholar]
- 6.Sturgess R, Day P, Ellis HJ, et al. l. Wheat peptide challenge in coeliac disease. Lancet. 1994;343:758–61. doi: 10.1016/s0140-6736(94)91837-6. [DOI] [PubMed] [Google Scholar]
- 7.Shidrawi RG, Day P, Przemioslo R, et al. In vitro toxicity of gluten peptides in coeliac disease assessed by organ culture. Scand J Gastroenterol. 1995;30:758–63. doi: 10.3109/00365529509096324. [DOI] [PubMed] [Google Scholar]
- 8.Maiuri L, Troncone R, Mayer M, et al. In vitro activities of A-gliadin-related synthetic peptides. Damaging effect on the atrophic coeliac mucosa and activation of mucosal immune response in the treated coeliac mucosa. Scand J Gastroenterol. 1996;31:247–53. doi: 10.3109/00365529609004874. [DOI] [PubMed] [Google Scholar]
- 9.Unsworth DJ, Walker-Smith JA, Holborow EJ. Gliadin and reticulin antibodies in childhood coeliac disease. Lancet. 1983;i:874–5. doi: 10.1016/s0140-6736(83)91411-3. [DOI] [PubMed] [Google Scholar]
- 10.Troncone R, Ferguson A. Anti-gliadin antibodies. J Pediatr Gastroenterol Nutr. 1991;12:150–8. doi: 10.1097/00005176-199102000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Mäki M. The humoral immune system in coeliac disease. Baillière's Clin Gastroenterol. 1995;9:231–49. doi: 10.1016/0950-3528(95)90030-6. [DOI] [PubMed] [Google Scholar]
- 12.Sjöberg K, Alm R, Ivarsson SA, et al. Prevalence and clinical significance of gliadin antibodies in healthy children and adults. Scand J Gastroenterol. 1994;29:248–54. doi: 10.3109/00365529409090472. [DOI] [PubMed] [Google Scholar]
- 13.Ribes-Koninckx C, Giliams JP, Polanco I, et al. IgA antigliadin antibodies in celiac and inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 1984;3:676–82. doi: 10.1097/00005176-198411000-00006. [DOI] [PubMed] [Google Scholar]
- 14.George EK, Mearin ML, Bouquet J, et al. Screening for coeliac disease in Dutch children with associated diseases. Acta Paediatr. 1996;85(Suppl. 412):52–53. doi: 10.1111/j.1651-2227.1996.tb14251.x. [DOI] [PubMed] [Google Scholar]
- 15.George EK, Mearin ML, Bouquet J, et al. High frequency of celiac disease in Down syndrome. J Pediatr. 1996;128:555–7. doi: 10.1016/s0022-3476(96)70369-4. [DOI] [PubMed] [Google Scholar]
- 16.Gale L, Wimalaratna H, Brotodiharjo A, et al. Down's syndrome is strongly associated with coeliac disease. Gut. 1997;40:492–6. doi: 10.1136/gut.40.4.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stern M, Bender SW, Grüttner R, et al. Serum antibodies against gliadin and reticulin in a family study of coeliac disease. Eur J Pediatr. 1980;135:31–36. doi: 10.1007/BF00445889. [DOI] [PubMed] [Google Scholar]
- 18.Stenhammar L, Brandt A, Wagermark J. A family study of coeliac disease. Acta Paediatr Scand. 1982;71:625–8. doi: 10.1111/j.1651-2227.1982.tb09486.x. [DOI] [PubMed] [Google Scholar]
- 19.Mäki M, Holm K, Koskimies S, et al. Normal small bowel biopsy followed by coeliac disease. Arch Dis Child. 1990;65:1137–41. doi: 10.1136/adc.65.10.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mäki M, Holm K, Lipsanen V, et al. Serological markers and HLA genes among healthy first-degree relatives of patients with coeliac disease. Lancet. 1991;338:1350–3. doi: 10.1016/0140-6736(91)92234-s. [DOI] [PubMed] [Google Scholar]
- 21.Arranz E, Ferguson A. Intestinal antibody pattern of celiac disease: occurrence in patients with normal jejunal biopsy histology. Gastroenterol. 1993;104:1263–72. doi: 10.1016/0016-5085(93)90333-8. [DOI] [PubMed] [Google Scholar]
- 22.Collin P, Helin H, Mäki M, et al. Follow-up of patients positive in reticulin and gliadin antibody tests with normal small-bowel biopsy findings. Scand J Gastroenterol. 1993;28:595–8. doi: 10.3109/00365529309096094. [DOI] [PubMed] [Google Scholar]
- 23.Vitoria JC, Arrieta A, Astigarraga I, et al. Use of serological markers as a screening test in family members of patients with celiac disease. J Pediatr Gastroenterol Nutr. 1994;19:304–9. doi: 10.1097/00005176-199410000-00008. [DOI] [PubMed] [Google Scholar]
- 24.Bonamico M, Mariani P, Mazzilli MC, et al. Frequency and clinical pattern of celiac disease among siblings of celiac children. J Pediatr Gastroenterol Nutr. 1996;23:159–63. doi: 10.1097/00005176-199608000-00011. [DOI] [PubMed] [Google Scholar]
- 25.Bürgin-Wolff A, Gaze H, Hadziselimovic F, et al. Antigliadin and antiendomysium antibody determination for coeliac disease. Arch Dis Child. 1991;66:941–7. doi: 10.1136/adc.66.8.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lerner A, Kumar V, Iancu TC. Immunological diagnosis of childhood coeliac disease: comparison between antigliadin, antireticulin and antiendomysial antibodies. Clin Exp Immunol. 1994;95:78–82. doi: 10.1111/j.1365-2249.1994.tb06018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grodzinsky E, Jansson G, Skogh T, et al. Anti-endomysium and anti-gliadin antibodies as serological markers for coeliac disease in childhood: a clinical study to develop a practical routine. Acta Paediatr. 1995;84:294–8. doi: 10.1111/j.1651-2227.1995.tb13631.x. [DOI] [PubMed] [Google Scholar]
- 28.Rujner J, Socha J, Barra E, et al. Serum and salivary antigliadin antibodies and serum IgA anti-endomysium antibodies as a screening test for coeliac disease. Acta Paediatr. 1996;85:814–7. doi: 10.1111/j.1651-2227.1996.tb14157.x. [DOI] [PubMed] [Google Scholar]
- 29.Meeuwisse GW. Diagnostic criteria in coeliac disease. Acta Paediatr Scand. 1970;59:461–3. [Google Scholar]
- 30.Walker-Smith JA, Guandalini S, Schmitz J, et al. Revised criteria for diagnosis of coeliac disease. Arch Dis Child. 1990;65:909–11. doi: 10.1136/adc.65.8.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eterman KP, Hekkens WT, Peña AS, et al. Wheat grains: a substrate for the determination of gluten antibodies in serum of gluten-sensitive patients. J Immunol Methods. 1977;14:85–92. doi: 10.1016/s0022-1759(97)90025-9. [DOI] [PubMed] [Google Scholar]
- 32.Kasarda DD, Okita TW, Bernardin JE, et al. Nucleic acid (cDNA) and amino acid sequences of α-type gliadins from wheat (Triticum aestivum) Proc Natl Acad Sci USA. 1984;81:4712–6. doi: 10.1073/pnas.81.15.4712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Okita TW, Cheesbrough V, Reeves CD. Evolution and heterogeneity of the α-/β-type and γ-type gliadin DNA sequences. J Biol Chem. 1985;260:8203–13. [PubMed] [Google Scholar]
- 34.Lähdeaho ML, Vainio E, Lehtinen M, et al. Activation of celiac disease immune system by specific α-gliadin peptides. Cereal Chem. 1995;72:475–9. [Google Scholar]
- 35.Gjertsen HA, Lundin KEA, Sollid LM, et al. T cells recognize a peptide derived from α-gliadin presented by the celiac disease-associated HLA-DQ (α 1*0501, β 1*0201) heterodimer. Hum Immunol. 1994;39:243–52. doi: 10.1016/0198-8859(94)90267-4. [DOI] [PubMed] [Google Scholar]
- 36.Devery JM, Bender V, Penttila I, et al. Identification of reactive synthetic gliadin peptides specific for coeliac disease. Int Arch Allergy Appl Immunol. 1991;95:356–62. doi: 10.1159/000235473. [DOI] [PubMed] [Google Scholar]
- 37.Mantzaris G, Jewell DP. In vivo toxicity of a synthetic dodecapeptide from A gliadin in patients with coeliac disease. Scand J Gastroenterol. 1991;26:392–8. doi: 10.3109/00365529108996500. [DOI] [PubMed] [Google Scholar]
- 38.Picarelli A, Maiuri L, Frate A, et al. Production of antiendomysial antibodies after in-vitro gliadin challenge of small intestine biopsy samples from patients with coeliac disease. Lancet. 1996;348:1065–7. doi: 10.1016/S0140-6736(96)03060-7. [DOI] [PubMed] [Google Scholar]
- 39.Dieterich W, Ehnis T, Bauer M, et al. Identification of tissue transglutaminase as the autoantigen of celiac disease. Nature Med. 1997;3:797–801. doi: 10.1038/nm0797-797. [DOI] [PubMed] [Google Scholar]
- 40.Sollid LM, Molberg Ø, McAdam S, et al. Autoantibodies in coeliac disease: tissue transglutaminase—guilt by association? Gut. 1997;41:851–2. doi: 10.1136/gut.41.6.851. [DOI] [PMC free article] [PubMed] [Google Scholar]