Abstract
It is now becoming accepted that one is not tolerant to all the determinants of self proteins: the T cell repertoire directed to some sequences in self proteins is intact and can be activated. When a self protein is exclusively expressed by tumour cells, the T cell repertoire directed to the particular self antigen can potentially be activated to attack the tumour: this would amount to induction of a beneficial autoimmune response. Prostate cancer offers a unique opportunity for activation of a tumour-specific immune response owing to the exclusive synthesis of prostate-specific antigen (PSA) and prostate-specific membrane antigen (PSM) by prostatic tissue and prostate tumour cells. In this study we examine the CD4 and CD8 T cell repertoires specific for peptides of PSA and PSM in normal human male individuals, using short-term, peptide antigen-driven CD4 and CD8 T cell lines. We show that short-term, CD4 T cell lines derived from six HLA-DR4 individuals showed strong proliferative responses to six of 10 tested peptides of PSA, selected as to contain a DR4 binding motif. Short-term, CD8 T cell lines from three HLA-A1 individuals showed specific cytolytic activity for autologous targets loaded with five of five tested peptides of PSA and PSM, selected to possess an HLA-A1 binding motif. One of the peptides chosen is termed a ‘dual-motif’ peptide, as it encodes determinants for both CD4 and CD8 T cells. These results, indicating the existence of CD4 and CD8 T cells against determinants of the self proteins, PSA and PSM, in healthy male individuals reveal the potential of the T cell repertoire from the typical prostate cancer patient to eradicate prostate tumours upon being appropriately activated.
Keywords: CD4 T cell, CD8 T cell, T cell repertoire, antigenic determinant, PSA, PSM, human, prostate cancer
INTRODUCTION
There has been a determined search for therapies specifically aimed at eradicating tumour cells while leaving normal host cells unaffected. This goal can potentially be accomplished by engaging the tumour antigen-specific T cell repertoire to attack the tumour. This has led to a vigorous search for tumour-specific antigens over the past several years [1,2]. The concept has recently emerged that one is not tolerant to many determinants on self proteins, and that these peptide determinants could furnish target structures for an immune response against tumours [3]. Therefore, a variety of tumour-restricted self antigens can be considered potential targets of an immune attack. Under this paradigm, an effective immune response directed against tumours does not necessarily have to be targeted to either altered self or non-self tumour antigens as had originally been presumed. Furthermore, recent progress in the theoretical framework of antigen recognition has resulted in a shift in our understanding of tumour-related antigens that can be rendered into specific targets of attack by the immune system: most of the peptides that are bound in the groove of an MHC molecule on tumour cells could essentially be a potential target determinant for immune attack. Prostate cancer presents a unique opportunity for inducing a tumour-specific attack by the immune system because a number of proteins, including prostate-specific antigen (PSA) and prostate-specific membrane antigen (PSM), are produced by cells of nearly all prostate tumours, but not by other cells or tissues of the individual [4,5].
What is the nature of the peptide determinants of self proteins against which an intact T cell repertoire remains in the host? These sequences are less well processed parts of the protein and are defined as cryptic or subdominant determinants [6]. Presumably, it is the response to these self determinants that becomes activated in autoimmune diseases. The cryptic determinants within any protein are not non-immunogenic while part of the protein: in fact they are usually either at a poorly processable site in the protein or the activation of T cells specific for these determinants is prevented by neighbouring suppressive determinants. Once these cryptic determinants are removed from the protein molecule in which they reside, they have a similar range of binding affinities for the MHC as do dominant determinants. More importantly, these cryptic self peptides, under appropriate conditions of immunization, can also elicit equally vigorous T cell responses. It is generally accepted that the host is more readily tolerant to the well processed determinants of the self proteins which usually would otherwise have been dominant determinants [3,6].
A variety of evidence points toward the importance of raising both CD4 and CD8 T cells to attack the tumour [7]. In this study we investigate both the CD4 and CD8 T cell repertoires directed to determinants of PSA and PSM molecules in normal male individuals using short-term, peptide antigen-driven CD4 and CD8 T cell lines. We show that short-term, CD4 T cell lines derived from six HLA-DR4 individuals showed strong proliferative responses to six of 10 tested peptides of PSA, selected as to contain a DR4 binding motif. Short-term, CD8 T cell lines from three HLA-A1 individuals were specific for two of two tested peptide determinants of PSA and three of three tested peptides of PSM, peptides having been selected to possess an HLA-A1 binding motif. These results establish the existence of CD4 and CD8 T cells against determinants of the self proteins, PSA and PSM, in healthy male individuals. They disclose the potential for tumour eradication by appropriate vaccination with immunogenic peptides in the prostate cancer patient.
MATERIALS AND METHODS
Volunteers
Human subjects, aged 25–30 years, were recruited from the local community, HSPC no. 95-08-341. The serum PSA levels in normal male individuals within this age range are undetectable.
HLA typing
HLA typing was performed in the UCLA Tissue Typing Facility utilizing the Terasaki microdroplet testing technique [8].
Peptides
Peptides were selected from the sequences of PSA and PSM based upon HLA-A1 and HLA-DR4 binding motifs [9,10]. Peptides were synthesized by a solid-phase method using a Multiple Peptide Synthesizer (Advanced Chemtech, 396 MPS, Louisville, KY). Peptides were further purified by reverse phase high performance liquid chromatography (HPLC) at the UCLA Center for Molecular and Medical Sciences and analysed by mass spectrometry.
Medium and reagents
CD4 and CD8 T cells were maintained in complete RPMI 1640 (Sigma Chemical Co., St Louis, MO) supplemented with 10% autologous human serum, 0.1 mm non-essential amino acids, 2 mml-glutamine, 25 μm HEPES buffer and 5 × 10−5 m 2-mercaptoethanol (2-ME). Human recombinant IL-2 (rIL-2) was purchased from Becton Dickinson (Bedford, MA) and recombinant IL-4 (rIL-4) and recombinant IL-7 (rIL-7) from PharMingen (San Diego, CA). Keyhole limpet haemocyanin (KLH) was obtained from Sigma BioSciences (St Louis, MO) and concanavalin A (Con A) from Sigma Chemical Co.
CD4 isolation and culture
Peripheral blood mononuclear cells (PBMC) were isolated from venous blood on a Ficoll gradient as described by Boyum [11]. Three million cells were cultured in each well of a 24-well plate with 7 μm peptide at 37°C for 5 days. After 5 days, rIL-2 (4 U/ml) was added to each well. On day 14, an aliquot of T cells was removed from culture to be used in the proliferation assay described below. The remaining cells were restimulated using autologous, irradiated antigen-presenting cells (APC) and 7 μm peptide [12]. Alternatively, CD4 isolation was performed as described by Ota et al. [13]. PBMC (2 × 105) were cultured in each well of 96-well U-bottomed plates with 10 μg peptide per well. rIL-2 (10 U/ml) and 2 U rIL-4/ml (PharMingen) were added every third day and proliferation assays performed every 2 weeks. On day 13 an aliquot of cultured cells was assayed for peptide specificity as described below.
CD8 isolation and culture
Primary cytotoxic T lymphocytes (CTL) were generated by the method described by Piebanski et al. [14]. PBMC, prepulsed with 50–100 μg/ml peptide for 1 h at 37°C, were resuspended in complete RPMI culture medium at 2 × 106 cells/ml. Cells (3 × 106) were plated in 24-well plates with 25 ng/ml rIL-7 and 5 μg/ml KLH. On days 7 and 14 the cells were restimulated with autologous, irradiated APC loaded with peptide as above. On days 9, 12, 15 and 19, all cultures received 10 U/ml human rIL-2. On day 21, using a portion of bulk cultures, CTL assays were performed as described below. The remaining cells were restimulated with autologous, irradiated, peptide-loaded lymphoblasts, rIL-7 and KLH (described below). The above cycle of restimulation, addition of cytokines and analysis of cytotoxic activity was continued during selection of peptide-specific CD8 T cells.
Proliferation assay
The antigenic specificity of CD4 T cells was tested using proliferation assays [15] performed every 14 days. T cells (1 × 105) were removed from culture and placed in 96-well plates with 2 × 105 irradiated, autologous APC with or without 7 μm peptide. Cells were harvested in an automatic cell harvester (Skatron Instruments, Lier, Norway) 3 days later. Sixteen hours before harvesting, cells were pulsed with 3H-thymidine. The 3H-thymidine incorporation was assayed in a beta plate liquid scintillation counter (LKB, Gaithersburg, MD).
CTL assay
Chromium release assays were performed as described elsewhere [16]. Lymphoblasts were derived by incubating PBMC for 48 h with 5 μg/ml Con A at 37°C. Lymphoblasts, incubated with 100 μm peptide for 90 min at 37°C, were used as target cells. The targets were then incubated with 200 μCi Na51CrO4 (Dupont NEN, Boston, MA) for 50 min at 37°C and resuspended in medium at a final concentration of 1 × 105 cells/ml. Effector cells were removed from bulk culture and incubated with 51Cr-labelled target cells in V-bottomed 96-well plates, at final effector to target ratios of 40:1–0.3:1, for 5 h at 37°C. Radioactivity of the harvested 100-μl supernatants was measured in a Beckman Gamma 5000 counter (Fullerton, CA). Cytotoxic activity was calculated as follows: % specific killing =
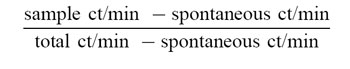 |
Spontaneous release was calculated from the supernatants of targets incubated with medium alone. Total release was the radioactivity measured using untreated and non-lysed 51Cr-labelled target cells.
RESULTS
CD4 T cells specific for PSA peptides in HLA-DR4 individuals
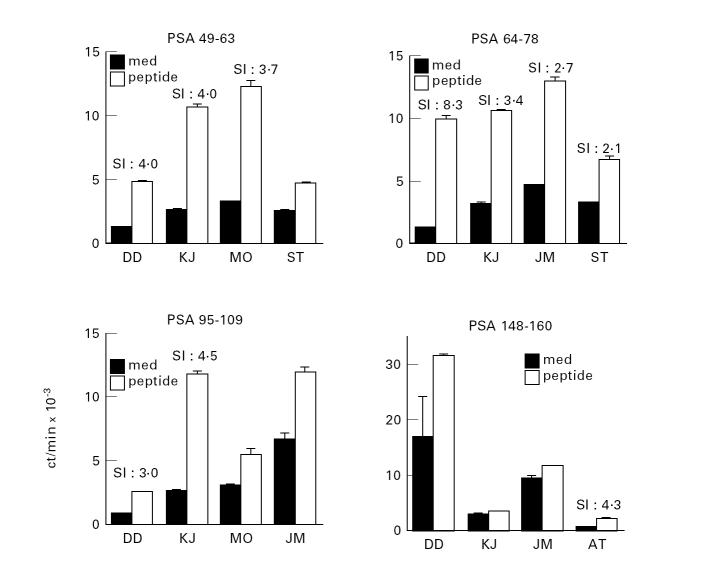
The repertoire of CD4 T cells directed to determinants of PSA in normal human males was examined using peptides of PSA to isolate short-term CD4 T cell lines from the peripheral blood lymphocytes of normal male individuals. Peptides were selected on the basis of the previously reported binding motifs for HLA-DR4. As the individual may be tolerant to the dominant determinants of a self protein, a minority of the peptides would therefore not be expected to be immunogenic; however, there should be a repertoire available to recognize and respond to the cryptic determinants on the self protein. Figure 1 demonstrates the peptide antigen-stimulated proliferative responses of 20 different short-term CD4 T cell lines derived from normal males. The top two and the middle left panels show that two individuals (DD and KJ) expressed a repertoire of CD4 T cells specific for peptides PSA 49–63, PSA 64–78 as well as PSA 95–109 (Fig. 1). The short-term T cell lines derived from these two individuals showed stimulation indices (SIs) between 3.0 and 8.3. Cell lines which showed SIs < 2.0 were not considered positive (Fig. 1). The results of CD4 T cell lines specific for 10 different PSA peptides and derived from six individuals are summarized in Table 1. All HLA-DR4 individuals, with the exception of ST, showed responsiveness to several peptides of PSA, usually 3–5. However, the best responses were noted with PSA peptides 49–63 and 64–78. Thus, CD4 T cells from normal males are clearly able to recognize many determinants of the self protein PSA.
Fig. 1.
Existence of CD4 T cell repertoire for prostate-specific antigen (PSA) peptides in normal HLA-DR4 male individuals. The Figure shows the peptide antigen-stimulated proliferative responses of 20 different short-term CD4 T cell lines derived from six different individuals. The stimulation indices (SIs) of the 11 cell lines considered specific for peptide antigen ranged from 2.1 to 8.3 (shown). The cell lines which showed SIs < 2.0 were not considered positive. As indicated in Materials and Methods, each cell line was tested at the end of each cycle of antigenic stimulation. The data shown are representative of multiple assays performed on each cell line. The peptides were selected on the basis of previously reported binding motifs for HLA-DR4. The sequences of all peptides used to induce CD4 T cells are shown in Table 1. The data in this Figure are expressed as mean ct/min ± s.e.m. for triplicate cultures. The SIs were calculated by ct/min incorporated by lymphocyte cultures incubated with peptide ÷ ct/min of cultures incubated without the peptide.
Table 1.
Summary of CD4 T cell lines derived from six HLA-DR4 individuals
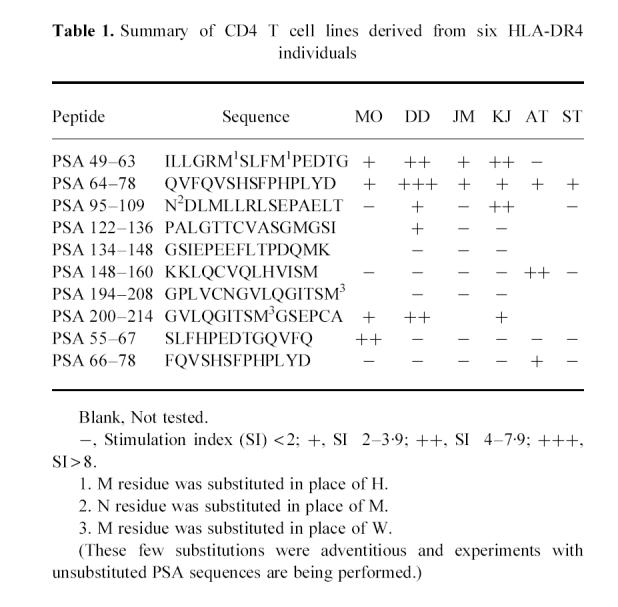
The heterogeneous pattern of responsiveness within the panel of HLA-DR4 individuals toward the set of PSA peptides tested is evident from Fig. 1 and Table 1. Table 1 shows that while MO, DD, JM and KJ showed positive responses to PSA 49–63 and PSA 64–78, their response to PSA 95–109, 148–160 and 66–78 differed considerably among individuals. Although KJ and DD expressed CD4 T cell responsiveness to the latter three peptides, MO and JM did not. Donor AT expressed CD4 T cell responses to PSA peptide 64–78 but did not respond to PSA 49–63 (see Table 1). This diversity of recognition and response is consistent with the fact that the group of DR4 individuals used in the study expressed different MHC molecules at loci other than DR. It is the whole complement of MHC molecules which shapes the peripheral T cell repertoire by positive and negative selection of T cells within the thymus [17].
Stability of peptide antigen-specific CD4 T cell lines
Figure 2 shows that certain peptide-specific CD4 T cell lines derived from HLA-DR4 individuals are stable only until 8–10 weeks. As shown for four different CD4 T cell lines with high proliferation indices, an increase in peptide-specific stimulation occurred over the initial 6–7 weeks. Subsequently, however, these cell lines showed a sharp decline in activation by their specific peptide antigens. This is similar to antigen-induced apoptotic cell death described in other systems [18]. Thus, activation of T cells with superantigens results in an analogous increase in proliferation of T cells followed by a dramatic decline in their proliferative responses.
Fig. 2.
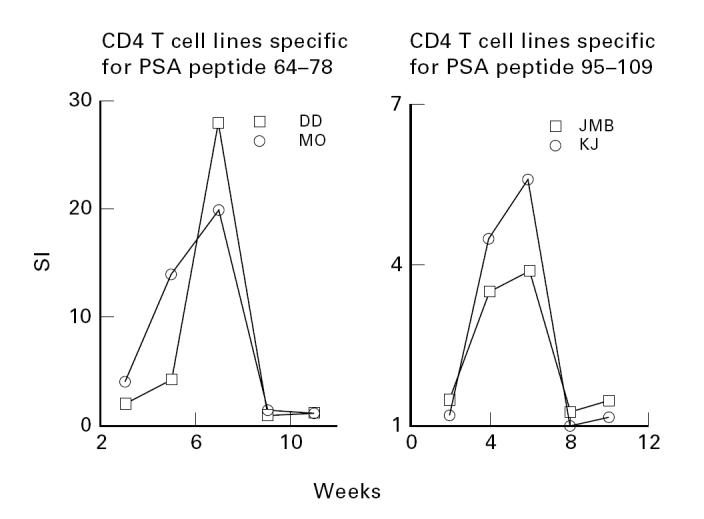
Stability of peptide antigen-specific CD4 T cell lines over time: peptide-specific CD4 T cell lines are stable only until 8–10 weeks. The data are shown as stimulation indices (SIs) derived from proliferation assays performed as described above. As shown for four different CD4 T cell lines, an increase in peptide-specific stimulation occurs over the initial 6–7 weeks, followed by a sharp decline in activation. This is reminiscent of antigen-induced apoptotic cell death described in various other systems, including stimulation of CD4 T cells by superantigens.
CD8 T cells specific for peptides of PSA and PSM in HLA-A1 individuals
As described for CD4 T cells, the CD8 T cell repertoire directed to PSA and PSM determinants was defined by using PSA and PSM peptides to isolate short-term CD8 T cell lines from PBMC of normal men. Peptides (9- or 10-mer) were selected on the basis of binding motifs for HLA-A1. It is interesting to note that the sequence of the PSA molecule (250 amino acids) contained only a total of two HLA-A1-binding peptides (Fig. 3 and Table 2). Though a total of six HLA-A1 binding peptides could be found within PSM (not shown), only three (shown, Table 2) were used in this study. Figure 3 shows that the HLA-A1 individual, JC, expressed a repertoire of CD8 T cells specific not only for PSA peptide 68–77, but also for PSM peptides 168–176, 347–356 and 557–566. The four short-term CD8 T cell lines depicted in this Figure demonstrate specific killing in the range of 16–68% at an effector to target ratio of 40:1. Interestingly, CD8 T cell lines specific for PSA 68–77 consistently showed strong specific killing (> 50%) in two different cell lines derived from JC (Fig. 2,Table 2) as well as from two other donors (Table 2).
Fig. 3.
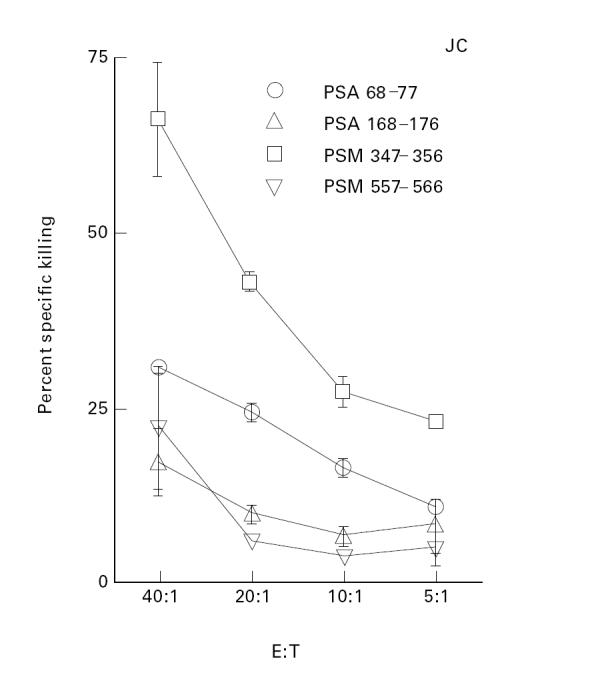
Existence of CD8 T cell repertoire for peptides of prostate-specific antigen (PSA) and prostate-specific membrane antigen (PSM) in normal HLA-A1 individuals. As described in Materials and Methods, 51Cr-labelled autologous, concanavalin A (Con A)-stimulated blasts loaded with the respective peptide were used as target cells. The sequences of peptides used to induce CD8 T cells are shown in Table 2. The data are expressed as percentage specific killing calculated as described in Materials and Methods. This Figure demonstrates the percentage specific killing mediated by four short-term CD8 T cell lines derived from one representative individual. The cell lines showed 18–68% specific killing at an effector to target ratio of 40:1. The data shown are representative of the multiple assays performed for each cell line at the end of each cycle of stimulation (see Materials and Methods and Fig. 4). The peptides were selected on the basis of binding motifs for HLA-A1. The CD8 T cell lines were periodically analysed for cell surface expression of CD8 molecules (data not shown). These analyses demonstrated a high percentage (> 70%) of CD8+ cells.
Table 2.
Summary of CD8 T cell lines derived from three HLA-A1 individuals
A progressive selection of CD8 T cells specific for the given peptide within the cell lines being stimulated in culture is evident from Fig. 4. By week 13, the CD8 T cell line specific for PSA 68–77 showed 98% specific cytotoxic activity at an effector to target ratio of 20:1 (Fig. 4a). Similarly, another cell line (Fig. 4b) specific for PSM peptide 557–566, derived from individual MM, developed an increased killing of peptide-loaded target cells over 13 weeks. It is clear from this Figure that CD8 T cell lines are specific for their respective peptides, as targets loaded with non-specific ovalbumin peptide elicited no CTL activity from these cell lines (Fig. 4a,b).
Fig. 4.
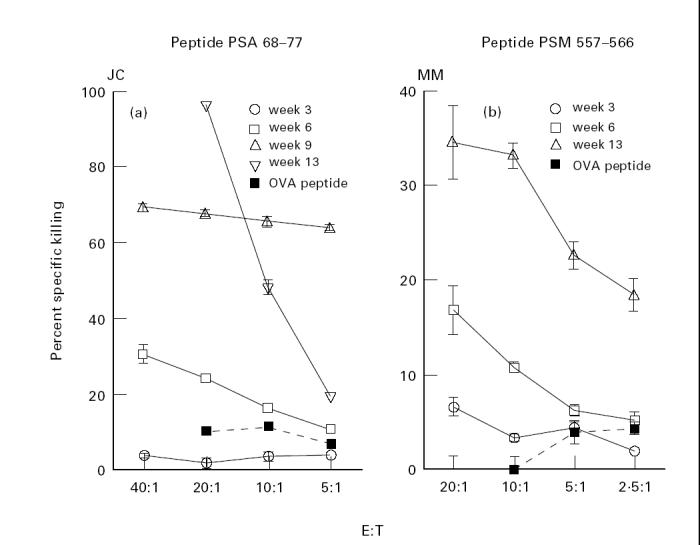
Progressive increase in peptide-specific cytolytic activity in CD8 T cell lines. Data are shown for two representative prostate-specific antigen (PSA) 68–77 (a) and prostate-specific membrane antigen (PSM) 557–566 (b) CD8 T cell lines. Ovalbumin 258–276 was used as a negative control. In addition, HLA-DR4-restricted peptides were also used as negative controls in these assays, the results were identical with the data obtained with ovalbumin 258–276 (data not shown). The data are expressed as percentage specific cytotoxicity as described for Fig. 3.
The results of CTL activity of CD8 T cell lines specific for PSA and PSM peptides, derived from three donors, are shown in Table 2. PBMC from JC were used to induce peptide-specific CD8 T cell lines on two separate occasions (JC1 and JC2). It is clear that of the five peptides tested, each HLA-A1 donor showed responsiveness to at least three, and as many as five of them. PSA peptide 68–77, and PSM peptides 347–356 and 557–566, induced the strongest responses, while PSA peptide 219–228 elicited the lowest responsiveness.
Most interestingly, PSA 64–78 was one of the strongest determinants for inducing CD4 T cells and PSA 68–77 revealed the best induction of CD8 T cells (Tables 1 and 2). As the sequence of the latter determinant is encoded within the former, we would like to refer to PSA 64–78 as a ‘dual motif’ peptide which contains overlapping motifs for inducing HLA-DR4-restricted CD4 T cells and HLA-A1-restricted CD8 T cells. We are currently investigating the ‘dual motif’ potential of this peptide using PBMC from individuals expressing both of the above-mentioned MHC molecules.
DISCUSSION
How intact is the T cell repertoire specific for one's own proteins in a given individual? The T cell repertoire directed to those self proteins which are available within the thymus undergoes deletion there. It is now generally accepted that it is the repertoire to the dominant determinants of such self proteins which gets deleted in the thymus. The existent T cell repertoire specific for thymus-accessible self proteins is thus expected to be directed mainly towards the cryptic determinants. This has been demonstrated in several instances [19] including in mice transgenic for hen egg-white lysozyme (HEL) [20]. The transgenic mice showed highly reduced T cell responses to those determinants of HEL which were dominant in the wild-type mice. HEL transgenic mice, however, had strong T cell responses to those HEL peptides which were cryptic or subdominant in the wild-type mice [20]. What about other self proteins, with either no or limited access to the thymus? T cell repertoire to these determinants may not be deleted there. These T cells may be deleted in the periphery when they recognize a self determinant in association with the MHC of the host cells, but in the absence of other activation factors such as costimulatory molecules (Matzinger and colleagues [21]). Despite the peripheral deletion of the T cell repertoire specific for this category of self proteins, there could be a transient period of responsiveness between the time of release of the T cells from the thymus to the periphery until the T cell encounters its self determinant and is affected by this interaction. During this time this T cell repertoire can be potentially activated by several factors, including the use of adjuvants. Thus, a self-directed T cell repertoire against most proteins exists in all individuals, for proteins which reach the thymus the existent T cell repertoire is directed to the cryptic determinants, and for those which do not, there is, perhaps, a potential T cell repertoire directed towards both the dominant and cryptic determinants.
This study examines the existence of CD4 and CD8 T cell repertoires specific for determinants of the self protein PSA and PSM in humans. It is not clear whether PSA and PSM should be regarded as sequestered or whether the developing thymocytes gain access to the dominant determinants of PSA and PSM. PSA concentrations in normal males range from 0 to 4 ng% and PSM is a membrane-bound protein. We present evidence for the existence of CD4 T cells specific for PSA and CD8 T cells specific for PSA and PSM. Almost all prostate tumour cells also express both these proteins and are likely to express determinants derived from these proteins associated with MHC molecules on their surface. We could derive CD4 and CD8 T cells specific for several peptides of PSA from all individuals included in the study (Table 1 and Table 2).
Why does the extensive T cell repertoire directed toward determinants of PSA and PSM that we find in male individuals fail to attack cells of the prostate tumours? There could be several explanations for its failure to attack the tumour: (i) it is unclear whether the determinants studied in this paper are derived by natural processing of PSA and PSM synthesized endogenously within the tumour cells. Experiments are currently in progress to address this issue directly, but based on our experience with other model protein antigens, we think it highly unlikely that of the five class-I-restricted and 10 class-II-restricted determinants that we have studied in this report, none is displayed within the MHC molecules expressed by the tumour cells; (ii) tumour cells have down-regulated their MHC molecules. Preliminary data from our laboratory (not shown) on the expression of class I and class II molecules on prostate tumours are not consistent with this hypothesis; (iii) tumour cells lack the expression of costimulatory molecules, which would result in either anergy or deletion of T cells due to lack of signal 2 (see above). There is extensive evidence in other systems that transfection of tumour cells with costimulatory molecules such as B7.1 and B7.2 increases their immunogenicity as well as rendering them highly susceptible to an attack by T cells. Active immunization using selected peptides along with appropriate adjuvants [3] is expected to overcome this problem by presentation of the peptides on professional APC. It is anticipated that the T cells thus activated, through a release of cytokines such as interferon-gamma (IFN-γ) and tumour necrosis factor-alpha (TNF-α), will induce up-regulation of costimulatory molecules as well as MHC molecules on the tumour cells themselves, thus rendering them susceptible to attack by PSA/PSM-specific T cells; (iv) it has recently been reported that the melanoma tumour cells express Fas ligand which results in apoptosis of activated T cells which express Fas [22]. It is not known whether prostate tumour cells express Fas ligand, but in case they do, all activated T cells present in the tumour milieu would be killed [22].
It is significant that PSA 64–78 is a ‘dual motif’ determinant for HLA-A1 and HLA-DR4 molecules. It has been shown earlier that induction of CD4 T cells is required for the generation and propagation of cytotoxic CD8 T cells [3,7]. Identification of more ‘dual motif’ determinants for other class I and class II HLA molecules for different proteins will enable us to bypass the need to introduce helper cytokines such as IL-2 in order to induce target-specific CD8 T cells.
In conclusion, there exists an extensive repertoire of T cells directed to determinants of self proteins, the prostate-specific antigen and prostate-specific membrane protein. Since expression of these self proteins is restricted to the cells of the prostate tumour and other cells of the prostatic tissue, this T cell repertoire can potentially be activated to attack the prostate tumours. This would amount to induction of a beneficial autoimmune response: current experience suggests that the autoimmune component directed towards the normal prostatic cells will not be devastating in the case of prostate cancer, as the prostate is not truly essential for life.
Acknowledgments
We thank Dr David Ucker for a critical reading of this manuscript. This work was supported in part by the Tina and Richard V. Carolan Foundation, the UCLA Prostate Disease Research Program and the UCLA Jonsson Cancer Center Foundation. We are grateful for the participation of healthy volunteers who donated their blood for this study.
References
- 1.Houghton AN. Cancer antigens: immune recognition of self and altered self. J Exp Med. 1994;180:1–4. doi: 10.1084/jem.180.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coulie PG, Brichard V, Van Pel A, et al. A new gene coding for a differentiation antigen recognized by autologous cytolytic T lymphocytes on HLA-A2 melanomas. J Exp Med. 1994;180:35–42. doi: 10.1084/jem.180.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nanda NK, Sercarz EE. Induction of anti-self immunity to cure cancer. Cell. 1995;82:13–17. doi: 10.1016/0092-8674(95)90047-0. [DOI] [PubMed] [Google Scholar]
- 4.Watt KWK, Lee PM, Timkulu T, et al. Human prostate-specific antigen: structural and functional similarity with serine proteases. Proc Natl Acad Sci USA. 1986;83:3166–70. doi: 10.1073/pnas.83.10.3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Israeli RS, Powell CT, Fair WR, et al. Molecular cloning of a complementary DNA encoding a prostate-specific membrane antigen. Cancer Res. 1993;53:227–30. [PubMed] [Google Scholar]
- 6.Sercarz EE, Lehmann PV, Ametani A, et al. Dominance and crypticity of T cell antigenic determinants. Ann Rev Immunol. 1993;11:729–66. doi: 10.1146/annurev.iy.11.040193.003501. [DOI] [PubMed] [Google Scholar]
- 7.Ostrand-Rosenberg S. Tumor immunotherapy: the tumor cell as an antigen-presenting cell. Curr Opin Immunol. 1994;6:722–7. doi: 10.1016/0952-7915(94)90075-2. [DOI] [PubMed] [Google Scholar]
- 8.Terasaki PI, Bernoco D, Park MS, et al. Microdroplet testing for HLA-A, -B, -C and -D antigens. Am J Clin Pathol. 1978;69:103–18. doi: 10.1093/ajcp/69.2.103. [DOI] [PubMed] [Google Scholar]
- 9.Rammensee HG, Friede T, Stevanovic S. MHC ligands and peptide motifs: first listing. Immunogenetics. 1995;41:178–228. doi: 10.1007/BF00172063. [DOI] [PubMed] [Google Scholar]
- 10.Sullivan DO, Arrhenius TS, del Guercio MF, et al. On the interaction of promiscuous antigenic peptides with different DR alleles. J Immunol. 1991;147:2663–9. [PubMed] [Google Scholar]
- 11.Boyum A. Isolation of mononuclear cells and granulocytes from human blood. Scand J Clin Lab Invest. 1968;(Suppl. 97):229–35. (paper IV): [PubMed] [Google Scholar]
- 12.Manca F, Li Pires G, Fenuglio D, et al. Dendritic cells are potent antigen presenting cells for in vitro induction of primary human CD4+ T-cell lines specific for HIV gp120. J Acq Immunol Def Syndr. 1994;7:15–23. [PubMed] [Google Scholar]
- 13.Ota K, Matsui M, Millford EL, et al. T-cell recognition of an immunodominant myelin basic protein epitope in multiple sclerosis. Nature. 1990;346:183–7. doi: 10.1038/346183a0. [DOI] [PubMed] [Google Scholar]
- 14.Piebanski M, Allsopp CEM, Aidoo M, et al. Induction of peptide specific primary cytotoxic T lymphocyte responses from human peripheral blood. Eur J Immunol. 1994;25:1783–7. doi: 10.1002/eji.1830250645. [DOI] [PubMed] [Google Scholar]
- 15.Nanda NK, Nath I. Characteristics of histamine receptors present on suppressor T cell in ‘healthy individuals’. Int J Immunopharmacol. 1985;7:587–95. doi: 10.1016/0192-0561(85)90081-5. [DOI] [PubMed] [Google Scholar]
- 16.Ostergaard HL, Clark WR. Evidence for multiple lytic pathways used by cytotoxic T lymphocytes. J Immunol. 1989;143:2120–6. [PubMed] [Google Scholar]
- 17.Pullen AM, Kappler JW, Marrack P. Tolerance to self-antigen shapes T cell repertoire. Immunologic Rev. 1989;107:125–39. doi: 10.1111/j.1600-065x.1989.tb00006.x. [DOI] [PubMed] [Google Scholar]
- 18.McCormack JE, Callahan JE, Kappler J, et al. Profound deletion of mature T cells in vivo by chronic exposure to exogenous superantigen. J Immunol. 1993;150:3785–92. [PubMed] [Google Scholar]
- 19.Lanzavecchia A. How can cryptic epitopes trigger autoimmunity? J Exp Med. 1995;181:1945–8. doi: 10.1084/jem.181.6.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cibotti R, Kanellopoulos JM, Cabaniols JP, et al. Tolerance to a self-protein involves its immunodominant but does not involve its subdominant determinants. Proc Natl Acad Sci USA. 1992;89:416–20. doi: 10.1073/pnas.89.1.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ridge JP, Fuchs EJ, Matzinger P. Neonatal tolerance revisited: turning on newborn T cells with dendritic cells. Science. 1996;271:1723–6. doi: 10.1126/science.271.5256.1723. [DOI] [PubMed] [Google Scholar]
- 22.Hahne M, Rimoldi D, Schroter M, et al. Melanoma cell expression of Fas (Apo-1/CD95) ligand: implications for tumor immune escape. Science. 1996;274:1363–6. doi: 10.1126/science.274.5291.1363. [DOI] [PubMed] [Google Scholar]