Abstract
Neonatal neutrophils express less membrane and cytoplasmic CR3 (iC3b-receptor, Mac-1, αMβ2-integrin) than do adult neutrophils, and it has been suggested that this renders neonatal neutrophils deficient in diapedesis and bactericidal activity. The reason(s) for this deficiency are unknown. In this study, CR3 expression and the CR3-dependent respiratory burst activity of individual neonatal neutrophils are quantified in comparison with adult leucocytes using flow cytometry. Monocytes and neutrophils are defined as CD14highCD15low and CD14lowCD15high, respectively. Although neonatal neutrophils bore less CR3 on average than did adult neutrophils, neonatal neutrophils were more heterogeneous and many neonatal neutrophils expressed adult levels of CR3. Because of higher neutrophil concentrations in cord versus adult blood, the calculated number of neutrophils in cord blood expressing high amounts of CR3 was equivalent to that of adult blood. Similar findings were made with monocytes. The size of the CR3-dependent respiratory burst stimulated by particulate β-glucan correlated directly with the expression of CR3 by individual neutrophils. With neonatal and adult neutrophils having comparable CR3 densities, the respiratory burst activities were equivalent. Wright–Giemsa differential staining of the subset of neonatal neutrophils with low CR3 levels isolated by fluorescence-activated cell sorting showed a higher proportion of immature cells than the sorted population expressing high CR3 levels. Therefore, higher proportions of immature cells in cord blood probably explain previous reports of deficient CR3 expression and function. The typical neutrophilia of cord blood may compensate for this apparent deficiency by providing adult concentrations of mature neutrophils.
Keywords: neutrophils, integrins, neonatology, infection, complement receptors
INTRODUCTION
Neonates are at risk of infection, and this is thought to result from a developmental deficiency in host defence mechanisms. Because passively acquired maternal antibodies provide a level of specific immunity, it has been proposed that neonates may have a deficiency in innate immunity. Supporting this hypothesis have been several previous investigations showing that neonatal neutrophils and monocytes are functionally deficient in transendothelial migration, chemotaxis, and phagocytosis compared with adult leucocytes [1–5]. Nevertheless, there have been conflicting reports as to whether neonatal neutrophils have a deficiency in bactericidal activity [6,7].
The effectiveness of neutrophils in preventing bacterial invasion depends on rapid migration into sites of infection followed by the destruction of bacteria either intracellularly after phagocytosis or extracellularly via granule exocytosis. CR3 plays a major role in these events, serving both as an iC3b-receptor and an adhesion molecule [8–11]. As a β2-integrin, CR3 mediates diapedesis of leucocytes into sites of infection through binding to endothelial cell intercellular adhesion molecule-1 (ICAM-1). As a receptor mediating cytotoxicity of bacteria, CR3 is responsible for recognition of microbial pathogens opsonized with iC3b. In addition, CR3 functions indirectly through coupling to other neutrophil receptors that do not have transmembrane domains, functioning as a signalling adapter for cytotoxic responses [12,13]. For example, neutrophil responses to IgG-opsonized bacteria occur via a membrane complex of FcγRIIIB (CD16) and CR3, and the phagocytosis of Gram-negative bacteria containing endotoxin is thought to occur because of CD14 signalling through attached CR3. The clinical importance of CR3 is particularly emphasized in the disease leucocyte adhesion deficiency, a genetic defect in CD18 synthesis associated with life-threatening bacterial infections [14–16].
In resting neutrophils and monocytes, most CR3 resides in cytoplasmic granules rather than on the membrane [17,18]. To measure total CR3 by flow cytometry, it is necessary to stimulate mobilization of cytoplasmic CR3 to the membrane with activators such as calcium ionomycin [5,19,20]. Using such techniques, previous studies have shown that neonatal neutrophils were deficient in total CR3 [5,21,22]. Moreover, this neutrophil CR3 deficiency was related to diminished adhesion and transendothelial migration [2,23]. Other studies have also suggested that this lack of CR3 may result in reduced opsonophagocytic killing of virulent organisms [3].
Most studies of neonatal neutrophils have utilized umbilical cord blood. Although representative of neonatal peripheral blood at birth, cord blood has also been used as a source of myeloid stem cells for bone marrow replacement therapy [24] and contains the entire series of differentiating myeloid cells [25,26]. Of particular relevance, studies on myeloid cell maturation have shown that CR3 increases co-ordinately with formation of cytoplasmic granules and a segmented nucleus [27–31]. Polymorphonuclear cells could be separated by density centrifugation into cell subsets containing increasing amounts of granules and membrane CR3. Accordingly, it seemed possible that some cells in cord blood that appeared to be fully mature polymorphonuclear cells might actually represent less mature cells with a lower CR3 content.
Since neonatal neutrophils express less CR3 than do adult neutrophils, it should be expected that they would also be deficient in CR3-dependent adhesion, as others have reported [1,2,23,32]. However, if cord blood were to contain a mixture of immature and mature neutrophil subsets, it follows that the CR3-dependent functions of these subsets would vary according to CR3 content.
The purpose of the current study was to determine if the previously reported ‘deficiency’ of CR3 in neonatal leucocytes actually represents an increased proportion of immature cells. A three-colour flow cytometry assay was devised that showed that cord blood contained a mixture of immature and mature neutrophils, as well as monocytes, that expressed varying amounts of CR3. Next, another type of flow cytometry assay was developed that allowed the assessment of individual neutrophils for CR3-dependent function relative to CR3 surface density. For cells with comparable CR3 expression in either cord or adult blood, the CR3-dependent respiratory burst function was the same.
MATERIALS AND METHODS
Adult and neonatal leucocyte preparations
Citrated venous blood was obtained simultaneously from the umbilical cords of healthy term newborn infants and adult volunteers. Total and differential leucocyte counts were performed prior to separation of leucocytes. After sedimentation of erythrocytes at room temperature by mixture of blood samples with an equal volume of 3% dextran T-500 (Amersham Pharmacia Biotech, Piscataway, NJ) in citrate anticoagulant and incubation for 30 min, the remaining erythrocytes in the leucocyte-rich plasma were lysed by briefly suspending the cells in 0.15 m ammonium chloride. After washing three times with 0.5% bovine serum albumin in PBS (BSA–PBS), the leucocytes were suspended at 1 × 106/ml in BSA–PBS. For the respiratory burst assay, all reagents were depleted of detectable endotoxin (lipopolysaccharide (LPS)), and sterile disposable plasticware was used. For solutions of dextran, ammonium chloride, PBS–BSA, and MoAb reagents, LPS was removed using Triton X-114 [33], followed by monitoring of LPS removal with LAL Test Kit 03 (Biowhitaker, Walkersville, MD).
MoAbs and immunofluorescence staining reagents
MoAb MN-41 specific for CD11b I-domain [34,35] was conjugated to FITC [36] for measurement of CR3 surface density. Alternatively, CR3 was analysed with anti-Leu-15–PE (Becton Dickinson Immunochemistry Systems, San Jose, CA). Anti-CD14–PE and non-specific mouse IgG–PE were purchased from Becton Dickinson. Mouse myeloma MOPC-21 IgG1 from ascites fluid [37] was coupled to FITC or biotin [38] and used as a control for non-specific staining. Biotinylated anti-CD15 MoAb and streptavidin-cychrome were purchased from Pharmingen (San Diego, CA).
Flow cytometry assay for CR3 expression by unseparated neutrophils and monocytes
To stimulate maximum expression of CR3, 1 × 106 leucocytes in a 12 × 75 mm tube were incubated for 20 min at room temperature in 100 μl of 10−7m calcium ionomycin in LPS-free Hanks' balanced salt solution (HBSS; Biowhitaker) and then placed in an ice bath [5,22]. The cells were stained on ice by addition of 20 μg each of MN-41–FITC, anti-CD15–biotin, and anti-CD14–PE and incubation for 20 min. After three washes with ice-cold PBS–BSA, 20 μl of streptavidin-cychrome (1:10 in PBS–BSA) were added and the resuspended cells were incubated on ice for 20 min. After three washes with ice-cold PBS–BSA, the cells were examined by flow cytometry. Controls included cells stained with the MoAbs separately for colour compensation, as well as cells stained with combinations of non-specific IgG coupled to FITC, PE and biotin. Five channels of listmode data were collected with a Coulter Profile II (Coulter Instruments, Miami Lakes, FL): (i) forward light scatter for cell size, (ii) side scatter light (SS) for granularity, (iii) log fluorescence one (LFL1) for FITC, (iv) LFL2 for PE, and (v) LFL3 for cychrome. Neutrophils were defined as CD14lowCD15high and monocytes as CD14highCD15low, effectively excluding any dead cells that would have been stained non-specifically as CD14highCD15high. Listmode data were analysed using WinList 3 software (Verity Software House, Topsham, ME). In some experiments, staining was carried out with 4 × 106 cells (using 4× more staining reagents) and FACS sorting of the stained cells was done using a Coulter Elite Analyser. Sorted fractions were collected directly onto microscope slides containing a drop of fetal bovine serum. After spreading the cells across the slide, the air-dried cells were stained with Wright–Giemsa and blind coded slides were examined for morphology by a certified haematology laboratory technician. The significance between data sets was calculated with Student's t-test using Instat software (Graphpad, San Diego, CA).
Flow cytometry assay for respiratory burst function versus CR3 surface density
A CR3-dependent intracellular respiratory burst stimulated with particulate β-glucan [38] was monitored by the fluorescein signal from cells loaded with 5 μm diacetyldichlorofluoresceine (DCF, or H2DCFDA, D-399; Molecular Probes, Inc., Eugene, OR). By combining the fluorescein burst signal with anti-Leu-15–PE staining for CR3, the burst within individual neutrophils (LFL1) could be related to CR3 surface density (LFL2). Neutrophils were analysed selectively by gating on CD15high cells using anti-CD15–biotin-streptavidin-cychrome as above (LFL3). Unlike the extracellular burst that corresponds to particle phagocytosis, the intracellular burst was stimulated with soluble LPS in the absence of phagocytosis. Accordingly, it was necessary to carry out this assay under LPS-free and sterile conditions. β-glucan from baker's yeast (Sigma, St Louis, MO) was suspended at 10 mg/ml in 0.2 m sodium hydroxide for 1 h at 37°C to destroy particle-associated LPS. After 10 washes with LPS-free HBSS and resuspension at 10 mg/ml in LPS-free 2% fetal calf serum (FCS) in HBSS, the pH was reduced to 7.5 and ≤ 0.05 endotoxin units were detectable. DCF, dissolved initially at 5 mm in ethanol, was diluted to 50 μm in LPS-free 2% FCS–HBSS before use in assays. Adult or cord leucocytes (2 × 106) were mixed with 80 μl anti-Leu-15–PE, 40 μl anti-CD15–biotin (1:10), 100 μl 50 μm DCF, and 100 μl β-glucan in a total volume of 1 ml in a sterile 12 × 75 mm plastic tube. After warming to 37°C in a water bath, the tubes were placed on a rotator with horizontal axis at 37°C for 20 min. The tubes were transferred to an ice bath, vortexed vigorously to disrupt β-glucan-clumped cells, and washed twice in ice-cold 2% FCS–HBSS at 400 g for 8 min. Next, 40 μl streptavidin-cychrome (1:10 dilution) were added and incubation was continued on ice for 20 min. Finally, the cells were vortexed vigorously, washed twice as before, and analysed by flow cytometry. Controls were included in which CR3 was blocked prior to β-glucan with 20 μg MN-41 anti-CR3 [39] or 500 μg/ml of soluble β-glucan [40]. Other controls were used for colour compensation and for spontaneous burst without β-glucan.
RESULTS
Expression of CR3 by neutrophils and monocytes in cord blood versus adult blood
As reported by others [2,5,22], neonatal neutrophils expressed approx. 66% as much CR3 as did adult neutrophils (Fig. 1). Similarly, neonatal monocytes expressed approx. 85% as much CR3 as did adult monocytes. Among neonatal neutrophils, a broad and homogeneous distribution of cells with very low to high surface densities of CR3 was observed in 10 out of 15 cord samples. In the remaining cord samples, the neutrophil CR3 surface density, although sometimes lower in mean value than that of adult neutrophils, had a narrow distribution resembling adult blood neutrophils (Fig. 1). Comparison of neonatal cells with high (adult levels) versus low levels of CR3 revealed no apparent differences in granularity (light side scatter), as both neutrophils and monocytes expressing high or low amounts of CR3 had a relatively similar side scatter profile (Fig. 2). However, when neutrophils expressing low versus high densities of CR3 were flow sorted, fixed, and stained with Wright–Giemsa for analysis of nuclear morphology, the sorted fraction of cells expressing low CR3 was found to contain a lower proportion of segmented polymorphonuclear cells (83%) than did the sorted fraction with high density of CR3 (94% polymorphonuclear).
Fig. 1.
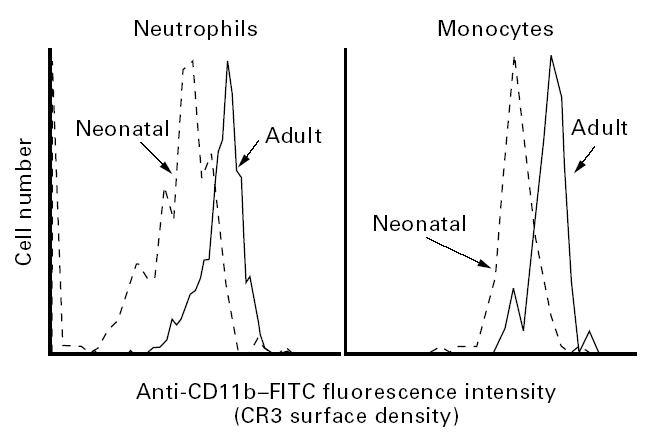
Three-colour flow cytometry analysis of CR3 surface density on neonatal versus adult leucocytes. The histograms compare the unseparated leucocytes from one cord and one adult blood sample in which the CD14lowCD15high neutrophils and CD14highCD15low monocytes were analysed individually for surface density of CR3.
Fig. 2.
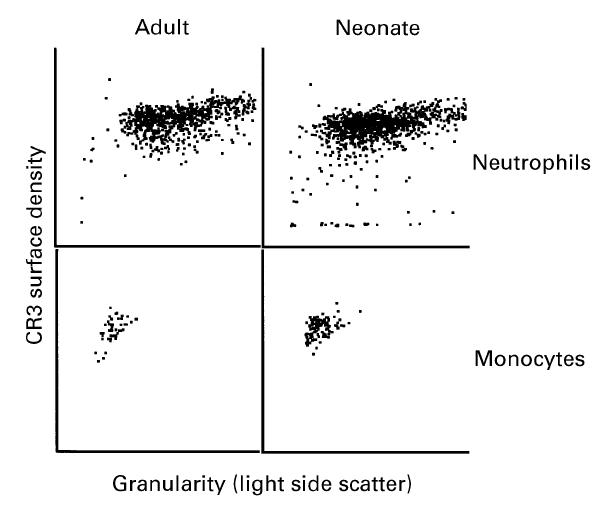
Assay of CR3 surface density versus surface granularity. The same sample data shown in Fig. 1 were analysed for CR3 density versus light side scatter. Among neonatal neutrophils, the small number of cells having low surface CR3 at the bottom of the histogram are heterogeneous in granularity (spread across the bottom of the histogram).
Although the mean amount of CR3 expressed by neonatal neutrophils and monocytes was lower than that of adult cells, when the two-to-three-fold higher leucocyte count of cord blood compared with adult blood was considered, it was calculated that cord blood actually contained slightly higher concentrations of both neutrophils and monocytes with high density of CR3 (defined as the mean ± 1 s.d. of 12 adult mean channel values for neutrophil or monocyte CR3 fluorescence intensity) than did adult blood (Fig. 3). However, among the 15 cord and 12 adult blood samples examined, there was such heterogeneity in the numbers of leucocytes with high CR3 expression that the difference between adult and cord blood in the absolute numbers of neutrophils or monocytes with high expression of CR3 was not statistically significant (P = 0.42 and P = 0.16, Fig. 3). It was particularly noteworthy that in a third of the cord samples, the distribution pattern of neutrophils with high and low numbers of CR3 versus granularity was indistinguishable from the average adult blood sample. Thus, the cord sample shown in Fig. 2 was representative of approximately two-thirds of cord samples examined and the remaining cord samples exhibited profiles that were indistinguishable from adult blood.
Fig. 3.
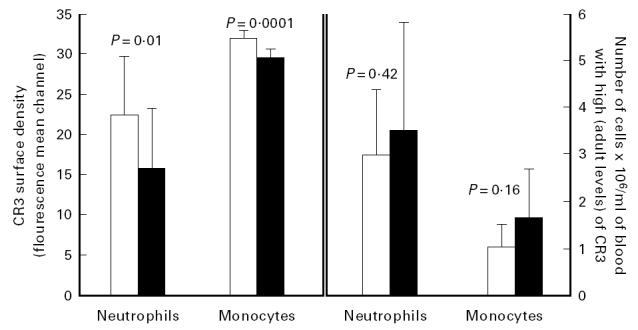
Comparison of adult (□) and neonatal (▪) leucocytes for CR3 density and for the concentration of cells with high CR3. The histogram on the left indicates the CR3 surface density of the entire populations of adult and neonatal leucocytes. The histogram on the right shows adult or cord blood concentrations of neutrophils and monocytes containing high densities (adult levels) of CR3. The mean leucocyte counts for 15 cord versus 12 adult blood samples were 10.8 ± 4.0 versus 5.5 ± 1.6 × 106 per ml, and differential counts for the cord versus adult blood samples were 3.9 ± 1.1 versus 2.6 ± 1.0 × 106 per ml for neutrophils and 3.3 ± 1.9 versus 1.1 ± 0.48 × 106 per ml monocytes.
Function of CR3 on neonatal neutrophils versus adult neutrophils
Since a subset of neonatal neutrophils expressed levels of CR3 equivalent to adult blood neutrophils, it was hypothesized that the CR3-dependent functions of this subset would be equivalent to those of adult blood neutrophils. The CR3-dependent respiratory burst stimulated by particulate β-glucan was examined using an assay in which a fluorescein signal representing an intracellular burst was generated in response to ligation of the lectin site of CD11b. Inhibition of this fluorescence signal by MoAbs that block the lectin site of CD11b confirmed the CR3 specificity of this response to particulate β-glucan (not shown). It was possible to measure this response on individual neutrophils in combination with measurement of CR3 surface density by use of anti-Leu-15–PE, since previous studies had shown that this CD11b MoAb was unique among > 20 other CD11b MoAbs examined, in that it neither blocked soluble β-glucan–FITC staining of neutrophil CR3 [40] nor inhibited the neutrophil respiratory burst stimulated by particulate β-glucan [39]. This analysis indicated that the size of the CR3-dependent respiratory burst was directly proportional to the surface density of CR3, and that the subset of neonatal neutrophils with high levels of CR3 exhibited the same sized respiratory burst as did adult blood neutrophils (Fig. 4). As expected, neonatal neutrophils exhibiting low expression of CR3 (average mean channel for four cord samples = 109 ± 8.01) also gave a proportionately lower respiratory burst (90.6 ± 30.5) than the four paired adult samples (CR3 = 173 ± 53.0, burst = 164 ± 59.0). The calculated burst to CR3 ratio of neonatal neutrophils (0.83 ± 0.3) was not significantly less (P = 0.25) than the ratio of 0.95 ± 0.15 observed with adult neutrophils (Fig. 4).
Fig. 4.
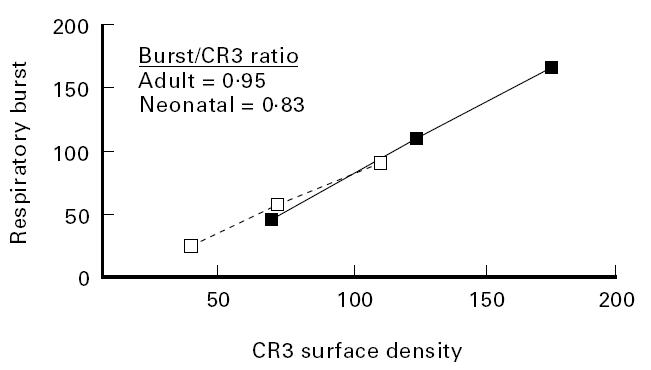
The CR3-dependent respiratory burst is proportional to the CR3 surface density of both adult (▪) and neonatal neutrophils (□). A three-colour flow cytometry assay was used to analyse CD15high neutrophils for the size of an intracellular respiratory burst triggered by β-glucan particles versus the surface density of CR3 expression.
DISCUSSION
Over the past 25 years, several investigators have reported that cord blood-derived neonatal neutrophils adhere to and migrate through microvascular endothelium inefficiently. These functional defects were subsequently linked to diminished CR3 expression. The purpose of the current study was to determine if this ‘deficiency’ of CR3 in neonatal leucocytes actually represents an increased proportion of immature cells rather than a population of defective cells. In particular, it is now appreciated that umbilical cord blood resembles bone marrow and contains the entire spectrum of myeloid cell maturation from CD34+ stem cells to mature polymorphonuclear neutrophils. The data confirm previous reports that the average cord blood neutrophil contained less CR3 than did the average adult blood neutrophil. However, a novel finding is that cord neutrophils were more variable in CR3 content than were adult neutrophils. Flow sorting and differential counts of neonatal neutrophils expressing low amounts of CR3 also indicated a less mature nuclear morphology. Because of a much higher neutrophil concentration in cord blood compared with adult blood, cord blood and adult blood contained equivalent concentrations of neutrophils with a high content of CR3. Finally, the size of the CR3-dependent respiratory burst of individual neutrophils was shown to be directly correlated with CR3 content, such that cord and adult blood neutrophils containing equivalent quantities of CR3 exhibited similar CR3-dependent respiratory bursts.
The expression of CR3 is detectable early in myeloid cell differentiation, and the process continues throughout the development of mature-appearing polymorphonuclear cells [29–31,41,42]. The terminal events in neutrophil maturation include the acquisition of cytoplasmic granules, which are responsible for increased cell density. Consequently, neutrophils in various stages of maturation may be separated by density gradient centrifugation [27,43]. Among mature-appearing neutrophils separated according to density, even mature appearing polymorphonuclear neutrophils demonstrated a further maturation manifested by a gradual increase in cytoplasmic granularity, cell density, and CR3 expression [28]. Thus, neutrophils that appear morphologically to be mature polymorphonuclear leucocytes may not have acquired their full content of CR3. Flow sorting and Wright–Giemsa staining revealed that the cord neutrophils with low CR3 were more likely to be immature than the cells with high CR3 content. This suggests that the cord neutrophils with lower CR3 represent less mature cells than the average adult blood neutrophil. At this time, there is no absolute definition of a mature neutrophil, but based on available information it appears likely that the acquisition of a full content of cytoplasmic granules containing CR3 represents one of the final phases of maturation.
Despite the widely accepted notion that neonatal neutrophils are deficient in CR3, there have been some inconsistencies in previous reports [2,5,21,23,44]. Some early investigations suggested that neonatal neutrophils contained normal amounts of CR3 but had an abnormality in their ability to mobilize granule stores of CR3 to the membrane surface in response to chemotactic factors or secretagogues [23]. Later, however, it was convincingly demonstrated by three different methods that neonatal neutrophils did contain less CR3 than did adult neutrophils and that their reduced mobilization of CR3 to the membrane surface corresponded to a reduced total amount of available cytoplasmic CR3 rather than to a reduced ability to respond to chemotactic stimuli [5,22]. In particular, when neonatal and adult neutrophils were compared, the amount of cell surface staining for CR3 obtained following stimulation with calcium ionomycin corresponded to the amount of CR3 detected following permeabilization of neutrophils and cytoplasmic staining for CR3 [5]. The current study that used the same method of measuring total CR3 content by surface staining following stimulation with calcium ionomycin also noted a significant variation among different cord samples with respect to CR3 content. The neutrophils in about one-third of cord blood samples contained equal or even higher levels of CR3 than did the paired adult blood samples. In contrast, neutrophils from the remaining cord blood samples contained less CR3 compared with the adult samples.
Similar data were acquired in the analysis of monocytes. The average neonatal monocyte was found to have slightly less CR3 than did the average adult monocyte. However, unlike the distinct band and segmented nucleus stages of neutrophil maturation, the terminal phases of monocyte maturation are not readily distinguished by Wright–Giemsa staining. Nevertheless, the concentration of cord blood monocytes with an adult content of CR3 per cell was the same as adult blood.
Because neonatal neutrophils on average express less CR3 per cell than do adult neutrophils, it follows that they should also be deficient in CR3-dependent functions when compared with adult blood neutrophils. For example, Anderson et al. [2] reported that CR3-dependent adhesion activity of cord blood neutrophils was less than that of adult blood neutrophils. In the current study, a flow cytometry assay indicated that a diminished CR3-dependent respiratory burst was observed only in the subpopulation of cord blood neutrophils that contained a lower amount of CR3.
Despite an equivalent concentration of cord blood leucocytes with similar CR3 content and function as adult blood, it is unknown whether the predominant immature leucocytes of cord blood might contribute to the known increased susceptibility of neonates to infection. Most previous studies of neutrophil function were done using equal numbers of isolated leucocytes from cord and adult blood. This study suggests that neonates at birth may have sufficient concentrations of mature neutrophils to compensate for the immature neutrophils with lower CR3 content and function. However, it is unknown whether the immature cells in cord blood might produce some form of physical interference or cytokine-mediated suppression of mature cell functions. Because the CR3-dependent respiratory burst was found to correlate with CR3 density, future assays for β2-integrin-dependent adhesion and bactericidal activity should attempt to correlate these functions with the CR3 content of individual cells. Alternatively, assays designed to compare the relative functional capacity of neonatal leucocytes with adult leucocytes should use a proportionately higher concentration of neonatal cells than adult cells, as is found in cord versus adult whole blood.
Acknowledgments
The authors are grateful to the delivery room nurses of Norton's Hospital for their help in obtaining the fresh cord blood samples used in this investigation. They would also like to thank Dr Herbert A. Lassiter of the University of Louisville for helpful suggestions. This work was supported by a grant from the National Institutes of Health, AI-27771-17.
References
- 1.Anderson DC, Hughes BJ, Smith CW. Abnormal mobility of neonatal polymorphonuclear leukocytes. Relationship to impaired redistribution of surface adhesion sites by chemotactic factor or colchicine. J Clin Invest. 1981;68:863–74. doi: 10.1172/JCI110341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson DC, Rothlein R, Marlin SD, et al. Impaired transendothelial migration by neonatal neutrophils: abnormalities of Mac-1 (CD11b/CD18)-dependent adherence reactions. Blood. 1990;76:2613–21. [PubMed] [Google Scholar]
- 3.Smith CL, Baker CJ, Anderson DC, et al. Role of complement receptors in opsonophagocytosis of group B streptococci by adult and neonatalneutrophils. J Infect Dis. 1990;162:489–95. doi: 10.1093/infdis/162.2.489. [DOI] [PubMed] [Google Scholar]
- 4.Schutze GE, Hall MA, Baker CJ, et al. Role of neutrophil receptors in opsonophagocytosis of coagulase-negative staphylococci. Infect Immun. 1991;59:2573–8. doi: 10.1128/iai.59.8.2573-2578.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abughali N, Berger M, Tosi MF. Deficient total cell content of CR3 (CD11b) in neonatal neutrophils. Blood. 1994;83:1086–92. [PubMed] [Google Scholar]
- 6.Miller ME. Phagocyte function in the neonate: selected aspects. Pediatrics. 1978:709–12. [PubMed] [Google Scholar]
- 7.Ambruso DR, Bentwood R, Henson PM, et al. Oxidative metabolism of cord blood neutrophils: relationship to content and degranulation of cytoplasmic granules. Pediatr Res. 1984;18:1148–53. doi: 10.1203/00006450-198411000-00019. [DOI] [PubMed] [Google Scholar]
- 8.Ross GD, Vetvicka V. CR3 (CD11b,CD18): a phagocyte and NK cell membrane receptor with multiple ligand specificities and functions. Clin Exp Immunol. 1993;92:181–4. doi: 10.1111/j.1365-2249.1993.tb03377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith CW. Leukocyte–endothelial cell interactions. Semin Hematol. 1993;30(Suppl. 4):45–55. [PubMed] [Google Scholar]
- 10.Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–14. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 11.Hogg N, Berlin C. Structure and function of adhesion receptors in leukocyte trafficking. Immunol Today. 1995;16:327–30. doi: 10.1016/0167-5699(95)80147-2. [DOI] [PubMed] [Google Scholar]
- 12.Petty HR, Todd RF. Integrins as promiscuous signal transduction devices. Immunol Today. 1996;17:209–12. doi: 10.1016/0167-5699(96)30013-3. [DOI] [PubMed] [Google Scholar]
- 13.Todd RF III, Petty HR. β2 (CD11/CD18) integrins can serve as signaling partners for other leukocyte receptors. J Lab Clin Med. 1997;129:492–8. doi: 10.1016/s0022-2143(97)90003-2. [DOI] [PubMed] [Google Scholar]
- 14.Anderson DC, Springer TA. Leukocyte adhesion deficiency: an inherited defect in the Mac-1, LFA-1, and p150,95 glycoproteins. Annu Rev Med. 1987;38:175–94. doi: 10.1146/annurev.me.38.020187.001135. [DOI] [PubMed] [Google Scholar]
- 15.Todd RF III, Freyer DR. The CD11/CD18 leukocyte glycoprotein deficiency. Hematol Oncol Clin N Am. 1988;2:13–31. [PubMed] [Google Scholar]
- 16.Walport MJ, Ross GD. Deficiency of the LFA-1 family of molecules. In: Asherson GL, Zembala M, editors. Human monocytes. 1. London: Academic Press, Inc.; 1989. pp. 417–27. [Google Scholar]
- 17.Singer II, Scott S, Kawka DW, et al. Adhesomes: specific granules containing receptors for laminin, C3bi/fibrinogen, fibronectin, and vitronectin in human polymorphonuclear leukocytes and monocytes. J Cell Biol. 1989;109:3169–82. doi: 10.1083/jcb.109.6.3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jones DH, Schmalstieg FC, Dempsey K, et al. Subcellular distribution and mobilization of MAC-1 (CD11b/CD18) in neonatal neutrophils. Blood. 1990;75:488–98. [PubMed] [Google Scholar]
- 19.Berger M, O'Shea J, Cross AS, et al. Human neutrophils increase expression of C3bi as well as C3b receptors upon activation. J Clin Invest. 1984;74:1566–71. doi: 10.1172/JCI111572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones DH, Anderson DC, Burr BL, et al. Quantitation of intracellular Mac-1 (CD11b/CD18) pools in human neutrophils. J Leukocyte Biol. 1988;44:535–44. doi: 10.1002/jlb.44.6.535. [DOI] [PubMed] [Google Scholar]
- 21.Bruce MC, Baley JE, Medvik KA, et al. Impaired surface membrane expression of C3bi but not C3b receptors on neonatal neutrophils. Pediatr Res. 1987;21:306–11. doi: 10.1203/00006450-198703000-00022. [DOI] [PubMed] [Google Scholar]
- 22.McEvoy LT, Zakem-Cloud H, Tosi MF. Total cell content of CR3 (CD11b/CD18) and LFA-1 (CD11a/CD18) in neonatal neutrophils: relationship to gestational age. Blood. 1996;87:3929–33. [PubMed] [Google Scholar]
- 23.Anderson DC, Becker-Freeman K, Heerdt B, et al. Abnormal stimulated adherence of neonatal granulocytes: impaired induction of surface MAC-1 by chemotactic factors or secretagogues. Blood. 1987;70:740–9. [PubMed] [Google Scholar]
- 24.Adamson JW. Cord blood stem cell banking and transplantation. Stem Cells. 1997;15:57–59. doi: 10.1002/stem.5530150809. [DOI] [PubMed] [Google Scholar]
- 25.Hasan R, Inoue S, Banerjee A. Higher white blood cell counts and band forms in newborns delivered vaginally compared with those delivered by cesarean section. Am J Clin Pathol. 1993;100:116–8. doi: 10.1093/ajcp/100.2.116. [DOI] [PubMed] [Google Scholar]
- 26.Schelonka RL, Yoder BA, Hall RB, et al. Differentiation of segmented and band neutrophils during the early newborn period. J Pediatr. 1995;127:298–300. doi: 10.1016/s0022-3476(95)70314-4. [DOI] [PubMed] [Google Scholar]
- 27.Ross GD, Jarowski CI, Rabellino EM, et al. The sequential appearance of Ia-like antigens and two different complement receptors during the maturation of human neutrophils. J Exp Med. 1978;147:730–44. doi: 10.1084/jem.147.3.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ross GD, Lambris JD. Identification of a C3bi-specific membrane complement receptor that is expressed on lymphocytes, monocytes, neutrophils, and erythrocytes. J Exp Med. 1982;155:96–110. doi: 10.1084/jem.155.1.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adinolfi M, Cheetham M, Lee T, et al. Ontogeny of human complement receptors CR1 and CR3: expression of these molecules on monocytes and neutrophils from maternal, newborn and fetal samples. Eur J Immunol. 1988;18:565–9. doi: 10.1002/eji.1830180412. [DOI] [PubMed] [Google Scholar]
- 30.Hickstein DD, Back AL, Collins SJ. Regulation of expression of the CD11b and CD18 subunits of the neutrophil adherence receptor during human myeloid differentiation. J Biol Chem. 1989;264:21812–7. [PubMed] [Google Scholar]
- 31.Rosmarin AG, Weil SC, Rosner GL, et al. Differential expression of CD11b/CD18 (Mo1) and myeloperoxidase genes during myeloid differentiation. Blood. 1989;73:131–6. [PubMed] [Google Scholar]
- 32.Klein RB, Fischer TJ, Gard SE, et al. Decreased mononuclear and polymorphonuclear chemotaxis in human newborns, infants, and young children. Pediatrics. 1977;60:467–72. [PubMed] [Google Scholar]
- 33.Aida Y, Pabst MJ. Removal of endotoxin from protein solutions by phase separation using Triton X-114. J Immunol Methods. 1990;132:191–5. doi: 10.1016/0022-1759(90)90029-u. [DOI] [PubMed] [Google Scholar]
- 34.Eddy A, Newman SL, Cosio F, et al. The distribution of the CR3 receptor on human cells and tissue as revealed by a monoclonal antibody. Clin Immunol Immunopathol. 1984;31:371–89. doi: 10.1016/0090-1229(84)90090-4. [DOI] [PubMed] [Google Scholar]
- 35.Diamond MS, Garcia-Aguilar J, Bickford JK, et al. The I domain is a major recognition site on the leukocyte integrin Mac-1 (CD11b/CD18) for four distinct adhesion ligands. J Cell Biol. 1993;120:1031–43. doi: 10.1083/jcb.120.4.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Winchester RJ, Ross GD. Methods for enumerating cell populations by surface markers using conventional microscopy. In: Rose NR, Friedman H, Fahey JL, editors. Manual of clinical laboratory immunology. 3. Washington, DC: American Society for Microbiology; 1986. pp. 212–25. [Google Scholar]
- 37.Myones BL, Dalzell JG, Hogg N, et al. Neutrophil and monocyte cell surface p150,95 has iC3b-receptor (CR4) activity resembling CR3. J Clin Invest. 1988;82:640–51. doi: 10.1172/JCI113643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bayer EA, Wilchek M. The use of the avidin-biotin complex as a tool in molecular biology. Meth Biochem Anal. 1980;26:1–45. doi: 10.1002/9780470110461.ch1. [DOI] [PubMed] [Google Scholar]
- 39.Ross GD, Cain JA, Myones BL, et al. Specificity of membrane complement receptor type three (CR3) for β-glucans. Complement Inflamm. 1987;4:61–74. doi: 10.1159/000463010. [DOI] [PubMed] [Google Scholar]
- 40.Thornton BP, Vetvicka V, Pitman M, et al. Analysis of the sugar specificity and molecular location of the β-glucan-binding lectin site of complement receptor type 3 (CD11b/CD18) J Immunol. 1996;156:1235–46. [PubMed] [Google Scholar]
- 41.Hickstein DD, Smith A, Fisher W, et al. Expression of leukocyte adherence-related glycoproteins during differentiation of HL-60 promyelocytic leukemia cells. J Immunol. 1987;138:513–9. [PubMed] [Google Scholar]
- 42.Hickstein DD, Howard M, Meuller L, et al. Expression of the β-subunit of the human leukocyte adherence receptor depends upon cell type and stage of differentiation. J Immunol. 1988;141:4313–7. [PubMed] [Google Scholar]
- 43.Winchester RJ, Ross GD, Jarowski CI, et al. Expression of Ia-like antigen molecules on human granulocytes during early phases of differentiation. Proc Natl Acad Sci USA. 1977;74:4012–6. doi: 10.1073/pnas.74.9.4012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smith JB, Campbell DE, Ludomirsky A, et al. Expression of the complement receptors CR1 and CR3 and the type III Fcγ receptor on neutrophils from newborn infants and from fetuses with Rh disease. Pediatr Res. 1990;28:120–6. doi: 10.1203/00006450-199008000-00009. [DOI] [PubMed] [Google Scholar]


