Abstract
A patient with von Hippel Lindau disease, bilateral symmetric renal cell carcinoma and pulmonary metastases treated with immunotherapy is the subject of this study. A left kidney and tumour mass were removed and the tumour cells used to make an autologous tumour/bacille Calmette–Guérin (BCG) vaccine as part of the treatment protocol. The patient's pulmonary nodules responded, but the remaining renal nodule subsequently grew. Samples of both tumours were obtained allowing for an internally controlled evaluation of the histological and immunohistologic differences between a responding and non-responding tumour nodule after therapy. The immunotherapy protocol is designed to promote a T cell response to autologous tumour. Cellular infiltrates were demonstrated in both responding and non-responding nodules compared with the pretreatment tumour specimen, but the responding nodule contained proportionately more T cells as well as markedly increased numbers of plasma cells and granulocytes. This suggested that several arms of the immune system may have been operative in the responding nodule.
Keywords: T cell, adoptive immunotherapy, autologous tumour vaccine, IL-2, in vitro, sensitization, renal cell cancer
INTRODUCTION
Immunotherapeutic approaches to the therapy of metastatic carcinoma have been associated with small but definite numbers of tumour responses [1–3]. Metastatic renal cell carcinoma is one of the tumour types more likely to respond to such therapy. Responses to therapy with cytokines [4–6], adoptive cellular transfer [1–3,7], or vaccination with autologous irradiated tumour cells or cellular fractions [8–10] have all been reported. To date, it has not been clarified which biological factors may be associated with tumour response. A protocol which combines autologous tumour vaccination and in vitro sensitization (IVS) of T cells during mixed lymphocyte tumour culture for subsequent adoptive transfer (termed IVS therapy) was completed at the University of Pittsburgh Cancer Institute [11]. This protocol is based on an extensively studied animal model [12–19] and on human preclinical studies [20]. The protocol is designed to promote the outgrowth of T cells for adoptive transfer therapy, with the hope that these cells might have specific anti-tumour reactivity and might yield long-lasting anti-tumour immunological memory. The patient described in this study underwent such adoptive transfer therapy. In this patient, regression of a metastatic pulmonary nodule was associated with a distinctive inflammatory response which was not present in a simultaneously progressive renal tumour nodule, suggesting a relationship between the response and the inflammatory cell infiltrate.
CASE REPORT
A 39-year-old white woman underwent screening for von Hippel Lindau disease after the diagnosis had been made in a sister who had retinal lesions and a renal mass. The sister's daughter had been diagnosed with retinal lesions 9 years previously. The mother had died of cerebral haemorrhage at age 31. Other involved members of the family included the maternal grandmother as well as an uncle and cousin on the mother's side. A computed tomography (CT) scan of the brain was negative; however, CT scanning revealed bilateral renal masses (left larger than right), pulmonary metastases, a large pancreatic lesion, and a hypervascular mass of the spinal cord at C2, consistent with haemangioblastoma. Both retinal examination and urine assays for catecholamines were negative (initial evaluation by G. M. Glenn MD, PhD, National Institutes of Health, Bethesda, MD). The left renal mass was removed by nephrectomy and found to be a renal cell carcinoma, clear cell type (T4 NO M1 lung). Fresh tumour tissue was obtained, and sufficient numbers of tumour cells were removed so that the patient could be entered upon the IVS protocol. She was subsequently vaccinated with irradiated autologous tumour cells admixed with bacille Calmette–Guérin (BCG), underwent vaccine-draining lymph node removal, and her vaccine-draining lymph node lymphocytes (2.2 × 109) were co-cultured with irradiated autologous tumour and IL-2 at 10 U/ml. After 2 weeks of culture, the lymphocytes were harvested and adoptively transferred with systemic IL-2, given at 30 000 U/kg every 8 h by bolus i.v. infusion. IVS cells (3.7 × 109) were infused. The population of IVS-generated lymphocytes transferred was characterized by the following phenotypic parameters: CD3+ 65%, CD4+ 52%, CD8+ 11% and CD56+ 25%. Cytotoxicity against K562 and Daudi targets was 126 and 129 lytic units, respectively. Cytotoxicity against fresh frozen autologous tumour cells was 196 lytic units. However, the spontaneous release of 51Cr from the autologous tumour target was elevated. Of a planned total of 15 doses of IL-2, the patient received only eight, and four of these were at 50% dose due to the side-effects of hypotension, severe rigour and hypoxia.
The pulmonary nodules and right renal mass were assessed as unchanged at monthly follow-up evaluations post-adoptive transfer. At 3 months post-treatment, the pulmonary nodules began to regress; however, at 5 months post-treatment (Fig. 1) the right renal mass was demonstrated to be progressive, with early hydronephrosis (increased to 193% of baseline). The pulmonary nodules were continuing to decrease in size to 48% and 66% of baseline on the right and left, respectively. The renal nodule was removed by wedge resection at approx. 7 months post-vaccination, and the responding right lower lobe pulmonary nodule was also excised at the same time. Subsequently, the patient developed obstructive jaundice and underwent a biliary bypass procedure with symptomatic relief. Biopsy of the pancreatic mass at that time was consistent with microcystic cystadenoma, a benign tumour of the pancreas associated with von Hippel patients. At 15 months post-treatment, there was no evidence of new lesions and the left lung nodule continued to shrink (to 23% of baseline).
Fig. 1.
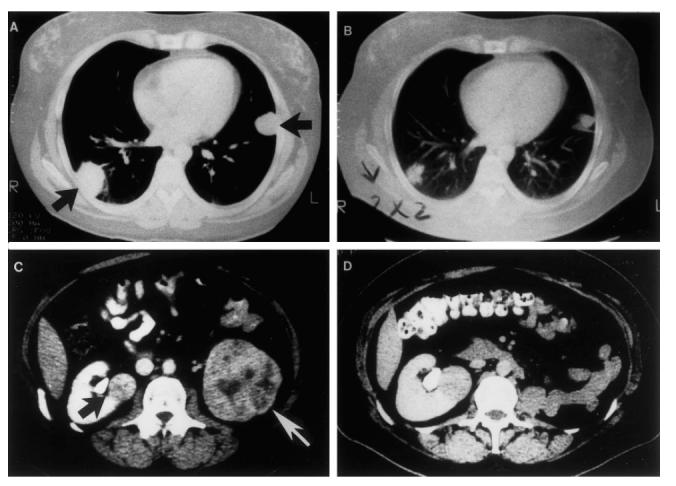
Comparison of comparable computed tomography (CT) scan images in this patient pre-protocol therapy A (2/90), C (1/90) and 5 months post treatment, B, D (9/90). Adoptive transfer occurred 22/3/90.
Subsequently, however, the left pulmonary nodule grew and tumour recurred at the site of prior right pulmonary nodule resection. The patient was treated again with autologous tumour (irradiated)/BCG vaccine and systemic IL-2. Twenty-four hours after second vaccination, a DTH-like recall response was noted at the original vaccination site (opposite arm) from > 15 months before. Ultimately disease progression was noted (metastasis to C2 haemangioblastoma) requiring different therapy. This case was selected for further study because of the availability of appropriate tumour tissue samples.
MATERIALS AND METHODS
Clinical protocol
The clinical protocol was designed to adoptively transfer sensitized autologous T cells in combination with systemic IL-2 for therapy. T cells were prepared by IVS, the method by which the T cells are grown in culture. To be eligible for the IVS protocol patients had metastatic cancer, adequate numbers of viable cryopreserved autologous tumour cells and intact cellular immunity as documented by the presence of DTH to standard recall antigens (mumps, candida, trichophyton and purified protein derivative (PPD)). Initial tumour processing included mechanical dissociation in the presence of enzymes collagenase 0.2% and DNase 0.02% (both Sigma, St Louis, MO). Viability of tumour cells obtained was estimated with trypan blue. Patients were vaccinated with autologous tumour (2 × 107 cells, irradiated with 100 Gy/5 × 107 lyophilized Tice BCG colony-forming units) 10 cm from a draining axillary lymph node.
Fourteen days post-vaccination the draining lymph nodes were excised and the lymphocytes obtained by mechanical and brief (< 15 min) enzymatic digestion with the same enzyme cocktail as above.
Viability was likewise determined by trypan blue dye exclusion. Subsequently, lymph node lymphocytes were co-cultured with irradiated (1–200 Gy) autologous tumour cells, IL-2 at 10 U/ml and culture medium consisting of RPMI 1640 supplemented by 2 mm glutamine and gentamicin (all from Gibco, Grand Island, NY) and 10% heat-inactivated human AB serum (NABI, Miami, FL) After 12–15 days in culture, lymphocytes only were present. These were harvested, an aliquot was removed for phenotypic analysis and cytotoxicity assay, and the remaining cells were adoptively transferred to the patient along with the concurrent systemic administration of IL-2 at a dose of 30 000 U/kg intravenously every 8 h for 15 doses. Recombinant IL-2 was provided by Hoffman LaRoche (Nutley, NJ) and had a specific activity of approx. 1.5 × 107 U/mg protein.
Phenotyping of IVS lymphocytes was performed by two-colour flow cytometry following staining with labelled MoAbs [21]. Those used included CD8, CD4, CD3, and CD56 (Becton Dickinson, San Jose, CA). Cytotoxicity of IVS cells against tumour targets was performed in standard 4-h 51Cr release assays as described. Cytotoxicity is quantified in lytic units. One lytic unit was defined as the number of effector cells required for 20% lysis of 5 × 103 target cells and the number of lytic units present in 107 effector cells was calculated using a computer program [22]. This protocol has been approved by the IRB of the University of Pittsburgh; informed consent was obtained from the patient prior to protocol treatment.
Histology
The left renal mass was excised prior to therapy and the right renal mass as well as the pulmonary nodule were removed 7 months after therapy. All three tumours were sampled randomly and processed routinely for light microscopy. For immunohistochemical staining, random samples from each tumour were embedded in OCT compound and snap frozen. Cryostat sections cut at 5 μm were air-dried and fixed in acetone before staining. An indirect avidin biotin staining method using the ABC Vectastain Kit (Vector, Burlingame, CA) was used. The chromogen was AEC (3-amino-9-ethyl carbazole) from Sigma. The following commercial primary antibodies were used at recommended dilutions: CD3 (T cells); CD4 (T helper); CD8 (T-suppressor); CD20 (B cells); CD15 (Leu-M1); CD56 (NK cells) (Becton Dickinson); CD54 (intercellular adhesion molecule-1 (ICAM-1)); CD11a (LFA-1); CD58 (LFA-3) (AMAC, Westbrook, ME); HLA-DP; HLA-DQ; HLA-DR; and HLA-ABC (Sera Lab, New York, NY). Negative controls utilizing non-specific mouse immunoglobulin were performed on each tumour sample.
The H–E-stained sections were evaluated with respect to growth pattern, cell type and nuclear grade of the tumours, the pattern and distribution of inflammation, and the types of inflammatory cells present. Nuclear grade of the tumour was classified on a subjective scale of 4, as described by Fuhrman et al. [23]. The pattern of inflammation was classified as patchy or diffuse, and the distribution as septal or intratumoural, or both.
The immunostained sections were evaluated in blind fashion for grade by a pathologist (B.B.) and positivity graded based on the scheme of Rubin et al. [24]. In summary, for inflammatory cells grade 1 + = cells confined to septa; grade 2 + = cells mainly in septa, few cells in tumour; grade 3 + = moderate to heavy cellular infiltrate in tumour. Positive staining of tumour cells was graded as 1 + if < 20% of cells were positive, 2 + if 20–50% of cells were positive and 3 + if > 50% of cells were positive. Positive endothelial cells were noted but not graded. In addition, two blinded observers counted positive cells in 1–2 high-power fields (HPF) where the number of positively staining cells was maximal. The results are reported as means of counted positive cells per HPF (× 40).
RESULTS
Gross pathology
The left renal tumour was 7.0 cm in maximum dimension. The cut surface was yellow tan. Necrosis and haemorrhage were present, but estimated to comprise < 10% of the tumour volume. Capsule and renal vein invasion were not present. The right renal tumour measured 3.0 cm and was a soft yellow-orange, partially haemorrhagic nodule confined to the capsule. The pulmonary nodule was 2.0 cm, subpleural and tan-white.
Light microscopy
Left renal tumour
The left renal tumour exhibited two histological patterns. The predominant pattern consisted of large clear cells in solid and tubular arrangement. Hyalin droplets were noted in the cytoplasm of many cells. In the second pattern, smaller clear cells were in compact clusters. In both patterns, nuclear grade was 3. Occasional single necrotic cells were noted.
Right renal tumour
The right renal tumour consisted of clear cells in compact clusters. Haemorrhage, but no necrosis, was present. The nuclear grade was 2.
Pulmonary tumour nodule
The pulmonary nodule consisted of large clear cells in sheets, with nuclear grade 3. Many contained hyalin cytoplasmic droplets and, in this regard, resembled the cells in the left kidney tumour. Single cell necrosis was prominent.
Inflammatory infiltrate
In addition to their cytologic differences, the three tumours differed with respect to the pattern of inflammation associated with them. Both renal tumours contained scant infiltrates of small lymphocytes and plasma cells, right > left. The inflammatory cells were mainly septal in the left tumour (unexposed to therapy), and intratumoural in the right renal tumour and the pulmonary nodule. Overall, the amount of inflammation in the lung nodule was greater than that in either kidney tumour and the histological pattern of inflammation was distinctive. The pulmonary nodule was infiltrated by small and large lymphocytes, plasma cells, and neutrophils in a distinctive pattern in which the lymphocytes and plasma cells tracked along septa and vessels, and the neutrophils infiltrated amongst tumour cells. Many tumour cells appeared to be degenerating. In addition, a zone of small lymphocytes and plasma cells encircled the nodule, tapering off towards the adjacent lung parenchyma. There was no evidence of infection in the lungs, either histologically or clinically. The histological features of the renal tumours and the pulmonary nodule are illustrated in Fig. 2.
Fig. 2.
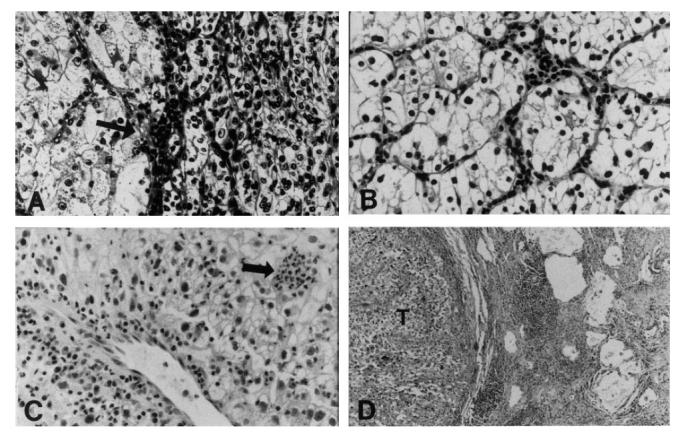
Light microscopic sections of the renal tumours and pulmonary nodule. (A) Left kidney tumour showing two growth patterns and scant inflammation in the septum (arrow) (H–E, × 160). (B) Right kidney tumour showing solid nests of clear cells with scant intratumoural inflammation (H–E, × 160). (C) Pulmonary nodule showing perivascular lymphocytes and plasma cells, and clusters of polys amongst the tumour cells (arrow) (H–E, × 160). (D) Zone of lymphoid aggregates and fibrosis at periphery of pulmonary tumour nodule (T) (H–E, × 40).
Immunohistochemistry
Inflammatory cells
T cells predominated in the inflammatory infiltrates in all three tumours. They varied in number and appeared to be increased in the right renal tumour and lung nodule over the original left renal tumour. The most striking increase was in the infiltration of CD4+ and CD8+ T cells in the responding lung nodule. Cells staining positive for CD11a (LFA-1) were also increased in both the right renal tumour and lung nodule. CD54 (ICAM-1)-positive cells were increased in the pulmonary nodule as opposed to the renal tumours, and CD58+ (LFA-3+) cells were also present in the nodule. Immunostaining for CD4, CD8 and LFA-1 is illustrated in Fig. 3.
Fig. 3.
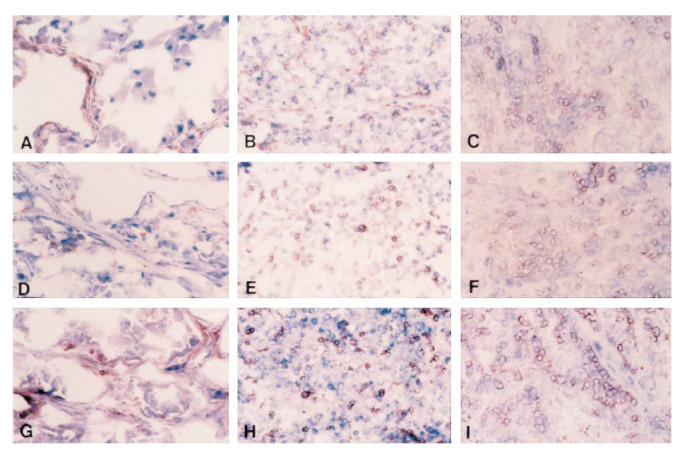
Immunostaining for CD4, CD8, and LFA-1 on frozen sections of the renal tumours and the pulmonary nodule (IPEX, × 160). (A) Left kidney tumour, 1 + CD4. (B) Right kidney tumour, 2 + CD4. (C) Pulmonary nodule, 3 + CD4. (D) Left kidney tumour, 1 + CD8. (E) Right kidney tumour, 2 + CD8. (F) Pulmonary nodule, 3 + CD8. (G) Left kidney tumour, 1 + LFA-1. (H) Right kidney tumour, 3 + LFA-1. (I) Pulmonary nodule, 3 + LFA-1.
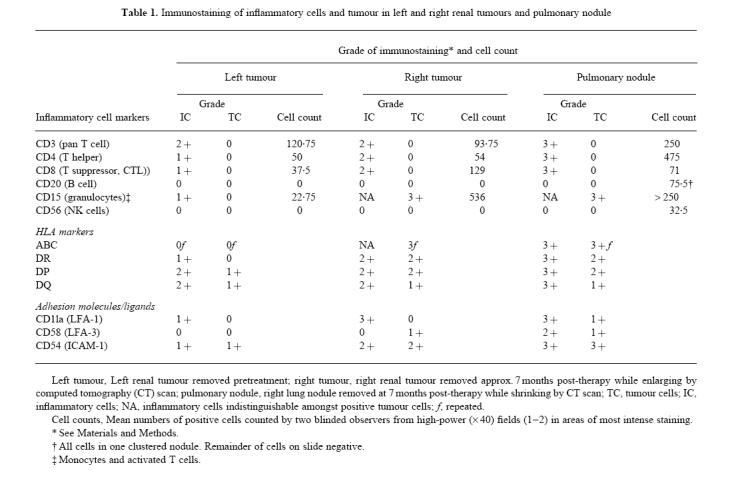
Cells staining positive for CD15 (neutrophils, monocytes and activated T cells) were occasionally present in the left renal tumour. In the right renal tumour and the lung nodule, inflammatory cells were difficult to distinguish against the high background staining on tumour cells. Neither CD20+ (B cells) nor CD56+ (NK cells) were found in significant numbers in either renal tumour. HLA class I and II antigens were up-regulated on the inflammatory cells in the lung nodule compared with the other two sites, possibly indicating cellular activation. The distribution of inflammatory cells within the tumours is summarized in Table 1.
Table 1.
Immunostaining of inflammatory cells and tumour in left and right renal tumours and pulmonary nodule
Tumour cells
HLA class I (ABC) antigens were detected in the right renal tumour and the lung nodule, but not in the left renal tumour. When present, these antigens were strongly and widely expressed and were graded as 3 +. Class II antigens DR and DP were up-regulated in the right renal tumour and lung nodule compared with the left renal tumour. ICAM-1+ tumour cells were also prominent in the right renal tumour and pulmonary nodule (Table 1).
Endothelial cells
Positive staining of endothelial cells was difficult to assess in the presence of strongly staining tumour and inflammatory cells. Nevertheless, endothelial cells were found to express HLA-ABC, -DP, -DQ and -DR antigens in the left renal tumour. In the right renal tumour and the pulmonary nodule, only HLA-ABC and -DR were observed.
DISCUSSION
Here we report a case of bilateral synchronous renal cell carcinoma with lung metastases in a patient with von Hippel Lindau disease who was treated with immunotherapy. Von Hippel Lindau disease has been extensively characterized [25,26]. This is an autosomal dominant disorder in which haemangioblastomas of retina, cerebellum, brain stem or spinal cord may be seen in association with cysts (kidney, pancreas or epididymis) as well as malignancies involving pancreas, kidney and neural crest (pheochromocytoma). Bilateral synchronous renal cell carcinoma in association with von Hippel Lindau is well known [27].
This patient's case allowed an interesting internally controlled comparison of primary tumour, shrinking pulmonary nodule (responding) and growing, probably synchronous, primary. Pathologic analysis of the tumour specimens from both right and left kidney as well as lung nodule by routine H–E staining demonstrated some interesting differences. The left renal carcinoma had not been previously exposed to therapy. In contrast, both the right renal tumour and the pulmonary nodule had been exposed to vaccination, adoptive transfer, and systemic IL-2. An intense inflammatory cell infiltrate was present in the responding pulmonary nodule, with fewer inflammatory cells noted in the right renal mass (treated but not responding) and fewest in the originally removed left renal primary (untreated). In the untreated left tumour, the inflammatory cells were primarily in tumour septae, while in the right tumour and pulmonary nodule they were interspersed amongst the tumour cells. The inflammatory cells were characterized immunohistologically and found to be predominantly T cells (CD4+ CD8+) and CD15+ cells (neutrophils, monocytes, and/or activated T cells). Plasma cells were prominent in H–E sections of only the responding lung nodule, suggesting local antibody production against the tumour. Neutrophils were also prominent on H–E staining of the lung nodule. Their dispersed pattern amongst the tumour cells was only seen in the responding lung nodule. B cells (CD20+) and NK cells (CD56+) were found in small numbers only in the lung nodule. The inflammatory cells appeared to be activated (increased HLA class 1 and II expression). Also noted was up-regulation of the lymphocyte adhesion molecule LFA-1 and its ligand (ICAM-1) on both inflammatory and tumour cells. Since LFA-1 is associated with lymphocyte adhesion and possibly T cell receptor function, T cells are further implicated in the described inflammatory response [28].
This case suggests but does not prove that the inflammatory infiltrate in the responding nodule was somehow associated with response to the therapy. To date, it has not been established that cellular infiltrates are causally related to anti-tumour response. Others have shown inflammatory cellular (lymphocytic) infiltrates in melanoma nodules after vaccination with autologous tumour [29] and IL-2-based immunotherapy [24]. Rubin et al. demonstrated a T cell/macrophage infiltrate in melanoma and breast cancer metastatic nodules after IL-2-based systemic treatment. Regression of metastases was associated with increased post-treatment DR antigen expression for tumour cells and the T cell/macrophage infiltrate. However, these factors were also found in some non-responding nodules [24].
Several features of the current case support a relationship between the inflammatory infiltrate and tumour response. First, the simultaneously growing right renal tumour did not possess an inflammatory cellular response of the same type or intensity as that in the lung nodule. In the responding pulmonary nodule, there was clearly a more intense inflammatory response (including T cells, plasma cells and neutrophils) compared with the non-responding right renal tumour. The pattern was distinctive, with partitioning of lymphocytes and plasma cells along vessels, and neutrophils and lymphocytes, among the tumour cells.
Tumour cell necrosis was spotty, and the histological picture was not one of an inflammatory response to infarcted tissue. Further, there was no evidence of infection. The IVS protocol was designed to allow for the in vitro outgrowth of T cells which theoretically would be capable of specifically responding to autologous tumour and of conferring long-term anti-tumour immunological memory after adoptive transfer. A cytotoxicity assay suggested that the infused IVS T cells were able to lyse fresh frozen autologous tumour cells. However, this result is not definitive given the elevated spontaneous release of the autologous tumour target cells used in the assay. In any case, quantitatively the amount of killing was not impressive.
Cytotoxicity against allogeneic tumour was not tested in this case and no further comment can be made regarding specificity. We [30] and others [31] have found that cytotoxicity may not be reflective of specific response to autologous tumour, as it is often minimal when determined by 4-h 51Cr release assay. Specific proliferation or cytokine release studies [32] have suggested specificity of IVS T cells when cytotoxicity against autologous tumour is minimal [30]. A population of predominantly CD4+ T cells was adoptively transferred to this patient. This transfer was followed by systemic IL-2 which may have expanded the adoptively transferred T cells in vivo. T cells, among other cell types, were increased in the post-treatment specimens. However, the relationship, if any, between the adoptively transferred IVS T cells and the T lymphocytes infiltrating the responding pulmonary nodule cannot be discerned from the data available.
Alternatively, the therapy administered may have induced HLA class 1 and ICAM-1 expression, causing localization of immune T cells and other effectors as a consequence of the alterations in tumour antigen and other surface molecules expressed.
Second, the time to measurable clinical response was 3 months. This is a longer period than is usually observed for clinical response to IL-2 or chemotherapy. However, the time course to response has been a matter of months using other vaccine-based therapies [29].
In parallel studies, the tumour-infiltrating lymphocytes (TIL) from the responding lung nodule and the non-responding renal nodule were cultured in IL-2 for 8 days. Of the TIL outgrown from the responding lung nodule, 93% were CD4+ T cells, with most of the cells expressing the high-affinity IL-2 receptor, whereas TIL outgrown from the non-responding renal nodule were less homogeneous (34% CD8+ T cells, 18% CD4+ T cells and 45% CD56+ NK cells). Autologous tumour cytotoxicity in 18-h 51Cr release assays was somewhat higher than that against Raji targets (Daudi) (1102 versus 1041 lytic units) for TIL outgrown from the responding lung nodule in contrast to that for TIL from the non-responding renal nodule (1733 versus 2622 lytic units). This suggested that the lung nodule contained auto-tumour-reactive CD4+ T cells [33]. Furthermore, lymphocytes from the responding lung nodule, the non-responding renal tumour and peripheral blood were analysed for T cell receptor (TCR) Vβ gene usage by polymerase chain reaction. Of oligoclonal T cells in the responding nodule Vβ13.1 clones represented 28% (range in eight normals from PBL 0.5–4.4%) and Vβ5.2 clones 14% (range in eight normals from PBL 1.3–7.7%). Many Vβ genes were not expressed. From the non-responding renal tumour T cells were restricted and more similar to PBL, although Vβ13.1 was somewhat increased at 12.3%. Vβ5.2 was not increased [33]. These findings suggest differences in the T cell infiltrate of responding and non-responding nodules at the level of the TCR.
The lack of response in the right renal tumour might be attributable to antigenic differences between right and left spontaneously arising renal cell carcinomas. Such antigenic differences have been noted [34].
Alternatively, lung metastases from renal cell carcinoma may be more likely to respond than primary cancers, but the basis for this clinical observation has not been established [35]. The observations in this study do not prove that regression in the pulmonary nodules was caused by the therapy. Whether the response in this case represented spontaneous regression, and if so, its relationship to the therapy also cannot be determined. Spontaneous regression is rare (0.8%) [36], and even if it were responsible for these observations, would not change the conclusions drawn.
This case report demonstrates a differential response of metastatic renal cell carcinoma after immunotherapy and suggests, but does not prove, an association between an inflammatory cell infiltrate and clinical response.
Acknowledgments
We would like to acknowledge T. Hakala MD, R. B. Herberman MD, M. Lotze MD, J. Rubin MD and D. Sheahan MD for useful suggestions. We thank R. Mascari and D. Watzman for technical support. We would also like to thank the following for valuable assistance: E. Elder PhD, L. Straw RN, S. Scialla MD, M. Schlesinger MD and the house staff nursing and support staff of Unit 12-3 of the Presbyterian University Hospital. We thank Ms Marcia Schmitz for her excellent secretarial support. T.F.L. was supported during this work by a Clinical Oncology Career Development Award from the American Cancer Society. Presented in part at the annual meeting of the Society for Biological Therapy, November 11, 1991, Pittsburgh, PA.
References
- 1.Rosenberg SA, Lotze MT, Muul LM, et al. Observations on the systemic administration of autologous lymphokine-activated killer cells and recombinant interleukin-2 in patients with metastatic melanoma. N Engl J Med. 1985;313:1485–92. doi: 10.1056/NEJM198512053132327. [DOI] [PubMed] [Google Scholar]
- 2.Rosenberg SA, Lotze MT, Muul LM, et al. A progress report on the treatment of 157 patients with advanced cancer using lymphokine-activated killer cells and interleukin-2 or high-dose interleukin-2 alone. N Engl J Med. 1987;316:889–97. doi: 10.1056/NEJM198704093161501. [DOI] [PubMed] [Google Scholar]
- 3.Fisher RI, Coltman CA Jr, Doroshow JH, et al. Metastatic renal cancer treated with interleukin-2 and lymphokine-activated killer cells. A phase II clinical trial. Ann Intern, Med. 1988;108:518–23. doi: 10.7326/0003-4819-108-4-518. [DOI] [PubMed] [Google Scholar]
- 4.Sosman JA, Kohler PC, Hank JA, et al. Repetitive weekly cycles of interleukin-2. II. Clinical and immunologic effects of dose, schedule, and addition of indomethacin. J Natl Cancer Inst. 1988;80:1451–61. doi: 10.1093/jnci/80.18.1451. [DOI] [PubMed] [Google Scholar]
- 5.Parkinson DR, Fisher RI, Rayner AA, et al. Therapy of renal cell carcinoma with interleukin-2 and lymphokine-activated killer cells: phase II experience with a hybrid bolus and continuous infusion interleukin-2 regimen. J Clin Oncol. 1990;8:1630–6. doi: 10.1200/JCO.1990.8.10.1630. [DOI] [PubMed] [Google Scholar]
- 6.Ernstoff MS, Nair S, Bahnson RB, et al. A phase la trial of sequential administration recombinant DNA-produced interferons: combination recombinant interferon gamma and recombinant interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol. 1990;8:1637–49. doi: 10.1200/JCO.1990.8.10.1637. [DOI] [PubMed] [Google Scholar]
- 7.Topalian SL, Solomon D, Avis FP, et al. Immunotherapy of patients with advanced cancer using tumor-infiltrating lymphocytes and recombinant interleukin-2: a pilot study. J Clin Oncol. 1988;6:839–53. doi: 10.1200/JCO.1988.6.5.839. [DOI] [PubMed] [Google Scholar]
- 8.Tykka H. Active specific immunotherapy with supportive measures in the treatment of advanced palliatively nephrectomised renal adenocarcinoma. A controlled clinical study. Scan J Urol. 1981;63(Suppl.):1–110. [PubMed] [Google Scholar]
- 9.McCune CS, O'Donnell RW, Marquis DM, Sahasrabudhe DM. Renal cell carcinoma treated by vaccines for active specific immunotherapy: correlation of survival with skin testing by autologous tumor cells. Cancer Immunol Immunother. 1990;32:62–66. doi: 10.1007/BF01741726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sahasrabudhe DM. Specific immunotherapy with suppressor function inhibition for metastatic renal cell carcinoma. J Biol Response Mod. 1986;5:581–94. [PubMed] [Google Scholar]
- 11.Logan T, Kirkwood J, Wolmark N, et al. Pilot study of in vitro sensitized (IVS) T cells in adoptive transfer therapy of cancer. ASCO. 1990;9:188. (Abstr.) [Google Scholar]
- 12.Shu S, Chou T, Sakai K. Lymphocytes generated by in vivo priming and in vitro sensitization demonstrate therapeutic efficacy against a murine tumor that lacks apparent immunogenicity. J Immunol. 1989;143:740–8. [PubMed] [Google Scholar]
- 13.Shu S, Rosenberg SA. Adoptive immunotherapy of newly induced murine sarcomas. Cancer Res. 1985;45:1657–62. [PubMed] [Google Scholar]
- 14.Shu S, Rosenberg SA. Adoptive immunotherapy of a newly induced sarcoma: immunologic characteristics of effector cells. J Immunol. 1985;135:2895–903. [PubMed] [Google Scholar]
- 15.Shu S, Chou T, Rosenberg SA. In vitro, sensitization and expansion with viable tumor cells and interleukin 2 in the generation of specific therapeutic effector cells. J Immunol. 1986;136:3891–8. [PubMed] [Google Scholar]
- 16.Shu S, Chou T, Rosenberg SA. In vitro differentiation of T-cells capable of mediating the regression of established syngeneic tumors in mice. Cancer Res. 1987;47:1354–60. [PubMed] [Google Scholar]
- 17.Shu S, Chou T, Rosenberg SA. Generation from tumor-bearing mice of lymphocytes with in vivo therapeutic efficacy. J Immunol. 1987;139:295–304. [PubMed] [Google Scholar]
- 18.Chou T, Chang AE, Shu S. Generation of therapeutic T lymphocytes from tumor-bearing mice by in vitro sensitization. J Immunol. 1988;140:2453–61. [PubMed] [Google Scholar]
- 19.Chou T, Bertera S, Chang AE, Shu S. Adoptive immunotherapy of microscopic and advanced visceral metastases with in vitro sensitized lymphoid cells from mice bearing progressive tumors. J Immunol. 1988;141:1775–81. [PubMed] [Google Scholar]
- 20.Logan TF, Shu S, Bahnson RR, Leong SPL, Banner BB. Generation of specific cytolytic cells from patients with melanoma (ME) and renal cell carcinoma (RCC) by in vitro sensitization (IVS) AACR. 1989;30:342. (Abstr.) [Google Scholar]
- 21.Whiteside TL, Heo DS, Takagi S, Johnson JT, Iwatsuki S, Herberman RB. Cytolytic antitumor effector cells in long-term cultures of human tumor-infiltrating lymphocytes in recombinant interleukin 2. Cancer Immunol Immunother. 1988;26:1–10. doi: 10.1007/BF00199840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Whiteside TL, Bryant J, Day R, Herberman RB. Natural killer cytotoxicity in the diagnosis of immune dysfunction: criteria for a reproducible assay. J Clin Lab Anal. 1990;4:102–14. doi: 10.1002/jcla.1860040207. [DOI] [PubMed] [Google Scholar]
- 23.Fuhrman SA, Lasky LC, Limas C. Prognostic significance of morphologic parameters in renal cell carcinoma. Am J Surg Pathol. 1982;6:655–63. doi: 10.1097/00000478-198210000-00007. [DOI] [PubMed] [Google Scholar]
- 24.Rubin JT, Elwood LJ, Rosenberg SA, Lotze MT. Immunohistochemical correlates of response to recombinant interleukin-2-based immunotherapy in humans. Cancer Res. 1989;49:7086–92. [PubMed] [Google Scholar]
- 25.Maher ER, Yates JRW, Harries R, et al. Clinical features and natural history of von Hippel-Lindau disease. Q J Med. 1990;283:1151–63. doi: 10.1093/qjmed/77.2.1151. [DOI] [PubMed] [Google Scholar]
- 26.Lamiell JM, Salazar FG, Hsia YE. Von Hippel-Lindau disease affecting 43 members of a single kindred. Medicine. 1989;68:1–29. doi: 10.1097/00005792-198901000-00001. [DOI] [PubMed] [Google Scholar]
- 27.Connor JM. Von Hippel-Lindau Disease. Q J Med. 1990;77:1099–100. doi: 10.1093/qjmed/77.2.1099. [DOI] [PubMed] [Google Scholar]
- 28.Tomita Y, Nishiyama T, Watanabe H, Fujiwara M, Sato S. Expression of intercellular adhesion molecule-1 (ICAM-1) on renal-cell cancer: possible significance in host immune responses. Int J Cancer. 1990;46:1001–6. doi: 10.1002/ijc.2910460609. [DOI] [PubMed] [Google Scholar]
- 29.Berd D, Maguire HC Jr, McCue P, Mastrangelo MJ. Treatment of metastatic melanoma with an autologous tumor-cell vaccine: clinical and immunologic results in 64 patients. J Clin Oncol. 1990;8:1858–67. doi: 10.1200/JCO.1990.8.11.1858. [DOI] [PubMed] [Google Scholar]
- 30.Logan T, Elder E, Whiteside T, et al. In vitro sensitized T cells given in a phase II study demonstrate differential proliferation and cytokine release to autologous tumor. J Immunother. 1995;18:133. (Abstr.) [Google Scholar]
- 31.Chang AE, Yoshizawa H, Sakai K, Cameron MJ, Sondak VK, Shu S. Clinical observations on adoptive immunotherapy with vaccine-primed T-lymphocytes secondarily sensitized to tumor in vitro. Cancer Res. 1993;53:1043–50. [PubMed] [Google Scholar]
- 32.Barth RJ, Mule JJ, Spiess PJ, Rosenberg SA. Interferon-gamma and tumor necrosis factor have a role in tumor regressions mediated by murine CD8+ tumor infiltrating lymphocytes. J Exp Med. 1991;173:647–58. doi: 10.1084/jem.173.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weidmann E, Logan TF, Yasumura S, Kirkwood JM, Trucco M, Whiteside TL. Evidence for oligoclonal T-cell response in a metastasis of renal cell carcinoma responding to vaccination with autologous tumor cells and transfer of in vitro-sensitized vaccine-draining lymph node lymphocytes. Cancer Res. 1993;53:4745–9. [PubMed] [Google Scholar]
- 34.Hashimura T, Tubs RR, Connelly R, et al. Characterization of two cell lines with distinct phenotypes and genotypes established from a patient with renal cell carcinoma. Cancer Res. 1989;49:7064–71. [PubMed] [Google Scholar]
- 35.Sarna G, Figlin R, de Kernion J. Interferon in renal cell carcinoma. The UCLA experience. Cancer. 1987;59:610–2. doi: 10.1002/1097-0142(19870201)59:3+<610::aid-cncr2820591306>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 36.Montie JE, Stewart BH, Straffon RA, Banowsky LH, Hewitt CB, Montague DK. The role of adjunctive nephrectomy in patients with metastatic renal cell carcinoma. J Urol. 1977;117:272–5. doi: 10.1016/s0022-5347(17)58429-3. [DOI] [PubMed] [Google Scholar]


