Abstract
Anti-liver cytosol 1 autoantibody (LC1) characterizes a severe form of autoimmune hepatitis (AIH), staining the cytoplasm of periportal hepatocytes and targeting an unidentified 60-kD liver cytosolic antigen. To identify its target, we used high-titre anti-LCI+ sera from two patients with AIH to screen 18 cytoplasm enzymes with periportal location by double immunodiffusion (DDI). Both sera gave a broad precipitin line against human liver cytosol, suggesting that they may recognize two distinct antigens, a possibility confirmed by the appearance of two precipitin lines when DDI conditions were optimized (0.8% agarose and 3% polyethylene glycol (PEG)). Experiments by DDI and Western blot (WB) identified a liver cytosolic autoantigen of 50 kD, different from LC1, giving a line of identity with argininosuccinate lyase (ASL). Reactivity to ASL was then investigated by DDI and WB in 57 patients with AIH, 17 with primary biliary cirrhosis (PBC), 15 with chronic hepatitis B virus (HBV) infection, 13 with αl-antitrypsin deficiency, 17 with Wilson's disease, 18 with extrahepatic autoimmune disorders, and in 48 healthy controls. Anti-ASL was found in 16% of AIH and 23% of PBC patients by DDI and in 14% of AIH, 23% of PBC and 20% of HBV patients by WB. No argininosuccinate was present in the urine of four anti-ASL+ patients tested, excluding an inhibition of enzymatic activity by anti-ASL. The addition of anti-ASL+ serum to human fibroblast cultures induced a significant increase in ASL activity. ASL is a new autoantigen in liver disease and its clinical relevance warrants further investigation.
Keywords: autoimmune hepatitis, argininosuccinate lyase, anti-liver cytosol antibody type 1
INTRODUCTION
Autoantibodies have a central diagnostic role in autoimmune liver diseases, including autoimmune hepatitis (AIH) and primary biliary cirrhosis (PBC) [1,2]. While the diagnostic antibody of PBC is the anti-mitochondrial antibody (AMA) [3], being present in approx. 95% of patients [4,5], diagnosis and classification of AIH relies on a panel of autoantibodies. AIH type I is defined by the presence of anti-nuclear (ANA) and/or anti-smooth muscle (SMA) antibodies [2], while AIH type II, affecting mainly children and reportedly running a more aggressive course [6], is characterized by anti-liver–kidney microsome antibodies type I (LKM1), the molecular target of which is cytochrome P4502D6 [7–10].
Recently, interest has focused on an autoantibody directed against an antigen contained in liver cytosol (LC1). In its original description by Martini et al. in 1988 [11], LC1 autoantibody (anti-LC1) was found in patients with clinical and laboratory manifestations of AIH and was considered to define a subgroup of this condition with a particularly aggressive behaviour. Since some 50% of these patients were simultaneously positive for LKM1, it was suggested that anti-LC1 could serve as a second marker of AIH type II. Anti-LC1, however, has been subsequently reported also in patients with ANA/SMA+ AIH [12].
Anti-LC1 can be detected by different techniques including immunofluorescence [11,13], double-dimension immunodiffusion [13,14] and Western blot (WB) [12,14]. The molecular identity of the antigen recognized by anti-LC1 remains unknown. We know, however, that anti-LC1 stains the cytoplasm of hepatocytes with a zonal distribution within rat liver, being particularly abundant in periportal areas, as demonstrated by the characteristic immunofluorescent staining. The LC1 antigen is contained in the cytosol, as shown by Martini et al. [11], who tested antibody-positive sera against subcellular liver cell fractions, and has a molecular weight of 60 kD.
In an attempt to identify the antigen targeted by the LC1 antibody we used two high-titre anti-LC1+sera and screened by double-dimension immunodiffusion (DDI) a large panel of enzymes localized in the periportal areas and with cytoplasmic distribution. Reactivity of the anti-LC1+sera was then compared with that of a panel of sera from patients with a variety of liver disorders.
MATERIALS AND METHODS
Patients
The two high-titre anti-LC1+ sera used for initial screening were obtained from a 10-year-old girl affected by LKM1+ AIH, and a 5-year-old girl with ANA/SMA+ AIH and will be henceforth referred to as serum 1 (S1) and serum 2 (S2). Anti-LC1 was present at a titre of 1:64 in S1, as determined by DDI, since in LC1/LKM1 double-positive sera the immunofluorescent LCI pattern is obscured by that of LKM1 [15], while in S2 it was 1:320 by immunofluorescence. A further 135 patients were subsequently studied; their demographic and clinical characteristics are summarized in Table 1. They fell into three groups: 72 had autoimmune liver diseases (55 AIH and 17 PBC), 45 other liver diseases (15 chronic hepatitis B virus (HBV) infection, 13 α1-antitrypsin deficiency, 17 Wilson's disease) and 18 extrahepatic autoimmune disorders (16 connective tissue disease, 1 autoimmune haemolytic anaemia, 1 autoimmune thyroiditis).
Table 1.
Demographic and clinical features of the patients with chronic liver diseases and of pathological and healthy controls at the time of testing*
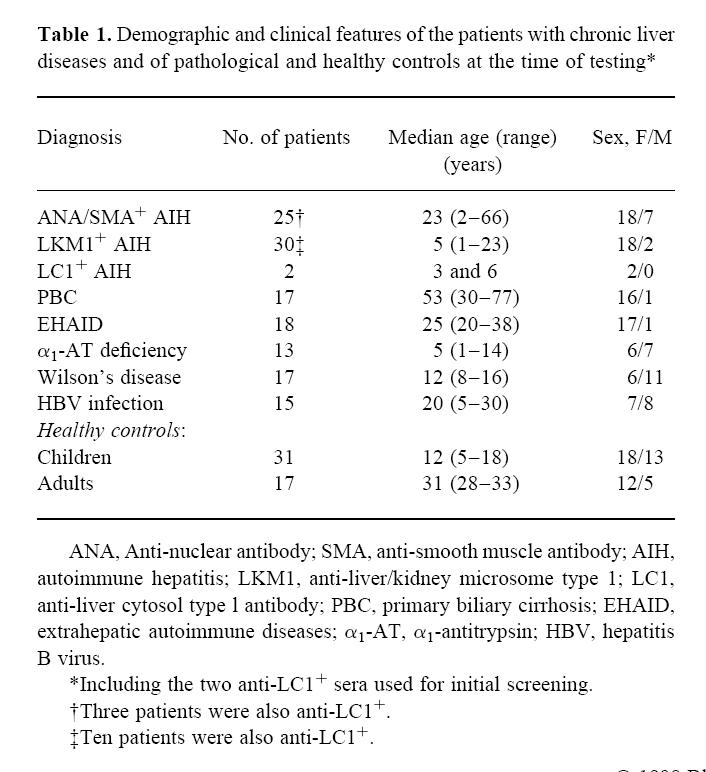
Of the 57 patients with AIH, including the two whose serum was used for initial screening, 20 were LKM1+, 10 LKM1/LC1+, two LC1+, 22 ANA/SMA+, three ANA/SMA+ and LC1+. All but four patients (one LKM1+, two LKM1/LC1+ and one ANA/SMA+) tested at presentation were on immunosuppressive treatment (prednisolone 0.2–1 mg/kg per day with or without azathioprine 0.5–1.5 mg/kg per day). Forty-eight sera from a healthy population were tested as control (Table 1).
Preparation of the cytosolic fraction
Liver cytosol was prepared from a piece of normal human liver, obtained after reduction for transplantation. Homogenized fresh tissue was fractionated by differential centrifugation [16], finally yielding the cytosolic fraction represented by the supernatant obtained following the removal of the nuclear and mitochondrial fractions, after a final centrifugation for 90 min at 105 000 g. On the basis of enzymatic activity [11,17] the contamination of mitochondrial succinodehydrogenase and microsomal NADPH cytochrome C reductase was < 1%. The protein concentration was measured by the method of Lowry et al. [18] and adjusted to 4 mg/ml.
Preparation of guinea pig anti-human cytosol antiserum
Guinea pig anti-human cytosol antiserum was raised by Eurogentec (Searing, Belgium) according to the following protocol. Human liver cytosol (20 mg) was injected into a guinea pig by the intradermic route. This procedure was repeated on days 14, 28 and 56. The guinea pig serum was tested during the immunization regimen and after 66 days for the presence of anti-cytosol antibodies using DDI and WB. The serum used in this study was obtained from blood obtained at day 80.
Enzymes
Enzymes from animal liver and other tissues were obtained commercially (Sigma, Poole, UK), being chosen on the basis of a close amino acid sequence homology (median 78%) with the human counterparts, as assessed interrogating the Swiss-Prot protein data base (Geneva University Hospital and University of Geneva, Geneva, Switzerland) and by their periportal location in the liver. The 18 enzymes, used at a final concentration of 4–20 mg/ml, comprised: lactic dehydrogenase (EC 1.1.28) from human liver, arginase (EC 3.5.3.1), argininosuccinate lyase (ASL; EC 4.3.2.1) and β-glucuronidase (EC 3.2.1.31) from bovine liver, fructose-1,6-diphosphatase (EC 3.1.3.11) from rabbit liver, malic dehydrogenase (EC 1.1.1.37), hexokinase (EC 2.7.1.1) and cytochrome oxidase (EC 1.9.3.1) from bovine heart, aspartate aminotransferase (EC 2.6.1.1) and alanine aminotransferase (EC 2.6.1.2) from porcine heart, adenosine-5-triphosphatase (EC 3.6.1.3) from dog kidney, γ-glutamyl transpeptidase (EC 2.3. 2.2) from bovine kidney, glutathione peroxidase (EC 1.11.1.9) from human erythrocytes, alkaline phosphatase (EC 3.1.3.1) from human placenta, malic enzyme (EC 1.1.1.40) from chicken liver, pyruvate kinase (EC 2.7.1.40) from rabbit liver, alcohol dehydrogenase (EC 1.1.1.1) from horse liver and 6-phosphogluconic dehydrogenase (EC 1.1.1.44) from sheep liver, the first 14 having a periportal, the other four perivenous localization.
Human ASL, which is not commercially available, was prepared as previously described [19], and kindly donated by Professor A. G. Palekar (Department of Paediatrics, Nassau County Medical Center, University of New York) for selected experiments. Purity of bovine ASL was ascertained by 12% SDS–PAGE (Fig. 1).
Fig. 1.
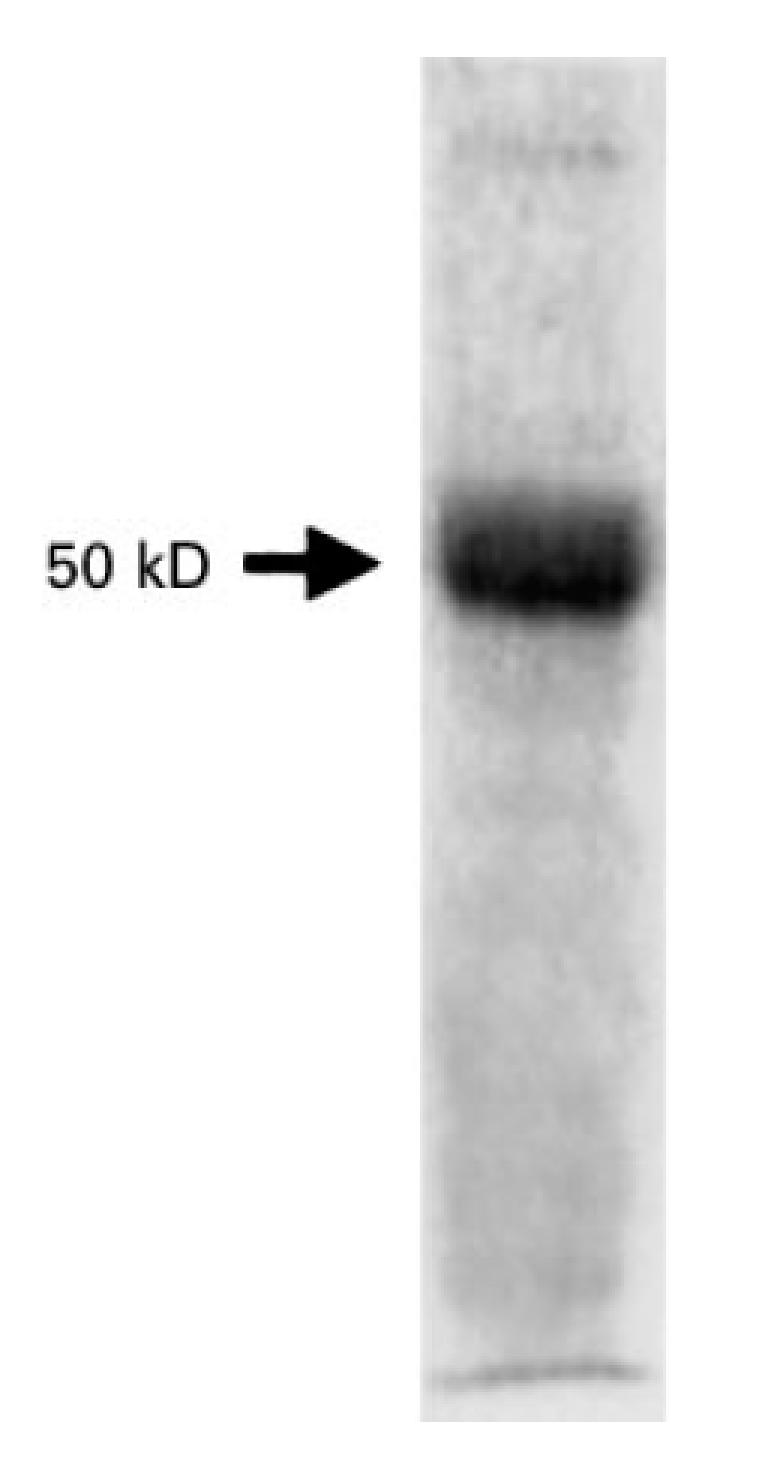
SDS–PAGE (12%) of bovine argininosuccinate lyase (ASL) (20 μg). A single band is visible at 50 kD.
DDI
DDI was used to assess whether patients' sera contained antibodies directed against any of the potential enzymatic targets. Different conditions were initially tested, including different agarose (0.5–0.7–0.8–1–1.5%) and polyethylene glycol 8000 (PEG) (0–1–2–3–4%) concentrations, serum dilutions (1:1 to 1:24) and gel configurations (well diameter 4–8 mm and distance between the wells 2–4–6 mm, circumference to circumference) to optimize the technique. After the initial experiments, the following conditions were found to be optimal and used to test the patient populations. Twenty millilitres of 0.8% agarose (Agarose M; Pharmacia Biotech, St Albans, UK) and PEG 3% (Sigma) in PBS 0.15 m pH 7.2 were poured in a 90-mm Petri dish. Wells of 5 mm in diameter, placed 4 mm apart, were punched into the gel and each filled with 30 μl of the potential antigen or undiluted patient serum. Plates were then incubated in a humidified atmosphere at room temperature for the first 24 h and then at 4°C. Formation of precipitin lines was assessed daily by two observers (N.P. and D.V.). The target of anti-LC1+ sera was investigated by testing these sera against liver cytosol and known periportal enzymes by DDI.
After screening experiments showed that both high-titre anti-LC1+ sera (S1 and S2) reacted with bovine liver ASL, but with none of the other 17 enzymes tested, we proceeded in three directions: (i) we compared the reactivity of S1 and S2 against bovine and human ASL and the liver cytosol; (ii) we compared the reactivity of the guinea pig serum raised against human liver cytosol with that of human sera containing the LC1 antibody; (iii) we tested a large number of patient and control sera against both the bovine ASL and the liver cytosol fraction to evaluate the antibody prevalence in the clinical setting.
Western blot
A commercial bovine liver ASL preparation was used as antigen, and separated electrophoretically in a 12% SDS–PAGE according to the method of Laemmli [20]. Approximately 160 μg of this preparation were loaded per minigel.
Polypeptides were transferred to a nitrocellulose sheet in a semidry electrophoretic transfer cell (BioRad Labs, Hemel Hempstead, UK). After transfer, non-specific binding was blocked by incubating the nitrocellulose paper with 5% milk in 10 mmol/l Tris buffer pH 8 containing 0.15 mmol/l NaCl and 0.05% Tween 20 (TNT) for 1 h. The sheet was cut into strips which were incubated with the patient sera diluted 1:100 in TNT buffer for 2 h, followed by horseradish peroxidase (HRP)-conjugated rabbit anti-human IgG (Dako, Copenhagen, Denmark) diluted 1:750 in TNT buffer. The antibody bound was revealed by addition of the chromogenic substrate 4-chloro1-naphthol (Sigma) until a blue colour appeared and the reaction was terminated by an excess of distilled water. A similar technique was used for the liver cytosol preparation.
Effects of anti-ASL+ sera on ASL enzymatic activity
To investigate whether anti-ASL has an effect on ASL enzymatic activity, we cultured fibroblast cell lines from two children being investigated for metabolic disorders other than urea cycle defects in the presence of 1% anti-ASL+ serum and anti-ASL−serum. ASL activity was evaluated by measuring the conversion of 14C-labelled citrulline via the two steps of the urea cycle that converts citrulline into arginine. Radioactivity in precipitated protein was measured and results were expressed in terms of total cellular protein [21].
Measurement of urine amino acid excretion
Amino acids were measured in the urine of four anti-ASL+patients using a modified high performance liquid chromatography (HPLC) method following derivatization with phenylisothiocyanate (PITC), according to Sherwood et al. [22]. Urine (50 μl) was deproteinized by the addition of sulphosalicylic acid (50 μl, 10% w/v) containing an internal standard (norleucine, 750 μmol/l) and centrifuged. An aliquot of the supernatant was reacted with PITC in the presence of a coupling agent to produce phenylthiocarbamyl derivatives of the amino acids. The derivatized samples were injected onto a 25-cm Hypersil ODS column (Jones Chromatography, Hengoed, UK) maintained at 30°C, using a SpectraSystem gradient HPLC system (Thermo Separation Products, Stone, UK) with UV (254 nm) and electrochemical detection (1.10 V).
RESULTS
DDI
In the initial experiments the two high-titre anti-LC1+ sera gave one single line when tested against liver cytosol under the conditions described by Han et al. [12]. In Fig. 2, a precipitin line is produced by S1 against ASL, while no precipitin line is observed against two of the 17 additional enzymes tested. A broad line is seen between S1 (well 1) and cytosol (well A), suggesting that it may be composed of two precipitin lines. To understand better the relationship between ASL and antigens contained in the liver cytosol, S1 at the dilutions of 1:16 and 1:24 was seeded in wells facing ASL and liver cytosol (Fig. 3). This configuration enabled S1 (i) to form two precipitin lines at the dilution of 1:24, (ii) to react with the two antigenic preparations, and (iii) to form precipitin lines informative on the relatedness of the two antigenic preparations. Of the two lines formed between anti-LC1 and liver cytosol, one is of partial identity and the other of non-identity with bovine ASL. The same results were obtained with S2. Under the same conditions, the guinea pig anti-human cytosol serum showed three lines against human liver cytosol, one of total identity with bovine ASL and two of non-identity (not shown). When S1 and S2 were tested against liver cytosol and purified human ASL, they gave a line of total identity (Fig. 4).
Fig. 2.
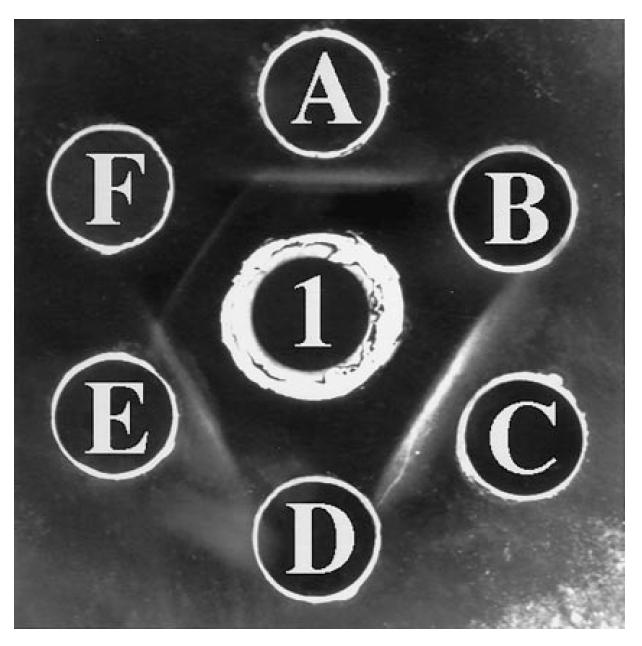
Reactivity of S1 against liver cytosol and three enzymes from a panel of 18 (see Table 2). Well 1 contains undiluted S1, wells A, C, E liver cytosol (the source of the anti-liver cytosol type 1 (LC1) antigen) at 4 mg/ml, well B glutathione peroxidase (4 mg/ml), well D lactic dehydrogenase (4 mg/ml), well F argininosuccinate lyase (ASL) 4 mg/ml. A precipitin line is formed against bovine ASL (F), but not against glutathione peroxidase (B) or lactic dehydrogenase (D). The line against the cytosolic fraction is broad, suggesting the possibility that it may contain two antigens, particularly so between wells 1 and A.
Fig. 3.
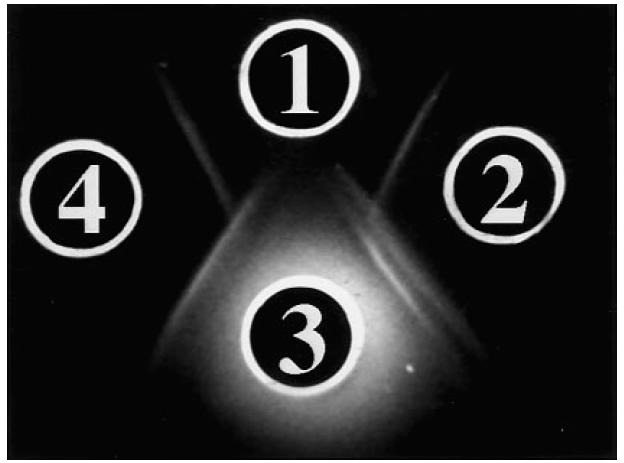
Reactivity of S1 against human liver cytosol fraction and bovine argininosuccinate lyase (ASL), when tested in 0.8% agarose and 3% polyethylene glycol (PEG). Well 1 contains bovine ASL 4 mg/ml, well 3 contains liver cytosol 4 mg/ml, wells 2 and 4 contain S1 diluted 1:24 and 1:16, respectively. The serum in well 2 gives a double line against liver cytosol (well 3) and a single line against ASL (well 1). Of the two precipitin lines formed against liver cytosol, the one nearer well 2 gives a pattern of non-identity with the line formed between wells 2 and 1, while the second line gives a pattern of partial identity. A partial mirror image is seen when well 4, containing S1 at 1:16 dilution, is considered. At this serum dilution the double line between S1 and cytosol is hardly visible.
Fig. 4.
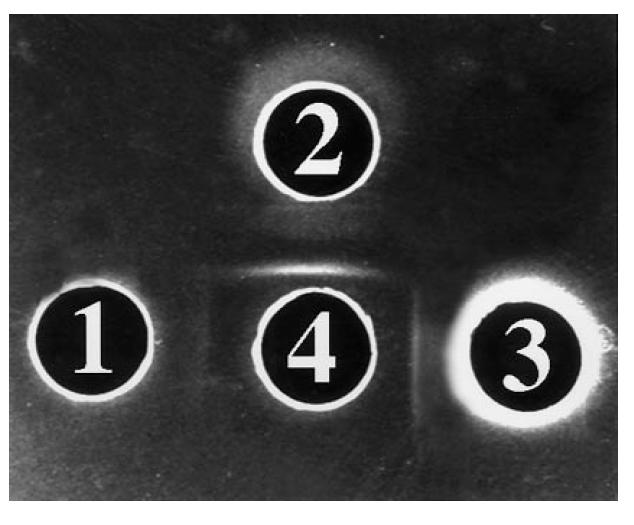
Reactivity of S1 against bovine argininosuccinate lyase (ASL), human ASL, and liver cytosol. Well 1 contains bovine ASL (4 mg/ml), well 2 human ASL (4 mg/ml), well 3 liver cytosol (4 mg/ml). In well 4 there is S1 diluted 1:4. The experiment was performed in 0.8% agarose, 3% polyethylene glycol (PEG). A pattern of identity is given by S1 (4) against human ASL (2) and cytosol (3). The lines between wells 1 and 2 meet at a sharp angle, a pattern compatible with that of partial identity seen in Fig. 3 between the same reactants.
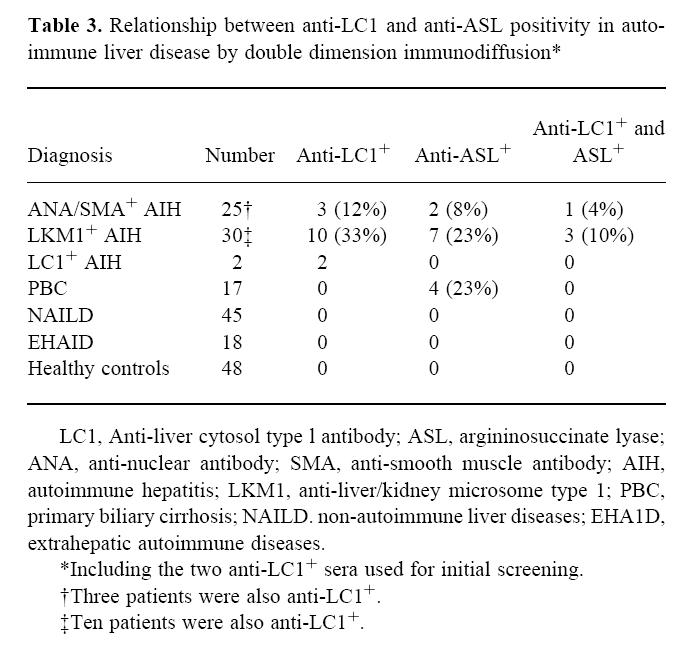
The results obtained testing the original two sera and those from 135 patients against bovine ASL are summarized in Table 2. Anti-ASL positivity was found in four patients with PBC (23%), in seven with LKM1+ AIH (23%) and in two with ANA/SMA+ AIH (8%). All four patients with AIH tested before treatment were anti-ASL+. All other patients and healthy controls were negative. The distribution of anti-LC1 and anti-ASL in various disorders is shown in Table 3. Among the 74 patients with autoimmune liver diseases, 15 (20%) were anti-LC1+, 13 (17%) were anti-ASL+ and four (5%) LC1 and ASL double-positive. There was no detectable difference in severity of liver disease and response to treatment between anti-ASL+ and anti-ASL−patients.
Table 2.
Reactivity against bovine argininosuccinate lyase (ASL) by double-dimension immunodiffusion (DDI) and Western blot (WB) in patients and controls*

Table 3.
Relationship between anti-LC1 and anti-ASL positivity in autoimmune liver disease by double dimension immunodiffusion*
Western blot
Both bovine and human ASL have a molecular weight of 50 kD [19], while LC1 has a molecular weight of 60 kD [12]. When S1 and S2 were tested against purified ASL they gave a 50-kD band, but both a 50-kD and a 60-kD band when tested against human liver cytosol. Using bovine ASL as target we found reactivity against a 50-kD peptide in four patients with ANA/SMA+ AIH (16%), four with LKM1+ AIH (13%), four with PBC (23%) and three with chronic HBV infection (20%) (Table 2). Ten of the 13 sera positive in DDI were also positive in WB. Five additional sera (two ANA/SMA+ AIH and three chronic HBV infection), negative by DDI, were positive by WB. All sera from patients with αl-antitrypsin deficiency, Wilson's, extrahepatic autoimmune diseases and from the 48 healthy controls were negative. Representative WB results are presented in Fig. 5.
Fig. 5.
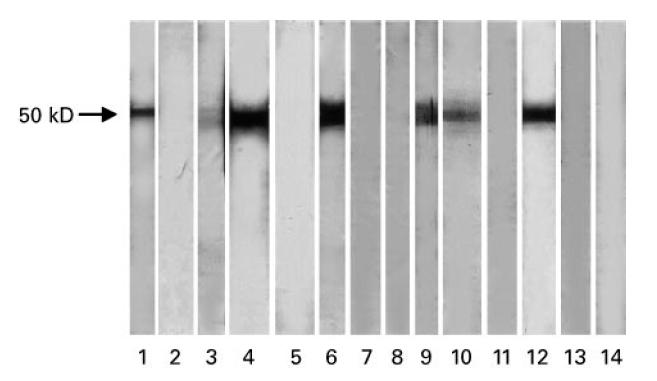
Representative Western blot results. Bovine argininosuccinate lyase (ASL; 160 μg per minigel) was subjected to a 12% SDS–PAGE. Patient sera were diluted 1:100. Lane 1, anti-ASL+ serum from anti-nuclear antibody (ANA)/smooth muscle antibody (SMA)-positive autoimmune hepatitis (AIH); lane 2, anti-ASL−serum from ANA/SMA+ AIH; lane 3, weakly anti-ASL+ serum from ANA/SMA+ AIH; lane 4, anti-ASL+ serum from ANA/SMA+ AIH; lane 5, anti-ASL−serum from liver–kidney microsome (LKM)-positive AIH; lane 6, anti-ASL+ serum from LKM/liver cytosol (LC1)-positive AIH; lane 7, anti-ASL−serum from LKM+ AIH; lane 8, anti-ASL−serum from LKM+AIH; lane 9, anti-ASL+ serum from LKM+ AIH; lane 10, anti-ASL+ serum from primary biliary cirrhosis (PBC); lane 11, anti-ASL−serum from PBC; lane 12, anti-ASL+ serum from PBC; lane 13, anti-ASL−serum from normal control; lane 14, anti-ASL−serum from normal control.
Effects of anti-ASL+ sera on ASL enzymatic activity
The incorporation of radio label in the first fibroblast cell line was 1198 p atom/6 h per mg protein in the presence of normal serum and 2690 in the presence of anti-ASL+serum. Similar results were obtained in the second fibroblast cell line, with an incorporation of 857 in the presence of normal serum and an incorporation of 2127 in the presence of anti-ASL+serum.
No urinary argininosuccinate excretion was detected in the four anti-ASL+patients studied (limit of detection 5 μmol/l).
DISCUSSION
In this study we demonstrate the existence of a new autoantibody in autoimmune liver disease. Such autoantibody is directed against an enzyme, argininosuccinate lyase (ASL; EC 4.3.2.1), that catalyses the terminal reversible reaction in arginine biosynthesis (argininosuccinic acid = arginine + fumarate). The existence of this new antigen–antibody system was suspected during the first phase of this study aimed at defining the target antigen of anti-LC1 using two high-titre anti-LC1+ sera. In DDI both these sera gave a broad precipitin line against human liver cytosol, the antigenic preparation used for the detection of anti-LC1, raising the possibility that two antigen–antibody systems may be responsible for the finding. The use of more discriminative DDI conditions enabled us to confirm the presence of two precipitin lines and to show in subsequent experiments, conducted both by DDI and WB, that human cytosol contains a new autoantigen of 50 kD in addition to the 60-kD antigen target of anti-LC1. This newly discovered antigen was identified as ASL by comparing sera reacting against unfractionated liver cytosol and purified periportal enzymes, leading to the identification of one of the two immunodiffusion lines observed in initial experiments as ASL. That ASL is a new autoantigen was confirmed in WB experiments, where the two high-titre anti-LC1+sera gave a 50-kD band against purified ASL, but both a 50-kD and a 60-kD band against human liver cytosol.
After identifying this new autoantibody we went on to examine its presence in a large series of patients with immune-mediated chronic liver disease and in pathological and normal controls. Anti-ASL is a liver disease-specific autoantibody, since it is absent in all the patients with extrahepatic autoimmune disease. This is not surprising, because ASL is an enzyme primarily found in the liver. However, it does not appear to be produced simply as a consequence of liver damage, since it was absent in patients with chronic liver disease due to αl-antitrypsin deficiency and Wilson's disease.
In fact, anti-ASL was confined to disorders with an immune component to their pathogenesis, such as AIH and PBC, and, at least in WB, in 20% of patients with chronic HBV infection. It is probable that the real prevalence of the antibody is higher than that reported in the present study, for two reasons. First, the patients with autoimmune conditions were those consecutively attending out-patient clinics, representing cross-sections of the various disorders, with the majority of them being under long-term immunosuppressive treatment at the time of study. This is known to abate autoantibody level [23]. Indeed, all four patients with AIH tested before starting treatment were positive for anti-ASL. Second, DDI, the main technique used in this study, has relatively low sensitivity. The prevalence of the autoantibody did not increase substantially, however, when we used WB, a technique more sensitive than DDI. One possibility is that, despite the difference in sensitivity, both techniques are able to detect all the antibodies present in the patient sera. This does not appear to be the case, since the antibody-positive populations did not overlap completely, surprisingly with a slightly lower antibody prevalence using WB, the more sensitive of the two techniques. Alternatively, it is possible that the two techniques define antibodies reacting against different epitopes on the same molecule, DDI revealing mainly conformational epitopes, WB mainly linear epitopes. The fact that autoantibodies may preferentially target conformational epitopes is well documented for other autoantibodies such as LKM1 in AIH [24], anti-glutamic acid decarboxylase in insulin-dependent diabetes [25] and AMA in PBC [26]. Only the use of techniques such as radioligand assays, that are not only more sensitive than DDI but also preserve conformational epitopes, will enable us to estimate the real prevalence of anti-ASL antibodies in liver disease.
An obvious question raised by this study is whether anti-ASL has pathogenic potential. ASL is a cytosolic enzyme and, according to classical views, is unlikely to invoke damaging autoimmune reactions, since its location is inaccessible to the effector molecules of the immune system, only the cell membrane being considered vulnerable to an immune attack. The view that antigens target of damaging immune reactions must reside on the membrane has recently been challenged, however, by the finding that autoantibodies directed to intracellular components, such as nuclear antigens, have the ability to inflict damage in vivo [27]. The clinical significance of ASL autoantibody remains to be determined. Although in this cross-sectional study we found no difference in severity of liver disease and response to treatment between anti-ASL+ and anti-ASL− patients, whether anti-ASL+ patients have a different clinical behaviour remains to be determined in prospective studies.
We wondered whether anti-ASL might be able to impair the enzymatic activity of its target. We searched for argininosuccinate in the urine, since this product appears when the enzyme is inactive, as is the case in the inborn error of ASL deficiency (argininosuccinic aciduria). Urinary argininosuccinate was absent in four anti-ASL+ patients tested, excluding that anti-ASL impairs significantly the enzymatic activity of its target in vivo. Additional experiments performed in vitro on fibroblast line cultures, a means of studying the integrity of the urea cycle enzymes, including ASL [21], gave unexpected results. The incorporation of radiolabelled arginine in the proteins was significantly increased when fibroblasts were cultured in the presence of 1% anti-ASL antibody-positive serum compared with normal serum. This finding was confirmed using a second fibroblast line. The results of these functional experiments indicate that anti-ASL autoantibody does not inhibit, but may stimulate ASL enzymatic activity. Although in the present study we have not investigated the possible effects of a stimulatory autoantibody, it is relevant that stimulatory antibodies occur in other autoimmune conditions, such as autoimmune thyroid disease. In Graves' disease in particular, anti-thyroid-stimulating hormone (TSH) antibodies stimulate the TSH receptor and account for the spectrum of clinical manifestations.
With the preliminary evidence we submit it is not possible to suggest a diagnostic or pathogenic role for anti-ASL. Organ-specific autoimmunity is composed of autoimmune reactions directed at different molecular targets of a given organ. In such an organ-specific scenario, the initial attack is focused on a single epitope of a key autoantigenic molecule, with autoimmunity subsequently spreading inter- and intramolecularly to other antigens within the organ, culminating in autoimmune disease. This framework is well documented in the experimental model of autoimmune diabetes, where glutamic acid decarboxylase is the first β cell-specific autoantigen targeted, followed by other islet autoantigens such as insulin and carboxypeptidase, preceding the development of insulin-dependent diabetes [28]. Manoeuvres directed at arresting autoimmunity against the initial target also prevent the development of insulin-dependent diabetes. In AIH a number of molecules have been described as targets of the autoimmune attack, but their hierarchical relationship is far from being understood. The position of anti-ASL, a newly discovered autoantigen, and indeed that of other liver-specific autoantigens in this hierarchy, remains to be defined.
Acknowledgments
The authors are indebted to Professor A. G. Palekar for his advice and the donation of human argininosuccinate lyase. The authors express their thanks to Dr F. Torre for assistance with iconographic material and to Dr E. Cançado for critical comments. G.M.-V. is supported by the Children's Liver Disease Foundation, Birmingham, UK.
References
- 1.Manns M. Autoantibodies and antigens in liver diseases—updated. J Hepatol. 1989;9:272–80. doi: 10.1016/0168-8278(89)90063-9. [DOI] [PubMed] [Google Scholar]
- 2.Meyer zum Büschenfelde KH, Lohse AW, Manns M, et al. Autoimmunity and liver disease. Hepatol. 1990;12:354–63. doi: 10.1002/hep.1840120225. [DOI] [PubMed] [Google Scholar]
- 3.Mackay IR, Gershwin ME. Primary biliary cirrhosis: current knowledge, perspectives, and future directions. Semin Liver Dis. 1989;9:149–57. doi: 10.1055/s-2008-1040507. [DOI] [PubMed] [Google Scholar]
- 4.Yeaman SJ, Fussey SP, Danner W, et al. Primary biliary cirrhosis: identification of two major M2 mitochondrial autoantigens. Lancet. 1988;1:1067–70. doi: 10.1016/s0140-6736(88)91894-6. [DOI] [PubMed] [Google Scholar]
- 5.Zurgil N, Bakimer R, Kaplan M, et al. Anti-pyruvate dehydrogenase autoantibodies in primary biliary cirrhosis. J Clin Immunol. 1991;11:239–45. doi: 10.1007/BF00918181. [DOI] [PubMed] [Google Scholar]
- 6.Homberg JC, Abuaf N, Bernard O, et al. Chronic active hepatitis associated with antiliver/kidney microsome antibody type 1: a second type of ‘autoimmune’ hepatitis. Hepatol. 1987;7:1333–9. doi: 10.1002/hep.1840070626. [DOI] [PubMed] [Google Scholar]
- 7.Alvarez F, Bernard O, Homberg JC, et al. Anti-liver-kidney microsome antibody recognizes a 50,000 molecular weight protein of the endoplasmic reticulum. J Exp Med. 1985;161:1231–6. doi: 10.1084/jem.161.5.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Manns MP, Johnson EF, Griffin KJ, et al. Major antigen of liver kidney microsomal autoantibodies in idiopathic autoimmune hepatitis is cytochrome P450dbl. J Clin Invest. 1989;83:1066–72. doi: 10.1172/JCI113949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ma Y, Peakman M, Lobo Yeo A, et al. Differences in immune recognition of cytochrome P4502D6 by liver kidney microsomal (LKM) antibody in autoimmune hepatitis and chronic hepatitis C virus infection. Clin Exp Immunol. 1994;97:94–99. doi: 10.1111/j.1365-2249.1994.tb06585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Czaja AJ, Manns MP. The validity and importance of subtypes in autoimmune hepatitis: a point of view. Am J Gastroenterol. 1995;90:1206–11. [PubMed] [Google Scholar]
- 11.Martini E, Abuaf N, Cavalli F, et al. Antibody to liver cytosol (anti-LCI) in patients with autoimmune chronic active hepatitis type 2. Hepatol. 1988;8:1662–6. doi: 10.1002/hep.1840080632. [DOI] [PubMed] [Google Scholar]
- 12.Han S, Tredger M, Gregorio GV, et al. Anti-liver cytosolic antigen type 1 (LC1) antibodies in childhood autoimmune liver disease. Hepatology. 1995;21:58–62. [PubMed] [Google Scholar]
- 13.Abuaf N, Johanet C, Chretien P, et al. Characterization of the liver cytosol antigen type 1 reacting with autoantibodies in chronic active hepatitis. Hepatol. 1992;16:892–8. doi: 10.1002/hep.1840160407. [DOI] [PubMed] [Google Scholar]
- 14.Muratori L, Cataleta M, Muratori P, et al. Detection of anti-liver cytosol antibody type 1 (anti-LC1) by immunodiffusion, counterimmunoelectrophoresis and immunoblotting: comparison of different techniques. J Immunol Methods. 1995;187:259–64. doi: 10.1016/0022-1759(95)00192-x. [DOI] [PubMed] [Google Scholar]
- 15.Abuaf N, Johanet C, Soulier E, et al. Meyer zum Büschenfelde KH. Immunology and liver, Falk symposium n. 70. Amsterdam: Kluwer Academic Publishers; 1992. Anti-liver cytosol antibodies in hepatology autoimmune hepatitis, viral hepatitis C and graft-versus-host disease; pp. 215–26. [Google Scholar]
- 16.De Duve C, Pressman BC, Gianetto R, et al. Intracellular distribution patterns of enzymes in rat liver tissue. Biochem J. 1955;63:604–17. doi: 10.1042/bj0600604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Homberg JC, Andre C, Abuaf N. A new anti-liver-kidney microsome antibody (anti-LKM2) in tienilic acid-induced hepatitis. Clin Exp Immunol. 1984;55:561–70. [PMC free article] [PubMed] [Google Scholar]
- 18.Lowry OH, Rosebrough NJ, Farr AL, et al. Protein measurement with the folin phenol reagent. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- 19.Palekar AG, Mantagos S. Human liver arginino succinase purification and partial characterization. J Biol Chem. 1981;256:9192–4. [PubMed] [Google Scholar]
- 20.Laemmli UK. Cleavage of structural proteins during the assembly of the head of the bacteriophage T4. Nature. 1970;227:683–5. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 21.Tedesco TA, Mellman WL. Argininosuccinate synthetase activity in cells cultured from a citrullinemic subject. Proc Natl Acad Sci USA. 1967;57:829. doi: 10.1073/pnas.57.3.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sherwood RA, Titheradge AC, Richards DA. Measurement of plasma and urine amino acids by high-performance liquid chromatography with electrochemical detection using phenylisothiocyanate derivatization. J Chomatogr Biomed Appl. 1990;528:293–303. doi: 10.1016/s0378-4347(00)82388-9. [DOI] [PubMed] [Google Scholar]
- 23.Gregorio GV, Portmann B, Reid F, et al. Autoimmune hepatitis in childhood: a 20-year experience. Hepatol. 1997;25:541–7. doi: 10.1002/hep.510250308. [DOI] [PubMed] [Google Scholar]
- 24.Duclos Vallee JC, Hajoui O, Yarnamoto AM, et al. Conformational epitopes on CYP2D6 are recognized by liver/kidney microsomal antibodies. Gastroenterol. 1995;108:470–6. doi: 10.1016/0016-5085(95)90076-4. [DOI] [PubMed] [Google Scholar]
- 25.Daw K, Ujihara N, Atkinson M, et al. Glutamic acid decarboxylase autoantibodies in stiff-man syndrome and insulin-dependent diabetes mellitus exhibit similarities and differences in epitope recognition. J Immunol. 1996;156:818–25. [PubMed] [Google Scholar]
- 26.Leung PS, Chuang DT, Wynn RM, et al. Autoantibodies to BCOADC-E2 in patients with primary biliary cirrhosis recognize a conformational epitope. Hepatol. 1995;22:505–13. [PubMed] [Google Scholar]
- 27.Alarcon Segovia D, Ruiz Arguelles A, Llorente L. Broken dogma: penetration of autoantibodies into living cells. Immunol Today. 1996;17:163–4. doi: 10.1016/s0167-5699(96)90258-3. [DOI] [PubMed] [Google Scholar]
- 28.Kaufman DL, Clare Salzler M, Tian J, et al. Spontaneous loss of T-cell tolerance to glutamic acid decarboxylase in murine insulin-dependent diabetes. Nature. 1993;366:69–72. doi: 10.1038/366069a0. [DOI] [PMC free article] [PubMed] [Google Scholar]


