Abstract
Permissively recognized peptides which can activate lymphocytes from subjects with a variety of class II HLA types are interesting diagnostic and vaccine candidates. In this study we generated T helper clones reactive to the permissively recognized p21–40 and p91–110 peptides of the 16-kD heat shock protein of Mycobacterium tuberculosis. All the clones specific for p91–110 secreted interferon-gamma (IFN-γ) and were of the Th1 phenotype. By contrast, the p21–40 peptide favoured the generation of IL-4-producing clones. Antibody blockade established that the peptide-specific Th clones could either be DR-, DP- or DQ-restricted. Thus, two permissively recognized sequences p21–40 and p91–110 from the same mycobacterial antigen can drive the differentiation of functionally distinct T helper subsets. Attempts to immunize against tuberculosis should bear in mind epitope specificity if a favourable Th subtype response is to be generated.
Keywords: Th1 and Th2 clones, tuberculosis, 16 kDantigen, peptides
INTRODUCTION
The mechanism controlling the functional differentiation of T helper cells into Th1 and Th2 effector T lymphocytes is incompletely defined [1]. There is evidence that peptide epitope variation can influence the generation of Th1 and Th2 clones [2–4]. Further, avidity differences during T cell activation can also shift selective differentiation into the Th1 or Th2 phenotype [5,6]. The preferential induction of Th1 or Th2 cells by T cell epitopes may have a great impact on future vaccination against infectious diseases [7,8].
Conventionally, it is believed that effective peptide-based vaccines would also need to contain large numbers of peptides, as the majority of antigenic peptides have been found to be MHC allele-specific [9–11]. However, in tuberculosis, peptides have been found which stimulate T cells from subjects expressing a variety of HLA class II types, a consequence of these peptides' ability to bind permissively to a number of MHC molecules with high affinity [12–14]. Such peptides can potentially overcome the problem of genetic restriction in an outbred population.
In a previous study we demonstrated that some peptides which bind permissively can also be recognized by Th clones when presented by antigen-presenting cells (APC) bearing either parental class II MHC type [15]. Such T cells are known as unrestricted or promiscuous T cells [16]. The functional advantage of promiscuous T cells recognizing antigen in association with more than one MHC molecule is that they may expand preferentially and thus mount a rapid immune response against the pathogen. Further insight into the function of promiscuous T cells is therefore of particular interest for the development of peptide-based vaccines.
We therefore generated Th clones, specific for the p21–40 and p91–110 peptides of the 16-kD protein of Mycobacterium tuberculosis from two purified protein derivative (PPD)+, HLA-heterozygous individuals, and investigated the clones' Th phenotype. We found that 16p91–110-specific clones were of high affinity for the peptide–MHC complex and were predominantly Th1, whereas 16p21–40-specific clones were of lower affinity and predominantly Th2 in phenotype. These data support the hypothesis that peptide structure can influence T helper differentiation.
MATERIALS AND METHODS
Cell lines
HT-2 cells and antibodies to HLA-DR (L243), -DQ (L2) and -DP (B7/21) were the gift of Professor R. Lechler (Department of Immunology, Imperial College School of Medicine, London, UK).
HLA typing
HLA typing at the DRB1 and DQB1 loci was performed by polymerase chain reaction using sequence-specific primers (PCR-SSP); and at DPB1 by PCR amplification and hybridization to sequence-specific oligonucleotide probes (SSOP) [17,18].
Peptide synthesis
Peptides were synthesized by 9-fluorenylmethoxycarbonyl (Fmoc) technology as previously described [13]. Their homogeneity was assessed by analytical reverse phase high performance liquid chromatography (HPLC) and molecular weight confirmed by mass spectrometry and estimated to be > 90%. Two peptides derived from the 16-kD protein of M. tuberculosis were used: p21–40, LFAAFPSFAGLRPTFDTRLM, and p91–110, SEFAYGSFVRTVSLPVGADE.
Generation of T cell clones
T cell clones were derived from the buffy coats of two PPD+ blood donors. The lymphocytes for cloning from buffy coats were selected on the basis of high proliferative responses to the peptides p21–40 and p91–110 of the 16-kD protein of M. tuberculosis. Donor J2 showed maximum response to p91–110, and J9 to p21–40. Peripheral blood mononuclear cells (PBMC) from donors J2 and J9 were separated from the buffy coats using Ficoll–Paque (Pharmacia, Uppsala, Sweden). PBMC were then washed and cultured in 96-well (Nunc, Roskilde, Denmark) plates at a density of 1.5 × 105 PBMC per well in the presence of peptide p21–40 for J9 and p91–110 for J2, in RPMI 1640 (Gibco, Paisley, UK) supplemented with 10% AB + serum, 2 mml-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin. The remaining PBMC were kept frozen in liquid nitrogen and were used as a subsequent source of APC. The plates were incubated at 37°C in 5% CO2. After 7 days, T cell cloning was performed by limiting dilution analysis. From each of the 96 wells, 0.1 cell/well was plated in 20 Terasaki plates in the presence of 20 U/ml recombinant human (rh)IL-2 (Boehringer, Mannheim, Germany) and 3000 R-irradiated autologous PBMC (1 × 104/well) which had been precultured with 50 μg/ml of peptide for 12–18 h. After 7 days culture, positive wells were identified. Only one clone was selected from each Terasaki plate to avoid the selection of sister clones. The clones were expanded by restimulating biweekly with rhIL-2 (20 U/ml) and peptide-pulsed PBMC, in 96-, 48- and 24-well flat-bottomed plates. All the clones stained positive for CD4 and CD3 (data not shown).
Dose response
T cell clones were rested for 7–10 days before each assay. Clones (1 × 104/well) and 1 × 104 irradiated autologous PBMC pulsed with different concentrations of peptides (0.675, 1.26, 11.11, 33.33 and 100 μg/ml) were incubated in 200 μl of complete medium in 96-well U-bottomed microtitre plates for 72 h. The control wells consisted of Th clones incubated with medium alone or with unpulsed PBMC. The cultures were pulsed with 1 μCi of 3H-TdR (Amersham, Aylesbury, UK) and harvested after 16 h onto glassfibre filters for scintillation counting (Skatron, Lier, Norway). The results are expressed as mean ct/min from triplicate cultures.
HLA isotype analysis of Th clones by antibody inhibition assay
In culture conditions as described above, peptide-pulsed PBMC (1 × 105/well) were incubated with MoAbs (1 or 10 μg/ml) specific for the monomorphic class II isotype-specific determinants of HLA-DR (L243), DQ (L2) or DP (B7/21) at 4°C for 45 min [19]. Without washing out MoAb, cloned Th cells (2 × 104/well) were added and cultured for 72 h, followed by a 3H-TdR pulse for 16 h.
Cytokine ELISA
Supernatants were harvested either after 24 h (IL-4) or 48 h (interferon-gamma (IFN-γ)). Maxisorp (Nunc) plates were coated at 4°C overnight in 0.1 m NaHCO3 with 50 μl/well of the following MoAbs: murine anti-IFN-γ MoAb 1-DIK at 2.5 μg/ml (Mabtech, Vikdalsvagan, Nacka, Sweden); anti-IL-4 (mouse anti-human IL-4; Mabtech) at 1 μg/ml. The plates were blocked for 1 h at room temperature in PBS–0.05% Tween–3% w/v bovine serum albumin (BSA). Supernatant (100 μl/well) was incubated in duplicate for 4 h and then washed four times. The plates were then incubated at room temperature for 1 h in 100 μl of the following biotinylated detection MoAbs: anti-IFN-γ at 1 μg/ml (Mabtech); anti-human IL-4 at 0.5 μg/ml (Mabtech). After five further washes, 100 μl of streptavidin peroxidase (Sigma, Poole, UK; 1 μg/ml) were added to each well and incubated for 30 min. Six final washes were followed by 150 μl K-Blue substrate (ELISA Technologies, Lexington, KY) followed by K-Red stop solution (50 μl) when the colour development was optimal. The plates were read at 680 nm and the cytokine concentration in each sample was calculated by reference to a standard curve. The following reagents were used as standards in the cytokine ELISA: rhIFN-γ (Pharmingen, San Diego, CA); rhIL-4 (Sigma). The sensitivity of each ELISA was as follows: IFN-γ, 1–5 pg/ml; IL-4, 10–25 pg/ml.
RESULTS
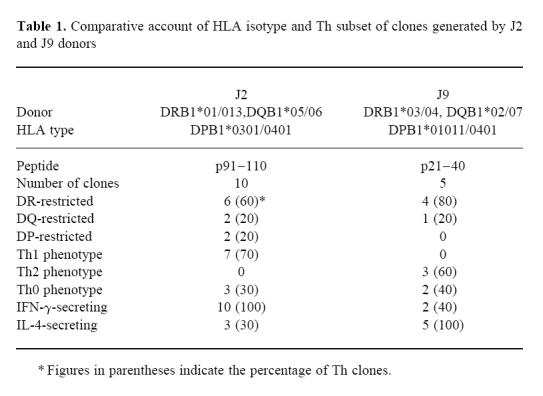
PBMC isolated from nine buffy coats were evaluated for the lymphoproliferation against peptides p21–40, p91–110 of the 16-kD; and p1–20, p350–369 and p65–83 of the 38-kD protein of M. tuberculosis (data not shown). The best response was seen in donors J2 and J9 to peptides p91–110 and p21–40, respectively, and the buffy coats from these donors were therefore chosen for Th cell cloning by limiting dilution. In total five and 10 Th clones were generated from J9 and J2, respectively. The clones obtained from donor J2 were specific for p91–110, whereas those from J9 were generated against p21–40. The HLA type of donor J2 was DR1, 13/DQ5, 6/DP3, 4; and J9 was DR3, 4/DQ2, 7/DP1, 4.
Dose response
The clones derived from both donors responded to the peptides in a dose-dependent manner. Interestingly, clones generated from the same individual showed marked variations in dose and proliferative response to the same peptide (Fig. 1a,b). Some of the clones responded maximally to low doses of the peptide, whereas others required highe r concentrations. None of the clones proliferated when cultured either with medium alone or with APC in the absence of peptide.
Fig. 1.
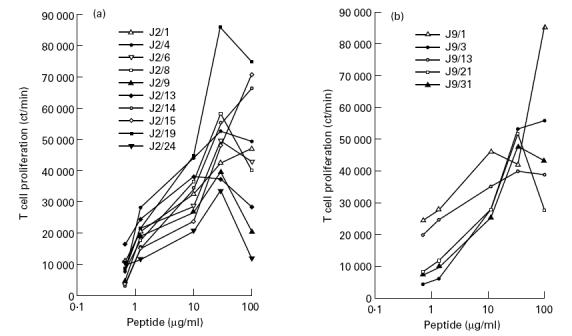
Th clones respond to peptide in a dose-dependent manner. Th clones were cultured in the presence of peptide p91–110 (a) and p21–40 (b) at various concentrations with irradiated autologous peripheral blood mononuclear cells (PBMC). After 72 h, 3H-TdR was added and 16 h later the cells were harvested and counted for β-emission. The results represent the ct/min of incorporated radioactivity. Control cultures, which showed < 2000 ct/min, were as follows: Th clones + medium, Th clones + PBMC without peptide, PBMC + peptide.
HLA isotype restriction of Th clones
Incubation of the cloned Th cells in the presence of peptide-pulsed, autologous PBMC and MoAb against DR, DQ and DP induced 67–93% inhibition of the proliferation using isotype-specific MoAb at a concentration of 1 and 10 μg/ml (Fig. 2a,b). Interestingly, Th clones restricted by HLA-DR, DQ or DP could be generated against the peptides. Each clone was inhibited only by one of the three tested antibodies, enabling unequivocal ascertainment of the relevant restricting HLA class II isotype. Six of the 10 clones obtained from donor J2 against peptide p91–110 were DR-restricted and two each by DP and DQ (Fig. 2a). In the case of J9, the proliferation of four clones was inhibited by anti-DR MoAb and one by anti-DQ MoAb (Fig. 2b). Thus the majority (66.67%) of Th clones were DR-restricted.
Fig. 2.
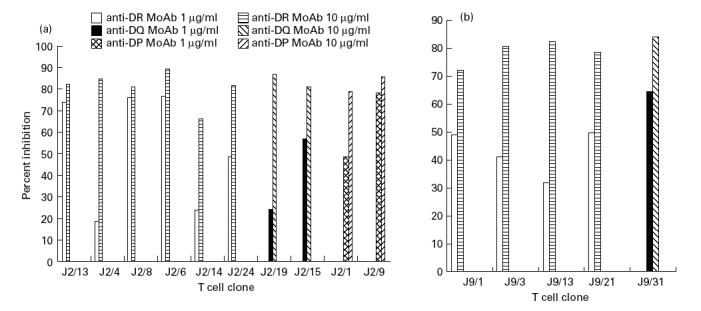
Inhibition of the proliferation of Th clones by anti-MHC antibodies. Irradiated autologous peripheral blood mononuclear cells (PBMC; 1 × 105/well) preincubated with MoAbs against HLA-DR (L243), -DQ (L2) and -DP (B7/21) were cultured with Th clones obtained from donors J2 (a) and J9 (b). Each Th clone was inhibited by only one of the three MoAbs. The results represent the percentage inhibition of Th clone proliferation by MoAb.
Cytokine phenotype of Th clones
We next ascertained the phenotype of Th clones on the basis of secretion of IFN-γ and IL-4 (Fig. 3). The production of cytokines by each Th clone derived from the same individual against the p91–110 peptide varied considerably (IFN-γ, 78–1284 pg/ml). All 10 Th clones specific for p91–110 generated from donor J2 produced IFN-γ (Fig. 3a). The majority (70%) of Th clones showed a pure Th1 phenotype secreting high levels of IFN-γ but no IL-4. None showed a pure Th2 pattern. Three clones exhibited a ‘Th0’ phenotype, producing both IL-4 and IFN-γ. Th1 clones, specific for p91–110, were either DR-, DP- or DQ-restricted. All three Th0 clones were DR-restricted (Table 1). Five p21–40-specific Th clones were generated from donor J9, all of which produced IL-4 (Fig. 3b). The clones varied in IL-4 production from 93 pg/ml (J9/3) to 2256 pg/ml (J9/13). Three clones showed the lymphokine profile of Th2 cells and two produced both IL-4 and IFN-γ. No purely Th1 clone was generated against p21–40. All the Th2 clones were DR-restricted, whereas the Th0 was DQ-restricted (Table 1). No clone from either donor secreted cytokines either in the presence of peptide-unpulsed APC or when cultured with medium alone.
Fig. 3.
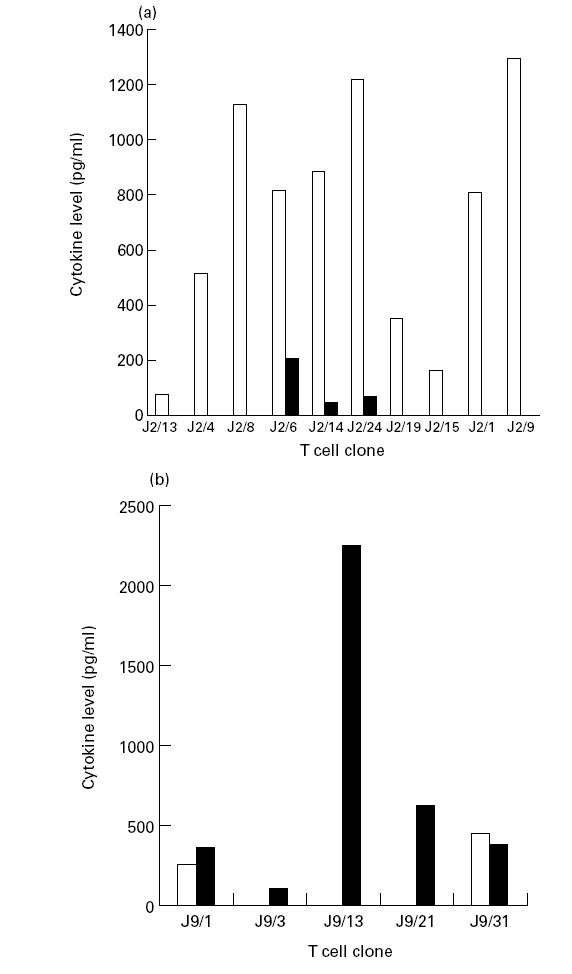
Cytokines production by Th clones. Supernates from clones stimulated with p91–110- (a) and p21–40- (b) pulsed antigen presenting cells (APC) were assayed for IFN-γ and IL-4 by ELISA. Control cultures consisting of Th clone + medium, and clones + APC in the absence of peptide did not induce any detectable cytokine secretion. □, IFN-γ; ▪, IL-4.
Table 1.
Comparative account of HLA isotype and Th subset of clones generated by J2 and J9 donors
Affinity of Th clones
The concentration of peptide required for optimum activation of Th clones was analysed and their affinity was estimated on the basis of the amount of peptide required to induce half maximal proliferation of Th clones. Peptide p91–110-specific Th clones were stimulated in the range of 0.77–12.09 μg/ml and p21–40-specific Th clones by 1.17–34.92 μg/ml. Six of seven Th1 clones were stimulated by < 1.95 μg/ml of peptide. By contrast, Th2 clones were stimulated in the range 16.43–34.92 μg/ml of peptide. ‘Th0’ clones were intermediate in affinity (range 1.30–13.18 μg/ml) (Fig. 4). Thus Th1 clones were stimulated by lower doses of peptide than Th2 clones.
Fig. 4.
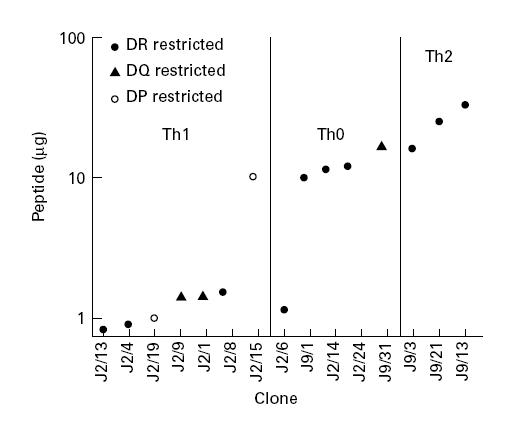
Affinity of Th clones. Th clones were cultured as in Fig. 1. Their affinity was calculated as the peptide concentration (μg/ml) required for half-maximal proliferation. The correlation between affinity and Th clone subtype and MHC restriction pattern is also shown.
DISCUSSION
The purpose of this study was to analyse the cytokine phenotype of T cell clones specific for two permissively recognized peptides (p21–40 and p91–110) of the 16-kD protein of M. tuberculosis. We found that: (i) all 10 p91–110-specific clones secreted IFN-γ. By contrast, all five Th clones specific for peptide p21–40 produced IL-4 and none displayed the Th1 phenotype; (ii) both peptides generated clones which could be DR-, DQ- or DP-restricted; (iii) there was hierarchy among the Th clones generated from the same individual with respect to the quantity of lymphokine produced, and in their peptide affinity.
Both p21–40 and p91–110 were recognized by Th clones in the context of parental DR and DQ alleles, and in the case of p91–110, DP also. This finding implies that these peptides not only bind to HLA-DR molecules but also to DQ and DP. An additional implication is that peptide epitope presentation by class II molecules is at least partially determined by the relatively conserved residues 94–286 in the β1 exon [20]. The majority of the Th clones were, however, DR-restricted, possibly as a consequence of lower cellular expression of DQ and DP [21], or lower peptide binding affinities for DQ and DP [22,23]. In addition, some Th clones proliferated when peptide was presented by one (restricted recognition); and the remaining clones in the context of (unrestricted recognition) parental HLA allele (data not shown). This phenomenon appears to be associated with peptide specificity and HLA type of the individual, consistent with our previous observations on promiscuous T cell clones [15]. The unrestricted p91–110-specific Th1 clones tended to require a lower dose of peptide in order to proliferate.
Peptide p91–110 binds HLA-DR1 with high affinity (IC50 ≤ 10 μm) and DR6 with moderate affinity [13]. Although the cytokine production of the peptide-specific Th clones from single individuals varied considerably, in this study the p91–110-specific clones isolated from a DR1,6 heterozygous donor were of high overall affinity for the peptide–MHC complex (as judged by the lower dose of peptide required for half maximal stimulation) and were predominantly Th1 in phenotype. By comparison, peptide p21–40 binds with only moderate affinity to DR4, and with low affinity to DR3 [13]. The p21–40-specific clones isolated from a DR3,4 heterozygous donor were of lower overall affinity for the peptide–MHC complex and were predominantly Th2 in phenotype. These data therefore support the hypothesis that the MHC binding affinity of peptides, leading to differential interactions at the T cell–APC interface, can be crucial for the differential development of cytokine patterns in T cells [24]. A functional hierarchy has been proposed among the peptides in a given antigen that interact with the T cell receptor (TCR) [25,26]. It has been reported that peptides can modulate the immune system to preferentially activate Th1 and Th2 cells, leading to either susceptibility or resistance to disease [27–29]. In Leishmania major, a repetitive peptide induces Th2 cells that exacerbate the disease [4].
As IFN-γ gene knock-out mice develop rapidly disseminated M. tuberculosis infection [30], and humans with a mutation in the IFN-γ receptor are susceptible to recurrent atypical mycobacterial infection [31], it is believed that the Th1 phenotype is protective in tuberculosis. In vitro, the 16-kD antigen is predominantly expressed by M. tuberculosis when subjected to oxygen deprivation and can account for up to 25% total bacillary protein expression in these circumstances [32,33]. As containment within the granuloma may induce similar conditions, the 16-kD protein may be an important antigenic target during bacillary latency. In this respect p91–110-specific IFN-γ-producing Th clones may contribute to premonition. However, vaccination with the whole antigen might equally lead to the generation of Th2 clones, thus predisposing a person more susceptible to tuberculosis. As different peptides from the same immunogenic protein can induce a different phenotype of response, attempts to vaccinate against tuberculosis may meet with limited success unless the epitope specificity, as well as the phenotype, of the response evoked is considered.
Acknowledgments
The authors are thankful to Professor J. Ivanyi and Professor R. Lechler for providing facilities and guidance throughout the study; to Dr N. Davy (RPMS) for HLA-DR and DQ typing; to Dr P. J. Dunn (UKTSSA, Bristol) for DP typing; and to Mr A. Hill for peptide synthesis.
References
- 1.O'Garra A. Cytokines induce the development of functionally heterogenous T helper subsets. Immunity. 1998;8:275–83. doi: 10.1016/s1074-7613(00)80533-6. [DOI] [PubMed] [Google Scholar]
- 2.Milich DR, Peterson DL, Schodel F, et al. Preferential recognition of hepatitis B nucleocapsid antigens by Th1 or Th2 cells is epitope and major histocompatibility complex dependent. J Virol. 1995;69:2776–85. doi: 10.1128/jvi.69.5.2776-2785.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mustafa M, Vingsbo C, Olsson T, et al. The major histocompatibility complex influences myelin basic protein 63–88-induced T cell cytokine profile and experimental autoimmune encephalomyelitis. Eur J Immunol. 1993;23:3089–95. doi: 10.1002/eji.1830231207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liew FY, Millott SM, Schmidt JA. A repetitive peptide of Leishmania can activate T helper type 2 cells and enhance disease progression. J Exp Med. 1990;172:1359–65. doi: 10.1084/jem.172.5.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hosken NA, Shibuya K, Heath AW, et al. The effect of antigen dose on CD4+ T helper cell phenotype development in a T cell receptor-alpha beta-transgenic model. J Exp Med. 1995;182:1579–84. doi: 10.1084/jem.182.5.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Secrist H, DeKruyff RH, Umetsu DT. Interleukin 4 production by CD4+ T cells from allergic individuals is modulated by antigen concentration and antigen-presenting cell type. J Exp Med. 1995;181:1081–9. doi: 10.1084/jem.181.3.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boom WH, Liebst L, Abbas A, et al. Patterns of cytokine secretion in murine leishmaniasis: correlation with disease progression or resolution. Infect Immun. 1990;58:3863–70. doi: 10.1128/iai.58.12.3863-3870.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Surcel H-M, Troye-Blomberg M, Paulie S, et al. Th1/Th2 profiles in tuberculosis based on proliferation and cytokine response of blood lymphocytes to mycobacterial antigens. Immunology. 1994;81:171–6. [PMC free article] [PubMed] [Google Scholar]
- 9.Falk K, Rotzschke O, Stevanovic S, et al. Allele-specific motifs revealed by sequencing of self-peptides eluted from MHC molecules. Nature. 1991;351:290–6. doi: 10.1038/351290a0. [DOI] [PubMed] [Google Scholar]
- 10.Babbitt BP, Allen PM, Matsueda G, et al. Binding of immunogenic peptides to Ia histocompatibility molecules. Nature. 1985;317:359–61. doi: 10.1038/317359a0. [DOI] [PubMed] [Google Scholar]
- 11.Buus S, Colon S, Smith C, et al. Interaction between a ‘processed’ ovalbumin peptide and Ia molecules. Proc Natl Acad Sci USA. 1986;83:3968–71. doi: 10.1073/pnas.83.11.3968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vordermeier HM, Harris DP, Friscia G, et al. T cell repertoire in tuberculosis: selective anergy to an immunodominant epitope of the 38 kDa antigen in patients with active disease. Eur J Immunol. 1992;22:2631–7. doi: 10.1002/eji.1830221024. [DOI] [PubMed] [Google Scholar]
- 13.Jurcevic S, Hills A, Pasvol G, et al. T cell responses to a mixture of Mycobacterium tuberculosis peptides with complementary HLA-DR binding profiles. Clin Exp Immunol. 1996;105:416–21. doi: 10.1046/j.1365-2249.1996.d01-791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Friscia G, Vordermeier HM, Pasvol G, et al. Human T cell responses to peptide epitopes of the 16-kD antigen in tuberculosis. Clin Exp Immunol. 1995;102:53–57. doi: 10.1111/j.1365-2249.1995.tb06635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Agrewala J, Deacock S, Jurcevic S, et al. Peptide recognition by T-cell clones of an HLA-DRB1*1501/*0901 heterozygous donor is promiscuous only between parental alleles. Hum Immunol. 1997;55:34–38. doi: 10.1016/s0198-8859(97)00056-6. [DOI] [PubMed] [Google Scholar]
- 16.Panina-Bordignon P, Tan A, Termijtelen A, et al. Universally immunogenic T cell epitopes: promiscuous binding to human MHC class II and promiscuous recognition by T cells. Eur J Immunol. 1989;19:2237–42. doi: 10.1002/eji.1830191209. [DOI] [PubMed] [Google Scholar]
- 17.Olerup O, Zetterquist H. HLA-DR typing by PCR amplification with sequence-specific primers (PCR-SSP) in 2 hours: an alternative to serological DR typing in clinical practice including donor-recipient matching in cadaveric transplantation. Tissue Antigens. 1992;39:225–35. doi: 10.1111/j.1399-0039.1992.tb01940.x. [DOI] [PubMed] [Google Scholar]
- 18.Versluis LF, Rozemuller E, Tonks S, et al. High-resolution HLA-DPB typing based upon computerized analysis of data obtained by fluorescent sequencing of the amplified polymorphic exon 2. Hum Immunol. 1993;38:277–83. doi: 10.1016/0198-8859(93)90555-f. [DOI] [PubMed] [Google Scholar]
- 19.Robbins PA, Evans EL, Ding AH, et al. Monoclonal antibodies that distinguish between class II antigens (HLA-DP, DQ, and DR) in 14 haplotypes. Hum Immunol. 1987;18:301–13. doi: 10.1016/0198-8859(87)90077-2. [DOI] [PubMed] [Google Scholar]
- 20.Andersson L, Gustafsson K, Jonsson AK, et al. Concerted evolution in a segment of the first domain exon of polymorphic MHC class II beta loci. Immunogenetics. 1991;33:235–42. doi: 10.1007/BF00230500. [DOI] [PubMed] [Google Scholar]
- 21.Robbins PA, Maino VC, Warner NL, et al. Activated T cells and monocytes have characteristic patterns of class II antigen expression. J Immunol. 1988;141:1281–7. [PubMed] [Google Scholar]
- 22.O'Sullivan D, Arrhenius T, Sidney J, et al. On the interaction of promiscuous antigenic peptides with different DR alleles. Identification of common structural motifs. J Immunol. 1991;147:2663–9. [PubMed] [Google Scholar]
- 23.Cease KB, Berkower I, York-Jolley J, et al. T cell clones specific for an amphipathic alpha-helical region of sperm whale myoglobin show differing fine specificities for synthetic peptides. A multiview/single structure interpretation of immunodominance. J Exp Med. 1986;164:1779–84. doi: 10.1084/jem.164.5.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kumar V, Bhardwaj V, Soares L, et al. Major histocompatibility complex binding affinity of an antigenic determinant is crucial for the differential secretion of interleukin 4/5 or interferon gamma by T cells. Proc Natl Acad Sci USA. 1995;92:9510–4. doi: 10.1073/pnas.92.21.9510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Evavold BD, Williams SG, Chen JS, et al. T cell inducing determinants contain a hierarchy of residues contacting the T cell receptor. Semin Immunol. 1991;3:225–9. [PubMed] [Google Scholar]
- 26.Murray JS, Pfeiffer C, Madri J, et al. Major histocompatibility complex (MHC) control of CD4 T cell subset activation. II. A single peptide induces either humoral or cell-mediated responses in mice of distinct MHC genotype. Eur J Immunol. 1992;22:559–65. doi: 10.1002/eji.1830220239. [DOI] [PubMed] [Google Scholar]
- 27.Jardim A, Alexander J, Teh HS, et al. Immunoprotective Leishmania major synthetic T cell epitopes. J Exp Med. 1990;172:645–8. doi: 10.1084/jem.172.2.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reiner SL, Wang ZE, Hatam F, et al. TH1 and TH2 cell antigen receptors in experimental leishmaniasis. Science. 1993;259:1457–60. doi: 10.1126/science.8451641. [DOI] [PubMed] [Google Scholar]
- 29.Liew FY, Millott S, Li Y, et al. Macrophage activation by interferon-gamma from host-protective T cells is inhibited by interleukin (IL) 3 and IL4 produced by disease-promoting T cells in leishmaniasis. Eur J Immunol. 1989;19:1227–32. doi: 10.1002/eji.1830190712. [DOI] [PubMed] [Google Scholar]
- 30.Cooper A, Dalton D, Stewart T, et al. Disseminated tuberculosis in interferon-γ gene-disrupted mice. J Exp Med. 1993;178:2243–7. doi: 10.1084/jem.178.6.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Newport MJ, Huxley CM, Huston S, et al. A mutation in the interferon-γ receptor gene and susceptibility to mycobacterial infection. N Engl J Med. 1996;335:1941–9. doi: 10.1056/NEJM199612263352602. [DOI] [PubMed] [Google Scholar]
- 32.Cunningham A, Spreadbury C. Mycobacterial stationary phase induced by low oxygen tension: cell wall thickening and localization of the 16-kilodalton alpha-crystallin homolog. J Bacteriol. 1998;180:801–8. doi: 10.1128/jb.180.4.801-808.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yuan Y, Crane DD, Barry III CE. Stationary phase-associated protein expression in Mycobacterium tuberculosis: function of the mycobacterial α-crystallin homologue. J Bacteriol. 1996;178:4484–92. doi: 10.1128/jb.178.15.4484-4492.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]


