Abstract
We have previously reported the presence of marked immune dysregulation with a dominant Th2 profile, in a population of Ethiopian immigrants (ETH) in Israel heavily infected with helminths. In order to characterize better this immune dysregulation we studied by flow cytometry the expression of several activation markers on peripheral T cell populations, and lymphocyte apoptosis, in blood samples obtained from 63 ‘new’ ETH (recently arrived), 18 ‘old’ ETH (> 5 years since immigration) and 34 non-Ethiopian Israelis. The main findings in the ‘new’ ETH group in comparison with the non-Ethiopian controls were: (i) decreased CD4 and increased CD8 lymphocyte counts; (ii) elevated levels of activated T cells (CD3, CD4 and CD8) expressing HLA-DR; (iii) decreased levels of ‘naive’ CD4+ cells (CD45RA+), with increased levels of ‘memory’ CD4+ cells (CD45RO+); (iv) decreased numbers of CD28+ CD8+ lymphocytes; (v) marked increase in lymphocyte apoptosis. These T cell alterations and activation profile remained unchanged in 10 ‘new’ ETH in whom the helminth infections persisted for 6–11 months. In contrast, in 18 ‘old’ ETH, without helminth infections, the T cell activation profile was within the normal range. These findings suggest that chronic helminth infections may have a profound effect on the immune system of the host that disappears after eradication of these infections and adjustment to the new environment. It should therefore be taken into consideration for every immunomodulation therapy and especially in vaccine design and trials, in regions endemic for helminth infections.
Keywords: chronic immune activation, helminth infection, T cell subsets, Ethiopian immigrants
INTRODUCTION
In the course of the last several years, a population of over 50 000 Ethiopian Jews immigrated from Ethiopia to Israel. They reached Israel in two major waves, in 1984–85, and in 1991, and since then a continued trickle of immigrants still reaches the country. Most of the immigrants came from rural areas in Ethiopia, with meagre sanitation and paucity of medical facilities. The prevalence of several helminth infections in this population was evident already from the first wave of immigration in 1985 and still holds true for most recently arrived immigrants [1,2]. These circumstances formed a unique ‘experimental’ situation in which a whole community of Ethiopians, with a high rate of helminth infections, has been ‘transplanted’ into a Western type of environment with very low prevalence of such infections.
We have previously described the presence of wide immune activation in this immigrant population, as characterized by serum markers and cytokine secretion pattern [3]. Those studies revealed a dominant Th2 type of immune profile in the vast majority of the newly arrived immigrants. Since we ascribed this marked immune dysregulation to the presence of helminth infections, it was obviously important to characterize further the immune activation profile of the peripheral blood lymphocytes. The remarkable alterations in the peripheral T cell subsets and activation profile that have been found prompted this study.
SUBJECTS AND METHODS
Human subjects
Three groups of clinically healthy HIV−adult individuals were enrolled to the study after obtaining an informed consent from each one of them: (i) 63 Ethiopian immigrants, shortly after their arrival in Israel (‘new’ ETH); (ii) 34 non-Ethiopian Israelis (non-ETH control); (iii) 18 Ethiopian immigrants that arrived in Israel over 5 years before the study (‘old’ ETH control). All immigrants arriving in Israel undergo several clinical screening tests with informed consent, which include also HIV testing by ELISA (Abbott HIV-1/HIV-2 3rd Generation Plus EIA; Wiesbadern-Delkenheim, Germany). Furthermore, this population has been also previously screened for HTLV-1 and HTLV-3 and found to be negative to these retroviruses [2]. Furthermore, all ETH participants were tested for tuberculosis, malaria and hepatitis and all were found to be negative for these infections. The prevalence of helminth infections in the ETH population was as follows: Schistosoma mansoni(40.8%), Necator americanus (27.9%), Ascaris lumbricoides(19.3%), Trichuris trichiura(10.7%), Taenia saginata (8.6%) and Hymenolepis nana(3.2%); 17.2% showed co-infection with two different helminths, 1.1% with three, 4.3% with four and 1.1% with five different helminths. No correlation was found between infection with any given helminth and the immunological parameters. The ‘old’ ETH participants received standard general treatment for helminth and parasite infections for total eradication of these infections, within 6 months after arriving in Israel. All non-ETH participants were also screened for HIV-1 and HIV-2 and other infectious diseases. In the course of the study, we found that a group of ‘new’ ETH that were previously investigated by us, close to their arrival in Israel, did not receive the anti-helminthic treatment that was prescribed for them, at the time of their restudy.
Lymphocyte phenotype and apoptosis analyses
For evaluation of the percentage and number of CD3, CD4 and CD8 cells, a single-colour immunophenotyping was used. Briefly, 100 μl of whole anti-coagulated blood were incubated at room temperature for 30 min with 10 μl of MoAbs directed against Leu-4 (CD3), Leu-3a (CD4) or Leu-2a (CD8) (all from Becton Dickinson, Mountain View, CA), washed and then incubated at room temperature for 30 min with an FITC-conjugated goat anti-mouse F(ab′)2 fragment of affinity-purified antibodies to Fab fragment of mouse IgG (Sigma, Rehovot, Israel). Lysing solution (2 ml; Becton Dickinson) were then added and incubated for a further 10 min at room temperature. Cells were washed twice in PBS pH 7.2, resuspended in 500 μl of 1% formaldehyde in PBS and analysed by flow cytometry using FACScan (Becton Dickinson). Lymphocytes were distinguished from monocytes on the basis of their forward versus side light scatter pattern. The monocyte-specific reagent CD14 (Becton Dickinson) was used to test for monocyte contamination of lymphocytes. The mean percentage of CD14+ cells within the lymphocyte gate was < 5%. A minimum of 10 000 cells/sample were acquired in list mode.
For evaluation of membrane expression of T cell activation markers, two-colour immunophenotyping was used. MoAbs conjugated with either FITC or PE were used. Briefly, 100 μl of blood samples were incubated with 10 μl of MoAbs in the dark at room temperature for 30 min, lysed, washed twice in PBS pH 7.2, and finally resuspended in 500 μl of 1% formaldehyde in PBS. The following pairs of MoAbs were used: HLA-DR(FITC)/CD3(PE), HLA-DR(FITC)/CD4(PE), HLA-DR(FITC)/CD8(PE), CD45RO(FITC)/CD4(PE), CD45RO(FITC)/CD8(PE), CD45RA(PE)/CD4(FITC), CD45RA(PE)/CD8(FITC) (all from Dako, Glostrup, Denmark) and CD28(PE)/CD8(FITC) (Becton Dickinson). Cells incubated with FITC- and PE-conjugated mouse IgG1/IgG2a (Dako) served as isotype control. The proportion of activated cells was expressed as a percentage in each T cell subset or as absolute numbers (cells/μl) calculated by multiplying the proportion of these cells by the appropriate lymphocyte subset cell count.
Apoptosis of peripheral blood lymphocytes was determined by flow cytometry using APOPTEST (YLEMSrl, Roma, Italy) based on the increased stainability of lymphocytes on the part of a newly developed fluorophore which occurs during the early phases of apoptosis. APOPTEST was performed on whole blood after erythrocyte lysis with APOLYSE lysing solution and APOSTAIN staining solution. Fluorescence was analysed on gated lymphocytes using the same forward and side scatter settings as described above for routine immunophenotyping and FL3 channel (650 nm) according to the manufacturer's instruction.
Determination of helminths in stool specimens
All ETH participants of the study were tested for intestinal helminth infections, by determining and quantifying parasite eggs. The stool samples were stored at 4°C until examined, usually within 7–14 days. Stool examinations and egg counts were performed according to Beaver et al. [4] and modification of the Richie et al. formalin-ether sedimentation method [5]. Hookworm larval identification was done on stool specimens by the Harada–Mori faecal culture method [6]. It is known that helminthiases are very rare among the Israeli population [1,2]. Therefore, stool examinations were performed only on six samples obtained from the non-ETH participants and all were found to be negative.
Statistical analysis
Statistical analysis was performed using SPSS-X software. Comparisons between groups with normal distribution were performed by Student's t-test. Study groups with asymmetric distribution were compared using non-parametric Mann–Whitney U-test. The results are presented as mean values ± s.d. of the mean. P < 0.05 was considered significant. Correlation analysis was performed with the Spearman's rank correlation test.
RESULTS
T cell subset characterization
Results of the determinations of the main peripheral blood T cell populations are depicted in Fig. 1. The most striking findings are the changes in the CD4 and CD8 subsets in the ‘new’ ETH. Though there was a wide distribution of CD4 counts in individuals, the mean value of this subset was significantly lower in the ‘new’ ETH in comparison with the non-ETH control (P < 0.0001).). It is noteworthy that 28.5% of the ‘new’ ETH had CD4+ counts of < 500 cells/μl, whereas only 3.1% of the non-ETH group, and 5.9% of the ‘old’ ETH group, had such low levels of these cells. The mean CD4 counts in the ‘old’ ETH were still significantly lower than in the non-ETH (P < 0.001) and not significantly different from that of the ‘new’ ETH. However, the percentage of CD4+ cells in the ‘new’ ETH (35.9 ± 7.2%) was significantly lower (P < 0.001) than that found in the non-ETH and ‘old’ ETH groups (41.3 ± 5.7% and 41.7 ± 4.1%, respectively; not shown). An increase in both number (Fig. 1) and percentage (not shown) of CD8+ cells is clearly seen in the ‘new’ ETH group in comparison with both other control groups. Moreover, 25.4% of the ‘new’ ETH had CD8+ counts > 900 cells/μl, whereas only 8.8% of the non-ETH control group and none of the ‘old’ ETH, had such high CD8 levels. It is interesting to note that in the ‘old’ ETH, there was a marked decrease of CD8 cell number (Fig. 1) and percentage (not shown) in comparison with both other groups (P < 0.0001). Accordingly, the mean CD4/CD8 ratio was lowest in the ‘new’ ETH and highest in the ‘old’ ETH groups, the difference between each group and the others being significant (P < 0.0001–0.02). Furthermore, 44.4% of the ‘new’ ETH had a CD4/CD8 ratio of < 1.0 compared with none in both control groups.
Fig. 1.
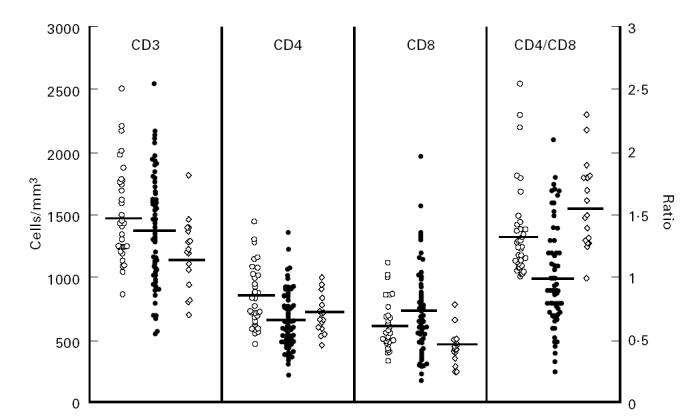
Peripheral T lymphocyte subsets and CD4/CD8 ratio in non-Ethiopian Israelis (non-ETH; ○), ‘new’ ETH (•) and ‘old’ ETH (⋄) groups. Each point represents a single individual. The horizontal bars indicate mean values.
Expression of activation markers on T cells and apoptosis of lymphocytes
The results of the studies of membrane activation markers on T lymphocytes are depicted in Figs 2 and 3. As can be clearly seen, HLA-DR, the universal marker for immune cell activation, was significantly elevated in all T cell subsets derived from the ‘new’ ETH, in comparison with the non-ETH and ‘old’ ETH control groups: for CD3 cells 13.3 ± 11.7%, 4.9 ± 2.4% and 5.1 ± 2.6% (P < 0.0001); for CD4 cells 6.5 ± 3.1%, 2.9 ± 1% and 3.5 ± 1.9% (P < 0.01–0.001); and for CD8 cells 16.2 ± 8.3%, 6.2 ± 1.9% and 9.8 ± 4.9% (P < 0.001), respectively (Fig. 2). These differences are particularly revealing when one looks at the mean absolute number of HLA-DR+ CD4+ cells in the three study populations: 44.8 ± 27.0 cells/μl in the ‘new’ ETH, 24.6 ± 11.1 cells/μl in the non-ETH controls and 22.1 ± 14.1 cells/μl in the ‘old’ ETH (not shown), despite the significant decrease in the absolute cell number of the CD4+ compartment in the ‘new’ ETH (see Fig. 1). The proportion of memory cells and naive cells within the CD4 compartment was also very clearly different in the ‘new’ ETH population in comparison with the non-ETH control group (Fig. 3). There was a sharp increase of memory CD45RO+ CD4+ cells (69.6 ± 11.7% versus 58.1 ± 8.7%, P < 0.001) and a parallel significant decrease of naive CD45RA+ CD4+ cells (23.5 ± 6.9% versus 34.8 ± 13.7%, P < 0.002). This is especially evident when one considers the very high number of individual cases in whom > 70% and < 20% of CD4 cells are memory and naive cells, respectively. The mean percentages of these cell populations in the ‘old’ ETH showed a clear trend towards the values found in the non-ETH controls. However, for the naive cells it was not significantly different from both ‘new’ ETH and non-ETH, while for memory cells it was still significantly higher than the percentage in the non-ETH group (P < 0.02). Within the CD8+ compartment the picture was quite different. No significant difference in the percentage of CD8+ lymphocytes expressing CD45RO and CD45RA antigens was observed between the ‘new’ ETH and non-ETH group. However, the mean proportion of these cells within the CD8 compartment of ‘old’ ETH was significantly different compared with the ‘new’ ETH: for naive CD8 cells 73.3 ± 9.2% versus 63.8 ± 13.8% (P < 0.045); for memory CD8 cells 28.1 ± 10.5% versus 34.8 ± 18.3% (P < 0.05), respectively. The difference between the ‘old’ ETH and non-ETH was not significant. The percentage (Fig. 3) and absolute number (not shown) of CD8+ cells expressing CD28 antigen was significantly lower in the ‘new’ ETH (39.4 ± 9.9%) in comparison with both the non-ETH controls and the ‘old’ ETH group (56.8 ± 16.2% and 58.4 ± 8.9%, respectively; P < 0.01). Finally, apoptosis of peripheral blood lymphocytes was clearly elevated in the ‘new’ ETH immigrants in comparison with the non-ETH controls: 7.8 ± 10.7% versus 2.5 ± 1.5% (P < 0.01) (Fig. 2). As can be seen, several of the ‘new’ ETH had very high levels of lymphocyte apoptosis and in a few it was > 15%.
Fig. 2.
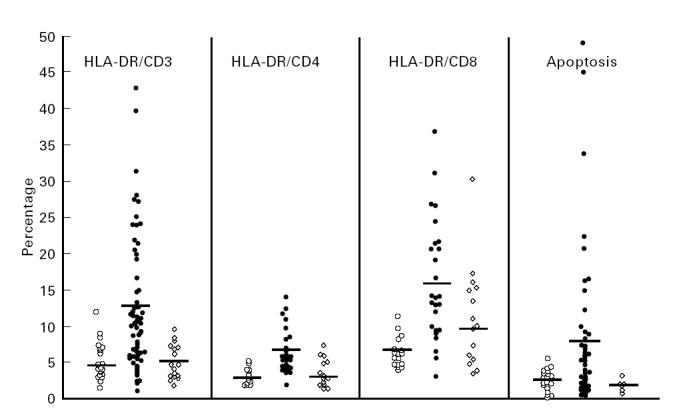
Expression of HLA-DR antigens on peripheral T lymphocyte subsets and apoptosis of peripheral lymphocytes in non-Ethiopian Israelis (non-ETH; ○), ‘new’ ETH (•) and ‘old’ ETH (⋄) groups. Each point represents a single individual. The horizontal bars indicate mean values.
Fig. 3.
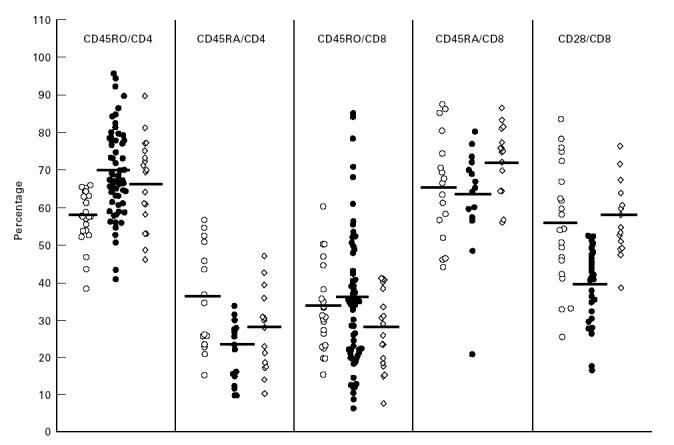
Expression of CD45RA and CD45RO antigens on peripheral CD4 and CD8 cells and of CD28 antigen on CD8 cells in non-Ethiopian Israelis (non-ETH; ○), ‘new’ ETH (•) and ‘old’ ETH (⋄) groups. Each point represents a single individual. The horizontal bars indicate mean values.
Correlation of activation markers with changes in peripheral lymphoid populations
We studied the correlation between the various activation markers and changes in peripheral lymphoid populations in two studied groups, ‘new’ ETH and non-ETH controls. The strongest positive correlations were found between the various activated T cell subsets obtained from the ‘new’ ETH study population, especially between HLA-DR+ CD3+ cells and either HLA-DR+ CD4+ or HLA-DR+ CD8+ cells (r = 0.78 and 0.88, respectively; P < 0.0001). Such correlations were not found in the non-ETH control population. A significant positive correlation was found between the activated HLA-DR+ CD3+ subset and the memory (CD45RO+) subsets of both CD4+ and CD8+ (r = 0.45 and 0.63, respectively; P < 0.0001), which was also not observed in the controls. No correlation (either positive or negative) was found between the activated subsets and naive (CD45RA+) cells. Lastly, a significant negative correlation was found between the proportions of CD28+ CD8+ cells and CD8+ and CD45RO+ CD4+ cells (r = − 0.41 and − 0.4, respectively; P < 0.05).
Persistent immune activation and helminth infections
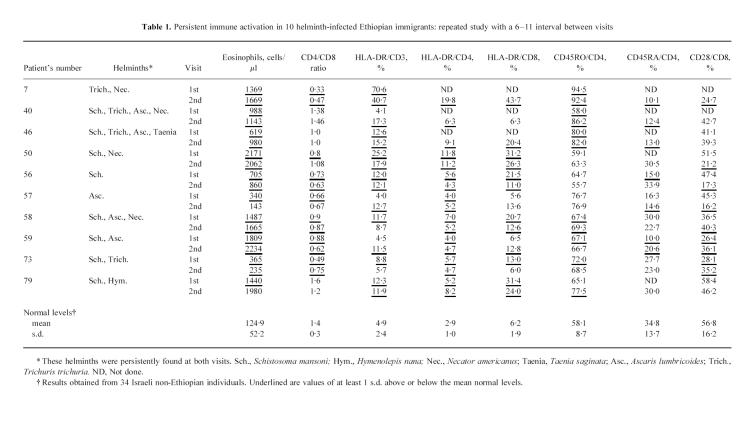
What is the cause of the findings described above, and can helminth infections account for them? In consecutive studies of the ‘new’ ETH population carried out on 116 subjects during the last 2 years, the prevalence of helminth infections was very high and consisted of several types (see Human subjects). We also studied consecutively a group of 10 ‘new’ ETH during a period of 6–11 months, following their arrival in Israel. We were not aware at the time of this study that some individuals of this group did not receive the anti-helminthic treatment that was prescribed for them. All of them were infected with one or more of the following helminths: S. mansoni, A. lumbricoides, Tr. trichiura, N. americanus, Tae. saginata or H. nana. They did not have any other type of chronic infection such as tuberculosis, malaria and viral hepatitis, and most importantly, at the time of their restudy they were still infected with the same helminths as at the time of their primary investigation. The results of the studies performed on blood obtained from these 10 individuals are summarized in Table 1. As can be seen, in the vast majority of individuals there was a significant blood eosinophilia, marked changes in peripheral T cell subsets (CD4/CD8 ratios) and pronounced membrane T cell activation pattern, that was maintained throughout the period of the study, and did not differ from that observed in the recently arrived immigrants, as described above. Thus, the answer to our primary question seems to be positive, i.e. helminth infection by itself may be a dominant factor responsible for the persistent immune activation and dysregulation that we have observed in the ‘new’ ETH.
Table 1.
Persistent immune activation in 10 helminth-infected Ethiopian immigrants: repeated study with a 6–11 interval between visits
DISCUSSION
The results of the present study have clearly shown that the recently arrived immigrants from Ethiopia have marked changes in their peripheral T cell populations as well as striking T lymphocyte membrane activation pattern. Thus, decreased CD4 levels and CD4/CD8 ratios with a trend to increased CD8 levels were found in most of the studied population. Concomitantly, marked increases of activated CD3+, CD4+ and CD8+ subsets together with diminished CD4 naive cells and increases in CD4 memory cells were also found in this population. These changes were associated with increased lymphocyte apoptosis and are most probably related to persistent helminth infection.
The changes in the major peripheral lymphocyte populations that we have observed are striking and are also in agreement with previous reports by Pollack et al. on other Ethiopian immigrants in Israel [7] and by Worku et al. on healthy individuals in Ethiopia [8]. Regretfully, only a small number of studies of this subject in African populations have been published. From some of these reports it seems that mean CD4 levels were indeed lower in HIV−individuals with either tuberculosis [9–11] or onchocerciasis [12]. Similar observations with a wider spectrum of infections associated with CD4 lymphocytopenia have been made outside of Africa [13–24]. A more recent study in Tanzania of a healthy population also showed that mean CD4 levels were lower in that population in comparison with levels in normal populations of Europe and North America, and that CD4 levels of < 500 cells/μl were found in > 10% of that population [25]. However, there are also studies in other African countries that have not found such changes [26–32]. The reasons for these differences are not clear and may reflect different infectious, environmental or genetic background. Since no evidence for retroviral infection was found in this population [2], it is difficult to ascribe the CD4 decrease to such infections. The fact that in the Ethiopian immigrant population these changes were no longer found after a few years of living in Israel clearly suggests that they are caused by environmental and not by genetic factors.
A major finding in our study is the marked activation profile of the peripheral T lymphocytes obtained from the ‘new’ ETH. It was more clearly seen in the CD4 compartment and was accompanied by a dramatic decrease in naive cells and an increase in memory cells. Thus, it seemed plausible to deduce that the marked activation of CD4 cells leads to depletion of CD4 naive cells and thereby results in the overall CD4 decline with insufficiency in CD4 replenishment. However, when we tried to correlate cell activation to the total CD4 levels, a significant positive rather than negative correlation was found between CD3+ DR+ and CD4+ levels in the ‘new’ ETH population. Furthermore, when we looked carefully at results obtained from individual subjects in this population, a wide distribution of activation levels with a wide range of CD4 levels was seen. Also, no significant correlation was observed between lymphocyte apoptosis and T cell activation. Though we do not have a clear explanation for this dissociation, there are a number of possible interpretations: (i) no direct relationships should necessarily exist between activation, apoptosis and CD4 decrease as measured at one time point in the peripheral blood; (ii) depletion of CD4 represents the net result of several coexisting events which may occur at different levels in different individuals; (iii) technical limitations of the methodology used to measure lymphocyte apoptosis may influence the results. Bearing all this in mind, we still favour the above suggested interpretation, that the chronic activation of the CD4 compartment by infectious disease is the cause for the final marked change in the lymphocyte populations. More careful sequential determinations and kinetics of changes in these variables, especially before and after the eradication of infections, should help resolve this issue.
An increase in activated CD8+ cells was also found in the ‘new’ ETH, that was not accompanied by similar changes in the naive and memory CD8+ compartments, but was accompanied by a marked decrease in CD28+ CD8+ cells. The reasons for these differences are not at all clear, and may be accounted for by differences in the activation signal, the character of the inciting antigen, or the immune response background. In our previous study we described the immune activation profile in a similar immigrant population, and found a dominant Th2 profile manifested by the characteristic cytokine secretion profile and the increased blood eosinophils and IgE [3]. Such a background may indeed influence and skew the immune activation towards a preferred CD4+ response and thereby account for the described differences between the CD4 and CD8 compartments. The decrease in CD28+ CD8+ cells, however, described particularly in HIV-infected individuals [33–37], has also been described in other clinical conditions characterized by immune activation, such as organ transplantation [38], autoimmune diseases [39], viral [40] and protozoan Trypanosoma cruzi infections [41], and may thus reflect the persistent chronic activation by helminth infections.
From the outset of our studies in the Ethiopian immigrant population, we have suggested that helminth infections are the major cofactor for the general immune activation seen in that population [42], and the results of the present study lend support to this suggestion. As described in detail above, a small group of ‘new’ immigrants did not receive any anti-helminthic treatment for a whole year, and as clearly demonstrated in Table 1, all parameters of immune activation were still present 6–11 months after immigration, while the helminth infection persisted. On the other hand, eradication of helminth infections has been associated with decrease in immune activation [43], and as demonstrated in the present study, an almost normal immune activation profile was found in the ‘old’ ETH, that do not have helminth infections. A more detailed study of time sequence relationships between helminth infection load and its eradication and the immune profile in individual patients is now in progress.
The general immune activation profile that we have found in the Ethiopian population is extremely reminiscent of that described in HIV-infected individuals [8,12,26,44–48]. Such immune activation has often been ascribed to HIV infection itself and to be the direct result of virus activity. Our findings at least cast some doubt on this interpretation, and would suggest that chronic immune activation of the host by chronic helminth infections, and not only by HIV, may result in similar changes of the host immune response. We are not suggesting that the chronically helminth infected host will develop the full blown picture of AIDS without HIV. However, chronic infection with helminths illustrates best some of the elements of chronic immune activation that may also be found in HIV infection, which lead most probably to CD4 decline, decreased CD4+ naive and increased CD4+ memory cells, lymphocyte apoptosis and decrease of CD28+ CD8+ cells [49]. The further study of this chronic infection may thus shed further light on the immunopathogenesis of HIV infection.
Last but not least, the marked immune activation that we have found in this chronically infected population is most probably representative of much larger African populations, and was also found in Asia as well as Central and South America [50–60]. Such wide immune activation may have far reaching implications. It is quite likely that it plays an important role in coping with infections, as well as in the generation and prevalence of autoimmune diseases and neoplasia. It is also highly likely, as we have suggested, that immune activation is a major factor in accounting for the increased susceptibility and progression of HIV infection in Africa and the developing countries [42]. No less important and directly relevant, however, the generation of protective immunity with vaccines in such populations must be different from that used in Western developed countries. Surprisingly, only very few studies have addressed this aspect. Our study clearly draws attention to the immediate need for further study of the effects chronic immune activation may have on vaccination, especially in the developing world and considering the terrible menace of the HIV pandemic.
Acknowledgments
The authors thank N. Yalin and G. Haroni for their expert statistical assistance. The work was supported in part by the grants from The Israel Academy of Sciences and Humanities (grant no. 490/96-1), The Israel Ministry of Health (grant no. 3776) and the Institute of Advanced Therapy (IAT) for Center of Excellence in AIDS Research in Israel.
References
- 1.Edman R, Greenberg Z. Intestinal parasitic infection in operation Solomon immigrants. J Isr Med Sci. 1993;29:374–6. [PubMed] [Google Scholar]
- 2.Nahmias J, Greenberg Z, Berger SA, Hornstein L, Bilgury B, Abel B, Kutner S. Health profile of Ethiopian immigrants in Israel: an overview. Isr J Med Sci. 1993;29:338–43. [PubMed] [Google Scholar]
- 3.Bentwich Z, Weisman Z, Moroz C, Bar-Yehuda S, Kalinkovich A. Immune dysregulation in Ethiopian immigrants in Israel: relevance to helminth infections. Clin Exp Immunol. 1996;103:239–43. doi: 10.1046/j.1365-2249.1996.d01-612.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beaver PC, Jung RC, Cupp EW. Clinical parasitology. Philadelphia: Lea & Febiger; 1984. Examination of specimens for parasites; pp. 733–58. [Google Scholar]
- 5.Knight WB, Hiatt RA, Cline BJ, Richie LS. A modification of the formol-ether concentration technique for increased sensitivity in detecting Schistosoma mansoni eggs. Am J Trop Med Hyg. 1976;25:818–23. doi: 10.4269/ajtmh.1976.25.818. [DOI] [PubMed] [Google Scholar]
- 6.Harada Y, Mori O. A new method for culturing hookworm. Yonago Acta Med. 1955;1:177–9. [Google Scholar]
- 7.Pollack S, Fuad B, Etzioni A. CD4 T-lymphocytopenia without opportunistic infections in HIV-seronegative Ethiopian immigrants to Israel. Lancet. 1993;342:50–51. doi: 10.1016/0140-6736(93)91912-6. [DOI] [PubMed] [Google Scholar]
- 8.Worku S, Bjorman A, Troye-Blomberg M, Jemaneh L, Farnert A, Christensson B. Lymphocyte activation and subset redistribution in the peripheral blood in acute malaria illness: distinct γδ+ T cell patterns in Plasmodium falciparum and P. vivax infections. Clin Exp Immunol. 1997;108:34–41. doi: 10.1046/j.1365-2249.1997.d01-981.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Egboga A, Corrah T, Todd J. Immunological findings in African patients with pulmonary tuberculosis and HIV-2 infection. AIDS. 1992;6:1045–6. doi: 10.1097/00002030-199209000-00025. [DOI] [PubMed] [Google Scholar]
- 10.Djomand G, Diaby L, N'Gbichi J-M, et al. Idiopathic CD4+ T-lymphocyte depletion in a West African population. AIDS. 1994;8:843–7. doi: 10.1097/00002030-199406000-00019. [DOI] [PubMed] [Google Scholar]
- 11.Martin DJ, Sim JGM, Sole GJ, et al. CD4+ lymphocyte count in African patients co-infected with HIV and tuberculosis. J Acqiur Immune Defic Syndr Hum Retrovir. 1995;8:386–91. [PubMed] [Google Scholar]
- 12.Soboslay PT, Dreweck CM, Hoffmann WH, et al. Ivermectin-facilitated immunity in onchocerciasis. Reversal of lymphocytopenia, cellular anergy and deficient cytokine production after single treatment. Clin Exp Immunol. 1992;89:407–13. doi: 10.1111/j.1365-2249.1992.tb06971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carney WP, Rubin RH, Hoffman RA, Hansen WP, Healey K, Hirsh MS. Analysis of T lymphocyte subsets in cytomegalovirus mononucleosis. J Immunol. 1981;126:2114–6. [PubMed] [Google Scholar]
- 14.Thomas HC, Brown D, Routhier G, et al. Inducer and suppressor T-cells in hepatitis B virus-induced liver disease. Hepatology. 1982;2:202–4. doi: 10.1002/hep.1840020203. [DOI] [PubMed] [Google Scholar]
- 15.Gomez E, Albercu I, Gonzalez M, Zaballos R, Vicente V. Effect of hepatitis C virus infection on the lymphoid population in hemophiliacs. Blood. 1991;77:1399–400. [PubMed] [Google Scholar]
- 16.Williams RC, Koster FT, Kilpatrick KA. Alterations in lymphocyte cell surface markers during various human infections. Am J Med. 1983;75:807–16. doi: 10.1016/0002-9343(83)90412-6. [DOI] [PubMed] [Google Scholar]
- 17.Pankhurst C, Peakman M. Reduced CD4+ T cells and oral candidiasis in absence of HIV infection. Lancet. 1989;i:1027–8. doi: 10.1016/s0140-6736(89)92176-4. [DOI] [PubMed] [Google Scholar]
- 18.Hansen ER, Lisby S, Baadsgaard O, Ho VC, de Villers EM, Veisgaard GL. Abnormal function of CD4+ helper/inducer T lymphocytes in a patient with widespread human papillomavirus type 3-related infection. Arch Dermatol. 1990;126:1604–8. [PubMed] [Google Scholar]
- 19.Cozon G, Greenland T, Revillard JP. Profound CD4+ lymphocytopenia in the absence of HIV infection in a patient with visceral leishmaniasis. N Engl J Med. 1990;322:132. doi: 10.1056/NEJM199001113220216. [DOI] [PubMed] [Google Scholar]
- 20.Seligmann M, Aractingi S, Oksenhendler E, Rabian C, Ferchal F, Gonnot G. CD4+ lymphocytopenia without HIV in patient with cryptococcal disease. Lancet. 1991;338:57–58. doi: 10.1016/0140-6736(91)93382-j. [DOI] [PubMed] [Google Scholar]
- 21.Leon ME, Ward B, Kanashiro R, et al. Immunologic parameters 2 years after high-titer measles immunization in Peruvian children. J Infect Dis. 1993;168:1097–104. doi: 10.1093/infdis/168.5.1097. [DOI] [PubMed] [Google Scholar]
- 22.Duncan RA, von-Reyn CF, Alliegro GM, Toossi Z, Sugar AM, Levitz SM. Idiopiathic CD4+ T-lymphocytopenia—four patients with opportunistic infections and no evidence of HIV infection. N Engl J Med. 1993;328:393–8. doi: 10.1056/NEJM199302113280604. [DOI] [PubMed] [Google Scholar]
- 23.Turett GS, Telzak EE. Normalization of CD4+ T-lymphocyte depletion in patients without HIV infection treated for tuberculosis. Chest. 1994;105:1335–7. doi: 10.1378/chest.105.5.1335. [DOI] [PubMed] [Google Scholar]
- 24.Laurence J. T-cell subsets in health, infectious disease, and idiopathic CD4+ T lymphocytopenia. Ann Intern Med. 1993;119:55–62. doi: 10.7326/0003-4819-119-1-199307010-00010. [DOI] [PubMed] [Google Scholar]
- 25.Urassa W, Pallangyo K, Lyamuya E, et al. Federation of African Immunological Societies Congress (FAIS), 9–13 March. 3. Cape Town, South Africa: Abstract book; 1997. Variability of levels of peripheral leukocytes, lymphocyte subsets and beta-2 microglobulin (B-2M) in HIV-1 seronegative adults. [Google Scholar]
- 26.Quinn TC, Piot P, McCormick JB, et al. Serologic and immunologic studies in patients with AIDS in North America and Africa. The potential role of infectious agents as cofactors in human immunodeficiency virus infection. JAMA. 1987;257:2617–21. [PubMed] [Google Scholar]
- 27.Katzenstein DA, Latif AS, Grace SA, et al. Clinical and laboratory characteristics of HIV-1 infection in Zimbabwe. J Acquir Immune Defic Syndr. 1990;3:701–7. [PubMed] [Google Scholar]
- 28.Le Guenno BM, Barabe P, Griffet PA, et al. HIV-2 and HIV-1 AIDS cases in Senegal: clinical patterns and immunological perturbations. J Acquir Immune Defic Syndr. 1991;4:421–7. [PubMed] [Google Scholar]
- 29.Kestens L, Brattegaard K, Adjorlolo G, et al. Immunological comparison of HIV-1, HIV-2 and dually-reactive women delivering in Abidjan, Cote d'Ivore. AIDS. 1992;6:803–7. doi: 10.1097/00002030-199208000-00006. [DOI] [PubMed] [Google Scholar]
- 30.Whittle H, Egboga A, Todd J, et al. Immunological responses of Gambians in relation to clinical stage of HIV-2 disease. Clin Exp Immunol. 1993;93:45–50. doi: 10.1111/j.1365-2249.1993.tb06495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chollet-Martin S, Simon F, Matheron S, Joseph CA, Elbim C, Gougerot-Pocidalo MA. Comparison of plasma cytokine levels in African patients with HIV-1 and HIV-2 infection. AIDS. 1994;8:879–84. doi: 10.1097/00002030-199407000-00003. [DOI] [PubMed] [Google Scholar]
- 32.Thea DM, Porat R, Nagimbi K, et al. Plasma cytokines, cytokine antagonists, and disease progression in African women infected with HIV-1. Ann Intern Med. 1996;124:757–62. doi: 10.7326/0003-4819-124-8-199604150-00009. [DOI] [PubMed] [Google Scholar]
- 33.Caruso A, Cantalamessa A, Licenziati S, et al. Expression of CD28 on CD8+ and CD4+ lymphocytes during HIV infection. Scand J Immunol. 1994;40:485–90. doi: 10.1111/j.1365-3083.1994.tb03494.x. [DOI] [PubMed] [Google Scholar]
- 34.Saukkonen JJ, Kornfeld H, Berman JS. Expansion of CD8+CD28- cell population in the blood and lung of HIV-positive patients. J Aquir Immune Defic Syndr. 1993;6:1194–204. [PubMed] [Google Scholar]
- 35.Brinchmann JE, Dobloug JH, Heger BH, Haaheim LL, Sannes M, Egeland T. Expression of costimulatory molecule CD28 on T cells in human immunodeficiency virus type 1 infection: functional and clinical correlations. J Infect Dis. 1994;169:730–8. doi: 10.1093/infdis/169.4.730. [DOI] [PubMed] [Google Scholar]
- 36.Borthwick NJ, Bofill M, Gombert WM, et al. Lymphocyte activation in HIV-1 infection. II. Functional defects of CD28- T cells. AIDS. 1994;8:431–41. doi: 10.1097/00002030-199404000-00004. [DOI] [PubMed] [Google Scholar]
- 37.Zanussi S, Simonelli C, D'Andrea M, et al. CD8+ lymphocyte phenotype and cytokine production in long-term non-progressor and in progressor patients with HIV-1 infection. Clin Exp Immunol. 1996;105:220–4. doi: 10.1046/j.1365-2249.1996.d01-746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kern F, Ode-Hakim S, Vogt K, Hoeflish C, Reinke P, Volk HD. The enigma of CD57+CD28− T cell expansion—anergy or activation. Clin Exp Immunol. 1996;104:180–4. doi: 10.1046/j.1365-2249.1996.d01-635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kaneko H, Saito K, Hashimoto H, Yagita H, Okumura K, Azuma M. Preferential elimination of CD28+ T cells in systemic lupus erythematosus (SLE) and the relation with activation-induced apoptosis. Clin Exp Immunol. 1996;106:18–29. doi: 10.1046/j.1365-2249.1996.d01-849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Borthwick NJ, Bofill M, Hassan I, et al. Factors that influence activated CD8+ T cell apoptosis in patients with acute herpes-virus infections: loss of costimulatory molecules CD28, CD5 and CD6 but relative maintenance of Bax and Bcl-x expression. Immunology. 1996;88:508–15. [PMC free article] [PubMed] [Google Scholar]
- 41.Dutra WO, Martins-Filho OA, Cancado JR, et al. Chagasic patients lack CD28 expression on many of their circulating T lymphocytes. Scand J Immunol. 1996;43:88–93. doi: 10.1046/j.1365-3083.1996.d01-9.x. [DOI] [PubMed] [Google Scholar]
- 42.Bentwich Z, Kalinkovich A, Weisman Z. Immune activation is a dominant factor in the pathogenesis of African AIDS. Immunol Today. 1995;16:187–91. doi: 10.1016/0167-5699(95)80119-7. [DOI] [PubMed] [Google Scholar]
- 43.Bentwich Z, Weisman Z, Grossman Z, Galai N, Kalinkovich A. Pathogenesis of AIDS in Africa: lessons from the Ethiopian immigrants in Israel. Immunologist. 1997;5:21–26. [Google Scholar]
- 44.Vanham G, Kestents L, Penne G, et al. Subset markers of CD8 (+) cells and their relation to enhanced cytotoxic T-cell activity during human immunodeficiency virus infection. J Clin Immunol. 1991;11:345–56. doi: 10.1007/BF00918800. [DOI] [PubMed] [Google Scholar]
- 45.Prince HE, Jensen ER. Three-color cytofluorimetric analysis of CD8 cell subsets in HIV-1 infection. J Acquir Immune Defic Syndr. 1991;4:1227–32. [PubMed] [Google Scholar]
- 46.Mahalingham M, Peakman M, Davies ET, Pozniak A, McManus TJ, Vergani D. T cell activation and disease severity in HIV infection. Clin Exp Immunol. 1993;93:337–43. doi: 10.1111/j.1365-2249.1993.tb08182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Landay AL, Mackewicz CE, Levy JA. An activated CD8+ T cell phenotype correlates with anti-HIV activity and asymptomatic clinical status. Clin Immunol Immunopathol. 1993;69:106–16. doi: 10.1006/clin.1993.1157. [DOI] [PubMed] [Google Scholar]
- 48.Benito JM, Zabay JM, Gil J, et al. Quantitative alterations of the functionally distinct subsets of CD4 and CD8 T lymphocytes in asymptomatic HIV infection: changes in the expression of CD45RO, CD45RA, CD11b, CD38, HLA-DR and CD25 antigens. J Acquir Immune Def Syndr Hum Retrovir. 1997;14:128–35. doi: 10.1097/00042560-199702010-00005. [DOI] [PubMed] [Google Scholar]
- 49.Bentwich Z, Kalinkovich A, Weisman Z, Grossman Z. Immune activation in the context of HIV infection. Clin Exp Immunol. 1998;111:1–2. doi: 10.1046/j.1365-2249.1998.00483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Freedman DO, Lujan-Trangay A, Steel C, Gonzalez-Peralta C, Nutman TB. Immunoregulation in onchocerciasis. Functional and phenotypic abnormalities in lymphocyte subsets and changes with therapy. J Clin Invest. 1991;88:231–8. doi: 10.1172/JCI115282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Munk ME, Soboslay PT, Arnoldi J, Brattig N, Schulz-Key H, Kaufmann SH. Onchocerca volvulus provides ligands for the stimulation of human γδT lymphocytes expressing Vδ1 chains. J Infect Dis. 1993;168:1241–7. doi: 10.1093/infdis/168.5.1241. [DOI] [PubMed] [Google Scholar]
- 52.Soboslay PT, Luder CGK, Hoffmann WH, et al. Ivermectin-facilitated immunity in onchocerciasis; activation of parasite-specific Th1-type responses with subclinical Onchocerca volvulus infection. Clin Exp Immunol. 1994;96:238–44. doi: 10.1111/j.1365-2249.1994.tb06548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Islam D, Bardhan PK, Lindberg AA, Christensson B. Shigella infection induces cellular activation of T and B cells and distinct species-related changes in peripheral blood lymphocyte subset during the course of the disease. Infect Immun. 1995;63:2941–9. doi: 10.1128/iai.63.8.2941-2949.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dutra WO, Martins-Filho OA, Cancado JR, et al. Activated T and B lymphocytes in peripheral blood of patients with Chagas disease. Int Immunol. 1994;6:499–506. doi: 10.1093/intimm/6.4.499. [DOI] [PubMed] [Google Scholar]
- 55.Sabin EA, Araujo Carvalho EM, Pearce EJ. Impairment of tetanus toxoid-specific Th1-like immune responses in humans infected with Schistosoma mansoni. J Infect Dis. 1996;173:269–72. doi: 10.1093/infdis/173.1.269. [DOI] [PubMed] [Google Scholar]
- 56.Araujo MI, de Jesus AR, Bacellar O, Sabin E, Pearce E, Carvalho EM. Evidence of a T helper type 2 activation in human schistosomiasis. Eur J Immunol. 1996;26:1399–403. doi: 10.1002/eji.1830260633. [DOI] [PubMed] [Google Scholar]
- 57.Martins-Filho OA, Dutra WO, Freeman GL, et al. Flow cytometric study of blood leukocytes in clinical forms of human schistosomiasis. Scand J Immunol. 1997;46:304–11. doi: 10.1046/j.1365-3083.1997.d01-119.x. [DOI] [PubMed] [Google Scholar]
- 58.Rizzardini G, Piconi S, Ruzzante S, et al. Immunological activation markers in the serum of African and European HIV-seropositive and seronegative individuals. AIDS. 1996;10:1535–42. doi: 10.1097/00002030-199611000-00012. [DOI] [PubMed] [Google Scholar]
- 59.Kalinkovich A, Maayan S, Weisman Z, Harpaz N, Bentwich Z. Immune activation, a cofactor for HIV transmission in Thailand. Lancet. 1994;343:1506–7. doi: 10.1016/s0140-6736(94)92618-2. [DOI] [PubMed] [Google Scholar]
- 60.Sundar S, Reed SG, Sharma S, Mehrotra A, Murray HW. Circulating T helper 1 (Th1) cell- and Th2 cell-associated cytokines in Indian patients with visceral leishmaniasis. Am J Trop Med Hyg. 1997;56:522–5. doi: 10.4269/ajtmh.1997.56.522. [DOI] [PubMed] [Google Scholar]