Abstract
Oral administration of DSS has been reported to induce an acute and chronic colitis in mice. The aim of our study was to evaluate if the chronic phase of DSS-induced colitis was characterized by a Th1/Th2 response and how this would relate to mucosal regeneration. Swiss Webster mice were fed 5% DSS in their drinking water for 7 days, followed by 2–5 weeks consumption of water. Control mice received only water. The animals were killed at 3 and 6 weeks after induction. Their colons were isolated for histology and immunohistochemistry, using specific MoAbs for T and B cells, macrophages, interferon-gamma (IFN-γ), IL-4 and IL-5. Colons were scored for inflammation, damage and regeneration. Two weeks after stopping DSS the colonic epithelium had only partially healed. Total colitis scores were still increased, especially in the distal colon, which was due to more inflammation, damage and less regeneration. In areas of incomplete colonic healing the basal parts of the lamina propria contained macrophages and CD4+ T cells. These CD4+ T cells showed a focal increase of IFN-γ and IL-4 staining compared with control animals. These findings were still observed 5 weeks after stopping DSS in some mice, albeit less extensive. Chronic DSS-induced colitis is characterized by focal epithelial regeneration and a Th1 as well as Th2 cytokine profile. We postulate that chronic immune activation mediated by both populations of Th cells can interfere with colonic healing and can play a role in the pathogenesis of chronic colitis.
Keywords: colitis, dextran sulphate, IL-4, interferon-gamma, mice
INTRODUCTION
Human inflammatory bowel disease (IBD), consisting of Crohn's disease (CD) and ulcerative colitis (UC), is a chronic condition, characterized by acute exacerbations followed by remissions. Despite many years of extensive research its pathogenesis is still poorly understood.
Several animal models for intestinal inflammation have been developed. Okayasu et al. [1] showed that oral administration of 5% DSS in drinking water of BALB/c mice was able to induce not only an acute, but also a chronic colitis after multiple cycles of DSS. The histopathology of this model showed some resemblance to UC and would enable research into the pathogenesis of this disease. This study was followed by a report from Cooper et al. [2], who showed that a similar and very reproducible DSS-induced colitis with an acute and a chronic phase could also be achieved in Swiss-Webster (SW) mice. In this study the authors gave a detailed description of the various histological changes, showing that the earliest lesions consisted of focal crypt loss, which was followed by acute and chronic inflammation. After stopping DSS administration the colon regenerates slowly over several weeks and the mucosa is infiltrated with chronic inflammatory cells.
In an earlier study we reported that neither T nor B cells are required for the induction of acute DSS-induced colitis, as this model could also be induced in severe combined immunodeficient (SCID) mice [3]. In order to establish the role of chronic inflammatory cells during the regenerative, chronic phase of DSS-induced colitis we investigated changes in distribution of lymphoid and non-lymphoid cells during the acute and chronic phase of DSS-induced colitis in SW mice. Furthermore, we investigated if the chronic phase was characterized by a dysregulation of T helper 1 (Th1)/T helper 2 (Th2) balance and how this would relate to mucosal regeneration.
MATERIALS AND METHODS
Mice
Pathogen-free female SW mice, 6–8 weeks old, weighing 20–25 g, were obtained from Harlan (Zeist, The Netherlands). These mice were kept under conventional conditions with free access to commercial food and water.
Experimental design
Swiss-Webster mice were fed 5% (w/v) DSS (mol. wt 40 kD; TdB Consultancy, Uppsala, Sweden) in their drinking water for 7 days, followed by 2–5 weeks consumption of water. The mice given DSS were divided into three groups and killed at day 7 of DSS administration (acute group) and after 2 weeks (DSS + 14) and 5 weeks (DSS + 35), respectively, after stopping DSS (regeneration groups). Each group consisted of six mice and was compared with control animals, which received only water. When the mice were killed the different parts of the colon, including caecum and lymphoid organs such as spleen and draining mesenteric lymph nodes, were isolated for histology and for immunohistochemistry on cryostat sections. In some animals the colons were removed for organ cultures to study interferon-gamma (IFN-γ) production.
Histology
Cryostat sections of 8 μm were picked up on slides and air-dried. After fixing the slides for 10 min in buffered formalin, they were stained with haematoxylin and eosin. The sections were graded by two blinded investigators with a range from 0 to 3 as to amount of inflammation (acute and chronic), depth of inflammation and with a range from 0 to 4 as to the amount of crypt damage or regeneration as indicated in Table 1. These changes were also quantified as to the percentage involvement by the disease process: (1) 1–25%; (2) 26–50%; (3) 51–75%; (4) 76–100%. Each section was then scored for each feature separately by establishing the product of the grade for that feature and the percentage involvement (in a range from 0 to 12 for inflammation and for extent, and in a range from 0 to 16 for regeneration and for crypt damage).
Table 1.
Histological grading of colitis

Immunohistochemistry
Colons and lymphoid organs were snap-frozen in liquid nitrogen. Cryostat sections of 8 μm were picked up on slides and air-dried. After fixating the slides for 10 min in pure acetone, a two-step immunoperoxidase staining was used. Slides were incubated horizontally for 60 min at room temperature with a solution of the first step MoAb in 0.01 m PBS pH 7.4, with 0.5% bovine serum albumin (BSA). The slides were washed three times in PBS and subsequently incubated with peroxidase-conjugated rabbit anti-rat serum, dilution 1:200 (Miles, Elkhart, IN) in PBS with 0.5% BSA and 1% normal mouse serum, for 30 min. After being rinsed in PBS (3 × 10 min), sections were stained for peroxidase activity with 3.3′-diaminobenzidine-tetra-hydrochloride (Sigma, St Louis, MO) in 0.5 mg/ml Tris–HCl pH 7.6 containing freshly added 0.01% H2O2 [4]. After the slides had been washed in PBS, they were lightly counterstained with haematoxylin, dehydrated and mounted in Entellan (Merck, Darmstadt, Germany). Control slides were incubated in PBS with 0.5% BSA in the first step, instead of the first specific antibody, and examined for non-specific staining. Staining procedures with MoAbs MOMA-2 which recognizes macrophages [5], MT-4 which recognizes CD4+ T cells [6], Lyt-2 marking CD8+ T cells [7] and B cell marker 6B2 [8] (all raised in our laboratory) were carried out on consecutive sections.
For the detection of IL-4, IL-5 and IFN-γ we used biotinylated MoAbs BVD6-24G2 [9], TRFK-4 [10] and MoAb R46A2 [9], respectively (kindly provided by Dr H. F. J. Savelkoul, Erasmus University, Rotterdam, The Netherlands). After fixation and rinsing with PBS the sections were incubated with biotinylated BVD6-24G2, TRFK-4 or R46A2, diluted in PBS containing 0.1% BSA (PBS–BSA) for 4 h at room temperature. Sections were then rinsed and incubated with streptavidin-coupled peroxidase (PO) diluted in PBS–BSA for 2 h. Histochemical revelation of PO activity using 3-amino-9-ethyl-carbazole (AEC; Sigma) as a substrate according to Claassen et al. [11] was performed. In this way cells containing the cytokine were stained red. Positive staining cells were counted in at least 5–10 representative areas per section of each tissue and they were expressed semiquantitatively.
Organ cultures
Full thickness biopsies from the colon of 3 mm diameter were obtained from the entire colon of control animals and DSS-treated mice at day 7 and day 21 post-induction using a dermal punch biopsy instrument as described before [3]. Tissue specimens were cultured at 37°C in 5% CO2, 95% O2 for 24 h in the presence or absence of 5 μg/ml concanavalin A (Con A). After 24 h the supernatants were harvested for bioassays. Prior to the bioassays, any residual Con A activity was inhibited by the addition of 20 mmα-methylmannoside.
Cytokine bioassays
IFN-γ was assayed using the murine WEHI 279 cell line [12]. The cells were incubated with samples for 48 h. Recombinant mouse IFN-γ was used to generate a standard curve and IFN-γ activity of the samples was interpolated. The activity was expressed as inhibition units in which a unit was defined as the IFN-γ concentration that resulted in inhibition equal to 50% of maximum.
Statistical analysis
All data are expressed as mean ± s.d. The statistical significance of the differences was evaluated using the non-parametric Wilcoxon rank sum test. Statistical significance was defined as P < 0.05.
RESULTS
Histopathology
Oral DSS administration for 7 days induced an acute colitis in SW mice. The histology of the colon at this time was characterized by multifocal dropouts of entire crypts in all parts of the colon and caecum compared with normal colon. In some animals this crypt damage was more severe in the proximal part of the colon, in others medial and distal portions were more affected. Especially in areas of focal lesions inflammatory cell infiltration was seen, including neutrophils and mononuclear cells. In some animals crypt abscesses were observed. The inflammation was mostly confined to mucosa, but in some areas extensive oedema of the submucosa was observed.
After stopping DSS for 2 weeks the colonic epithelium had only partly healed. Re-epithelialization usually started from the neighbouring epithelium as revealed by focal mitoses of neighbouring epithelial cells bridging over the lesions. The amount of regeneration varied from the presence of only a surface epithelium to (in)complete crypt formation, as indicated in the regeneration score (Fig. 1). In some parts of the colon, such as the proximal section, the lesions had almost completely healed, whereas in other parts, especially in the caecum and in the distal colon, regeneration of the epithelium was incomplete, both in depth and over a larger surface (Fig. 1). At this stage we also found large lymphoid aggregates located below the surface epithelium in the proximity of incompletely healed colonic lesions. Although these aggregates were also found in control mice, their size appeared greater at this stage.
Fig. 1.
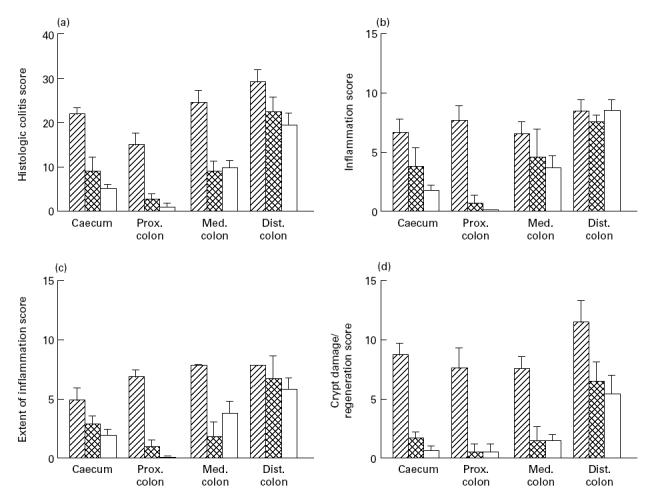
Histological grading of colitis. (a) Sum of the scores of b, c, and d. (b) Inflammation score. (c) Extent of inflammation. (d) Crypt damage/regeneration score, as indicated in Table 1. Hatched bars represent mice at day 7 of DSS feeding, cross-hatched bars represent mice killed 14 days after stopping DSS, open bars depict mice 35 days after stopping DSS.
Five weeks after stopping DSS epithelial restitution was almost complete and the majority of the crypts had regenerated. However, in some animals the colonic epithelium had not completely healed.
Distribution of lymphoid and non-lymphoid cells
During acute DSS-induced colitis the colonic mucosa and submucosa contained an increased number of MOMA-2+ macrophages and monocytes (Fig. 2A). Also, significantly more granulocytes were observed in the acute phase of DSS-induced colitis compared with controls. This increase was more prominent in the more proximal parts of the colon. The increase of granulocytes and macrophages was not accompanied by a comparable elevation of MT-4+ and 6B2+ lymphocytes in the colonic tissue at this stage (data not shown).
Fig. 2.

(A) DSS 7 days. Multiple MOMA-2+ macrophages in submocosal oedema. (B) Two weeks after stopping DSS, the submucosa still contains some MOMA-2+ macrophages. Large lymphoid aggregates in the lamina propria, consisting mainly of B cells (C), but also T cells in the periphery (D), are shown. (E) Two weeks after stopping DSS, below an incompletely regenerated epithelium the base of the lamina propria contains bands of MT4+ cells.
During the regenerative phase 2 weeks after stopping DSS the base of the lamina propria and submucosa below incompletely healed colonic lesions was still infiltrated with large numbers of mucosal MOMA-2+macrophages (Fig. 2B). The same area also contained focal accumulations of MT-4+T cells (Fig. 2E). We also observed large lymphoid aggregates in the damaged mucosa. These structures consisted mainly of B cells (Fig. 2C), while relatively few MT-4+cells were seen in the margins of these aggregates (Fig. 2D).
Cytokine pattern
Two weeks after stopping DSS administration the colonic mucosa of these animals showed focal staining of IFN-γ (Fig. 3A) as well as IL-4+cells at the base of the lamina propria (Fig. 3C). IL-4+ and IFN-γ+ cells were only present in areas of inflammation and regenerating crypt lesions and were absent in normal mucosa of the same animal. In contrast, we could not detect any IL-5 staining in the colons of these animals (Fig. 3B). In colonic tissue obtained from control mice staining for IFN-γ, IL-4 or IL-5 was virtually absent (data not shown).
Fig. 3.

Colonic tissue 2 weeks after stopping DSS (same area as in Fig. 2E). (A) IFN-γ-producing cells (arrows). (B) IL-5-producing cells are not present. (C) IL-4-producing cells at the base of the lamina propria.
Organ cultures from colonic tissue of DSS-treated animals confirmed the IFN-γ pattern during chronic DSS-induced colitis; there was a significant increase of IFN-γ production 2 weeks after stopping DSS, whereas we could not detect any IFN-γ production in organ cultures from colons during acute DSS-induced colitis or control animals (Fig. 4).
Fig. 4.

IFN-γ production in organ cultures from colons of indicated time points during DSS-induced colitis and control animals; solid bars represent healthy animals, hatched bars represent mice at day 7 of DSS feeding, cross-hatched bars represent mice at day 14 after stopping DSS. *Significantly different compared with healthy animals, P < 0.05.
Regarding cytokine-producing cells in the spleen of control and DSS-treated mice, no differences were found between healthy control mice and acute colitis mice after 7 days DSS. However, 14 days after stopping DSS a temporary rise in the number of IL-4-producing cells was found which was not present any more at day 35 after stopping DSS. A significant increase in IFN-γ+ cells and in IL-5-producing cells was found at day 35 after stopping DSS (Fig. 5).
Fig. 5.

Number of IFN-γ-, IL-5- and IL-4-positive cells in 5–10 representative areas per spleen section, expressed as number of cells/mm2; solid bars represent healthy animals, hatched bars show mice at day 7 of DSS feeding, cross-hatched bars represent mice at day 14 after stopping DSS, open bars indicate mice at day 35 after stopping DSS. *Significantly different compared with healthy controls, P < 0.05.
DISCUSSION
In the present study we show that 7 days DSS administration in drinking water of SW mice resulted in an acute inflammation of the colon, followed by a slow regeneration of the colonic epithelium with concomitant chronic inflammation after DSS was stopped.
The acute phase of DSS-induced colitis in SW mice is characterized by focal crypt lesions and secondary mucosal and submucosal inflammation extending into the colon and caecum, with granulocytes and macrophages in mucosa and submucosa. These findings are similar to acute DSS-induced colitis in BALB/c mice [1,3], in which mucosal production of macrophage-derived cytokines, such as tumour necrosis factor-alpha (TNF-α) and IL-6, was increased compared with animals that were not fed DSS [3]. However, in BALB/c mice the disease is mainly located in the distal colon. It seems therefore that strain differences can influence the induction phase of DSS colitis.
One cycle of DSS administration followed by drinking water resulted in extensive and relatively slow regeneration of the colonic epithelium after DSS injury. This regeneration was much slower than in acute injury models, which use toxic substances such as acetic acid [13,14], and ethanol [15], in which colonic healing is completed relatively rapidly after injury. The slow epithelial regeneration in this model may be due to DSS itself, which is toxic to the basal crypt cells, causing a complete crypt drop-out [2,3]. Epithelial regeneration will therefore have to start from the adjacent crypts bridging over sometimes large, focal defects, as shown in our results. Another explanation for slow regeneration is impaired phagocytosis by macrophages saturated with DSS [1,16]. Especially in the event of incomplete colonic healing, this would enable toxic bacterial products from the intestinal lumen to further perpetuate intestinal inflammation and delay epithelial regeneration.
Chronic immune activation can also impair epithelial regeneration. In the present and in a previous study [3] we showed that the acute phase of DSS-induced colitis was mediated by neutrophils and macrophages. The present study shows that during chronic phases at day 14 and day 35 lymphocytes were more prominent, as in areas of incomplete healing we observed large lymphoid aggregates. More strikingly, the basal part of the lamina propria contained an increased number of CD4+ T cells compared with control animals. This suggests that T cells could play an important role during the chronic, regenerative phase of DSS-induced colitis. In this way the chronic phase of DSS-induced colitis in SW mice resembles other induced models of chronic intestinal inflammation, such as the chronic phase of trinitro-benzene sulfonic acid (TNBS) colitis [17] and peptidoglycan-polysaccharide induced (PG/PS) enterocolitis [18], which are also T cell-mediated. Further evidence that chronic DSS-induced colitis is T cell-mediated is that cyclosporin A has been shown to suppress the production of IL-2, IFN-γ and IL-3 [19]. Murthy et al. [20] showed that intracolonic treatment with cyclosporin had a therapeutic effect during the chronic phase of DSS-induced colitis in SW mice.
Murine CD4+ (helper) T cells can be defined according to their pattern of cytokine secretion [21]. In the chronic phase of DSS colitis we found IFN-γ as well as IL-4 to be increased compared with colons of acute DSS-induced colitis or controls. In contrast, IL-5 staining was almost absent. The splenic cytokine pattern was not completely comparable to the cytokines in the colon, as in the spleen of chronic DSS-colitis mice IFN-γ, IL-5 and IL-4 showed a (partly temporary) increase. These data reveal the absence of a distinct Th1 cytokine pattern in the colonic mucosa and in the spleen during the chronic phase of DSS-induced colitis.
The Th1 versus Th2 cytokine profile has a profound influence on the chronicity and aggressiveness of inflammation and infection in mice [22] and almost certainly is an important determinant in chronic intestinal inflammation. Th1 lymphocytes appear to be selectively activated in CD [22,23] and during experimental models of chronic intestinal inflammation, such as PG/PS-induced chronic granulomatous enterocolitis [18], IL-10 knockout mice [24] and colitis in HLA-B27 transgenic rats [25]. Some IBD patients have a decreased mucosal IL-4 production [26] and in some experimental colitis models, such as in IL-2 knockout mice [27], IL-4 production is down-regulated. In contrast to CD, UC has no such typical Th1 cytokine pattern [23,28]. In DSS-induced colitis, a UC-like model, IL-4 secretion is up-regulated during its chronic phase. In IL-4-deficient mice the severity of acute DSS-induced colitis is reduced [29]. Recent reports also reveal increased IL-4 expression in early postoperative ileal recurrences in CD [30] as well as increased IL-4 levels in other experimental models, such as colitis developing in T cell receptor (TCR) α-chain-deficient mice [31]. These findings combined with our data suggest that IL-4 can also play an important proinflammatory role during DSS-induced colitis as well as in other models of UC-like chronic intestinal inflammation.
In conclusion, oral administration of DSS to SW mice causes a reproducible acute colitis, followed by a slow regeneration of the colonic epithelium with a concomitant chronic inflammation showing high mucosal IFN-γ and IL-4 levels. DSS-induced colitis is not a typical Th-1-like model and its chronicity could be due to slow regeneration after DSS damaged the colonic epithelial barrier followed by an aspecific immune activation in which both Th1 and Th2 cytokines play a role. Since impaired colonic regeneration can lead to chronic intestinal inflammation, the DSS model in SW mice can be useful to study colonic regeneration and thus contribute to unravelling the pathogenesis of human IBD.
References
- 1.Okayasu I, Hatakeyama S, Ohkusa T, et al. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology. 1990;98:694–702. doi: 10.1016/0016-5085(90)90290-h. [DOI] [PubMed] [Google Scholar]
- 2.Cooper H, Murthy SNS, Shah RS, et al. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest. 1993;69:238–49. [PubMed] [Google Scholar]
- 3.Dieleman LA, Ridwan BU, Tennyson GS, et al. Dextran sulfate sodium (DSS)-induced colitis occurs in severe combined immunodeficient (SCID) mice. Gastroenterology. 1994;107:1643–52. doi: 10.1016/0016-5085(94)90803-6. [DOI] [PubMed] [Google Scholar]
- 4.Graham RC, Karnovsky MC. The early stages of absorption of injected horseradish peroxidase in the proximal tubules of mouse kidney: ultrastructural cytochemistry by a new technique. J Histochem Cytochem. 1966;14:291–302. doi: 10.1177/14.4.291. [DOI] [PubMed] [Google Scholar]
- 5.Kraal G, Rep M, Janse M. Macrophages in T and B cell compartments and other tissue macrophages recognized by monoclonal antibody MOMA-2. Scan J Immunol. 1987;26:653–61. doi: 10.1111/j.1365-3083.1987.tb02301.x. [DOI] [PubMed] [Google Scholar]
- 6.Pierres A, Naquet P, van Agthoven A, et al. A rat anti-mouse T4 monoclonal antibody H129-19 inhibits proliferation of Ia-reactive T cell clones and delineates two phenotypically distinct (T4+,Lyt-2,3− and T4−/Lyt2,3+) subsets among anti-Ia cytotoxic T cell clones. J Immunol. 1984;132:2775–82. [PubMed] [Google Scholar]
- 7.Ledbetter JA, Herzenberg LA. Xenogenic monoclonal antibodies to mouse lymphoid differentiation antigens. Immunol Rev. 1979;47:63–90. doi: 10.1111/j.1600-065x.1979.tb00289.x. [DOI] [PubMed] [Google Scholar]
- 8.Coffman RL. Surface antigen expression and immunoglobulin gene rearrangement during mouse pre-B cell development. Immunol Rev. 1985;69:5–23. doi: 10.1111/j.1600-065x.1983.tb00446.x. [DOI] [PubMed] [Google Scholar]
- 9.Sander B, Hoiden I, Andersson U, et al. Similar frequencies and kinetics of cytokine producing cells in murine peripheral blood and sera. J Immunol Methods. 1993;166:201–14. doi: 10.1016/0022-1759(93)90361-a. [DOI] [PubMed] [Google Scholar]
- 10.Schumacher JH, O'Garra A, Schrader B, et al. The characterization of four monoclonal antibodies specific for mouse IL-5 and development of mouse and human IL-5 ELISA. J Immunol. 1988;141:1576–81. [PubMed] [Google Scholar]
- 11.Claassen E, Boorsma DM, Kors N, et al. Double enzyme conjugates producing an intermediate color for simultaneous and direct detection of three different intracellular immunoglobulin determinants with only two enzymes. J Histochem Cytochem. 1986;34:423–6. doi: 10.1177/34.4.2419394. [DOI] [PubMed] [Google Scholar]
- 12.Reynolds DS, Boom WH, Abbas AK. Inhibition of B lymphocyte activation by interferon gamma. J Immunol. 1987;139:767–73. [PubMed] [Google Scholar]
- 13.Dieleman LA, Elson CO, Tennyson GS, et al. Kinetics of cytokine expression during healing of acute colitis in mice. Am J Physiol. 1996;271:G130–6. doi: 10.1152/ajpgi.1996.271.1.G130. [DOI] [PubMed] [Google Scholar]
- 14.Yamada T, Marshall S, Specian RD, et al. A comparative analysis of two models of colitis in rat. Gastroenterology. 1992;102:1524–34. doi: 10.1016/0016-5085(92)91710-l. [DOI] [PubMed] [Google Scholar]
- 15.Wallace JL, Whittle BJR, Boughton-Smith NK. Prostaglandin protection of rat colonic mucosa from damage induced by ethanol. Dig Dis Sci. 1985;30:866–76. doi: 10.1007/BF01309518. [DOI] [PubMed] [Google Scholar]
- 16.Ohkusa T, Okayasu I, Tokoi S, et al. Changes in bacterial phagocytosis of macrophages in experimental ulcerative colitis. Digestion. 1995;56:159–64. doi: 10.1159/000201236. [DOI] [PubMed] [Google Scholar]
- 17.Palmen Mjhj, Wijburg OLC, Kunst IH, et al. CD4+ T cells from 2,4,6-trinitrobenzene sulfonic acid (TNBS)-induced colitis rodents migrate to the recipient's colon upon transfer; downregulation by CD8+ T cells. Clin Exp Immunol. 1998;112:216–25. doi: 10.1046/j.1365-2249.1998.00564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sartor RB, Bender DE, Allen JB, et al. Chronic experimental enterocolitis and extraintestinal inflammation are T lymphocyte dependent. Gastroenterology. 1993;104:A7. [Google Scholar]
- 19.Faulds P, Goa KL, Benfield P. Cyclosporin: a review of its pharmacodynamic and pharmacokinetic properties, and therapeutic use in immunoregulatory disorders. Drugs. 1993;45:953–1040. doi: 10.2165/00003495-199345060-00007. [DOI] [PubMed] [Google Scholar]
- 20.Murthy SN, Cooper HS, Shin H, et al. Treatment of dextran sulfate sodium-induced murine colitis by intracolonic cyclosporin. Dig Dis Sci. 1993;38:1722–34. doi: 10.1007/BF01303184. [DOI] [PubMed] [Google Scholar]
- 21.Mosmann TR, Coffman RL. Heterogeneity of cytokine secretion patterns and functions of helper T cells. Adv Immunol. 1989;46:111–48. doi: 10.1016/s0065-2776(08)60652-5. [DOI] [PubMed] [Google Scholar]
- 22.Sartor RB. Viewpoints on digestive diseases. Cytokines in intestinal inflammation: pathophysiological and clinical considerations. Gastroenterology. 1994;106:533–9. doi: 10.1016/0016-5085(94)90614-9. [DOI] [PubMed] [Google Scholar]
- 23.Mullin G, Lazenby AJ, Harris ML, et al. Increased interleukin-2 messenger RNA in the intestinal mucosal lesions of Crohn's disease but not ulcerative colitis. Gastroenterology. 1992;102:1620–7. doi: 10.1016/0016-5085(92)91722-g. [DOI] [PubMed] [Google Scholar]
- 24.Berg DJ, Davidson N, Kuhn R, et al. Enterocolitis and colon cancer in interleukin-10 deficient mice are associated with aberrant cytokine production and CD4+ Th1-like responses. J Clin Invest. 1996;98:1010–20. doi: 10.1172/JCI118861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Breban M, Hammer RE, Richardson JA, et al. Transfer of the inflammatory disease of HLA-B27 transgenic rats by bone marrow engraftment. J Exp Med. 1993;178:1607–16. doi: 10.1084/jem.178.5.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karttunnnen R, Breese EJ, Walker-Smith JA, et al. Decreased mucosal interleukin-4 (IL-4) production in gut inflammation. J Clin Pathol. 1994;47:1015–8. doi: 10.1136/jcp.47.11.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McDonald SAC, Palmen Mjhj, Van Rees EP, et al. Characterization of the cell-mediated immune response in IL-2 knock-out mice before and after the onset of colitis. Immunology. 1997;91:73–80. doi: 10.1046/j.1365-2567.1997.00217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mullin GE, Maycon ZR, Braun-Elwert L, et al. Inflammatory bowel disease mucosal biopsies have specialized lymphokine mRNA profiles. Inflammatory Bowel Dis. 1996;2:16–26. [PubMed] [Google Scholar]
- 29.Stevcea L, Pavli P, Doe WF, et al. The severity of dextran sulphate-induced colitis is significantly reduced in interleukin-4 deficient mice. Gastroenterology. 1996;110:A1019. [Google Scholar]
- 30.Desreumaux P, Brandt E, Gambiez L, et al. Distinct cytokine pattern in early and chronic ileal lesions of Crohn's disease. Gastroenterology. 1997;112:118–26. doi: 10.1016/s0016-5085(97)70116-1. [DOI] [PubMed] [Google Scholar]
- 31.Takahashi I, Kiyono H, Hamada S. CD4+ T-cell population mediates development of inflammatory bowel disease in T-cell receptor α-chain-deficient mice. Gastroenterology. 1997;112:1876–86. doi: 10.1053/gast.1997.v112.pm9178680. [DOI] [PubMed] [Google Scholar]


