Abstract
Type 1 diabetes is associated with autoimmunity to insulin. Genetic susceptibility to type 1 diabetes is polygenic and includes the INS VNTR-IDDM2 locus which may regulate the expression of insulin in pancreas and thymus. In order to determine whether insulin autoimmunity could be attributed to a genetic susceptibility conferred by the INS VNTR-IDDM2 locus, peripheral blood T cell proliferation to human insulin and insulin autoantibodies (IAA) was measured in patients with new onset type 1 diabetes and control subjects. IAA were detected in 21 of 53 patients and in none of 25 control subjects, while T cell responses were low (stimulation index range 0.4–7.2) and similar in both groups. Both antibody and T cell responses were higher in younger subjects and IAA were more prevalent in patients with the HLA-DR4 allele. No relationship was observed between humoral and cellular responses to insulin. No association was found between the INS VNTR-IDDM2-susceptible allele and insulin autoimmunity. Increased T cell responses and IAA were found in patients with either the diabetes-susceptible or the diabetes-protective INS VNTR-IDDM2 locus genotypes, and increased T cell responses were also found in control subjects with either susceptible or protective INS VNTR-IDDM2 locus genotypes. This study confirms that primary T cell proliferative responses to insulin are low and detectable also in control subjects. The detection of T cell proliferation and autoantibodies to insulin in subjects with and without the protective INS VNTR-IDDM2 locus genotypes does not support the hypothesis of an allele-specific capacity for tolerance induction which could determine a susceptibility to develop autoimmunity against the insulin protein and subsequently diabetes.
Keywords: type 1 diabetes, insulin, cell-mediated immunity, autoantibody, genetics
INTRODUCTION
Type 1 diabetes is characterized by humoral [1] and cellular [2] immune responses to pancreatic islet proteins. These immune responses are important markers of the immune process preceding disease onset [3] and have been used to identify individuals with preclinical disease, in whom intervention therapy may prevent or delay progression to disease onset [4].
Several islet autoantigen targets have been identified. One of these, insulin, is specifically expressed within pancreatic islets by the β cells. A humoral immune response to insulin is detected in the majority of patients with childhood disease [4,5]. Cell-mediated responses to insulin in peripheral blood are weak [6–13], but insulin-specific T cell clones isolated from animal models of diabetes have been shown to transfer disease [14]. Moreover, treatments with intravenous, subcutaneous, oral or intranasal insulin have been shown to prevent or delay disease onset in animals and in humans [15–19]. Thus insulin, or its prohormone proinsulin, is considered an important early autoimmune target in type 1 diabetes, and has therefore a potential role in antigen-specific immunotherapy [20].
The aberrant autoimmune response to insulin and other islet autoantigens associated with type 1 diabetes occurs very early in life [21]. These immune responses, and particularly those against insulin, are influenced by the IDDM1 HLA class II loci [22]. Recently it was suggested that the INS VNTR-IDDM2 locus may also influence the autoimmune response. The INS VNTR-IDDM2 locus contains a polymorphic region 5′ to the insulin gene coding sequence, and it has been shown that this region has linkage to type 1 diabetes [23,24]. Moreover, it has recently been reported that insulin is expressed in fetal thymus, and that the level of expression is higher in subjects with INS VNTR-IDDM2 genotypes associated with type 1 diabetes protection than in those with the susceptible genotype [25,26]. These studies have proposed a mechanism of an allele-specific capacity for tolerance induction which could determine a susceptibility to develop autoimmunity against the insulin protein and subsequently type 1 diabetes. If this hypothesis is correct, then it would be expected that autoimmune responses to insulin would be associated with the type 1 diabetes-susceptible INS VNTR-IDDM2 locus genotype. The aim of this study was to determine whether we could provide evidence for this hypothesis through the measurement of humoral and cellular responses to insulin in the peripheral circulation of new onset diabetic patients and control subjects.
PATIENTS AND METHODS
Patients and control subjects
The study was carried out on 54 newly diagnosed diabetic patients (median age 12 years, range 1–32 years; 36 males) and 27 healthy control subjects (median age 27 years, range 12–46 years; 16 males). Thirty-nine patients and nine control subjects were aged ≤ 15 years. Thirty-five patients and 27 control subjects were tested for T cell proliferation, 53 patients and 25 control subjects were tested for insulin autoantibodies (IAA), 50 patients and 26 control subjects were tested for INS VNTR-IDDM2 alleles. Patients were tested for T cell proliferation within 10 days from the beginning of insulin therapy. All control subjects were negative for islet cell antibodies (ICA) and IAA and had no family history of type 1 diabetes. Experimental protocols were approved by the local Ethical Committee.
Antigens
For T cell proliferation studies, human semi-synthetic crystallized Zn-insulin (Novo-Nordisk, Baegsverd, Denmark) was used at four concentrations (0.04, 0.4, 4 and 40 μm, corresponding to 0.23, 2.3, 23 and 230 μg/ml, respectively) which lie in the middle of the dose ranges used in previous studies (0.05–500 μg/ml). Tetanus toxoid without thiomersal (Connaught, Ontario, Canada) was used as a control antigen at a final concentration of 10 μg/ml. For autoantibody measurements, human recombinant (3-125iodotyrosylA14) insulin (2000 Ci/mmol, Amersham, Aylesbury, UK) was used.
T cell proliferation assay
Mononuclear cells were isolated from heparinized peripheral blood by Ficoll gradient centrifugation and depleted of CD8+ cells using Dynabeads M450 CD8 magnetic beads (Dynal, Oslo, Norway) according to the manufacturer's instructions. Cells were resuspended at a concentration of 1.5 × 106/ml in RPMI 1640 + 25 mm HEPES (Biowhittaker, Walkersville, MD) supplemented with 10% pooled human serum (PAA Labor- und Forschungsgesellschaft, Linz, Austria), 2 mm glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin (Biowhittaker) and 2 × 10−5 m 2β-mercaptoethanol (Sigma, St Louis, MO). A proliferation assay was carried out using 150 000 cells (100 μl) in triplicate wells incubated for 6 days in the presence or absence of increasing concentrations of Zn-human insulin (0.04, 0.4, 4, 40 μm) or tetanus toxoid (10 μg/ml) in a final volume of 150 μl. 3H-thymidine (1 μCi/well; Amersham) incorporation in the last 18 h was measured by liquid scintillation after automatic cell harvesting. Results were expressed as a stimulation index (SI = median ct/min of triplicate wells containing cells + antigen/median ct/min of 24 wells containing cells + medium alone).
Autoantibody measurements
All autoantibody determinations were performed on serum aliquots drawn on the same day of the proliferation test and stored at − 20°C. IAA were determined using a method based on the micro radio binding assay (RBA) of Williams et al. [27]. This method was modified by precipitating immune complexes with 50 μl of 50 mm Tris–HCl pH 8 with 1% v/v Tween 20, 10% v/v pre-swollen protein A-Sepharose (Pharmacia Biotech AB, Uppsala, Sweden) and 5% v/v pre-swollen protein G-Sepharose (Pharmacia Biotech AB). IAA results were converted into arbitrary units by use of a standard curve. The threshold and 99th centile of local control subjects was calculated at > 4.4 IAA units [28]. ICA in control subjects were measured in undiluted sera by indirect immunofluorescence on cryostat sections of blood group O human pancreas as previously described [29].
HLA typing
Typing for the HLA DRB1*03 and DRB1*04 alleles was carried out on DNA extracted from peripheral blood mononuclear cells (PBMC) and stored at − 20°C using polymerase chain reaction with sequence-specific primers (PCR-SSP) based on the method of Olerup et al. [30] with primer sets E (DR3) and G (DR4) as described by Bein et al. [31]. Subjects were typed as DR3/X, DR4/Y, DR3/4 or DRX/Y, where X is an allele other than DRB1*04 and Y is an allele other than DRB1*03.
INS VNTR- IDDM2 alleles
Subjects were typed as having INS VNTR class I alleles and/or INS VNTR class III alleles using HphI digestion of PCR amplification products of the region of interest, as previously described [24]. Subjects were divided into two groups: those with the type 1 diabetes-susceptible HphI +/+ class I homozygous genotype and those with the protective HphI −/− class III homozygous or HphI +/− class I/class III heterozygous genotypes, according to the previously reported association of the HphI +/+ class I homozygous genotype with type 1 diabetes [24].
Statistical analysis
For T cell responses, all comparisons of distribution between groups were made using the Mann–Whitney U-test. Antibody prevalences were compared using Fisher's test, and the distribution of INS VNTR-IDDM2 locus alleles in patients and control subjects using χ2 test for trend.
RESULTS
Cell-mediated responses
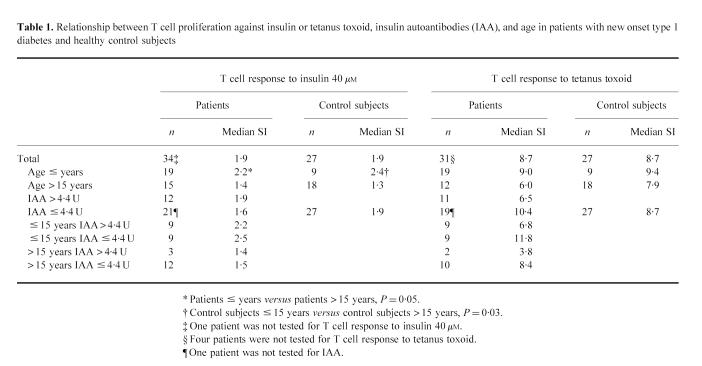
Responses to Zn-insulin were low and no significant differences between patients with type 1 diabetes (SI range 0.4–7.2) and control subjects (SI range 0.5–4.7) were observed for all concentrations (Fig. 1). Patients aged ≤ 15 years showed higher proliferative responses to 40 μm Zn-insulin (median SI 2.2) than those aged > 15 years (median SI 1.4, P = 0.05). An age-related response to the highest insulin dose was also seen in control subjects (median SI 2.4 in subjects ≤ 15 years versus 1.3 in those > 15 years, P = 0.03), and no differences were observed in the proliferative response to 40 μm Zn-insulin between patients aged ≤ 15 years and age-matched control subjects. All patients and all but one control subject responded to tetanus toxoid, with no differences between groups (median SI 8.7 in patients versus 8.7 in control subjects, P = NS), and no relationship with age (Table 1).
Fig. 1.
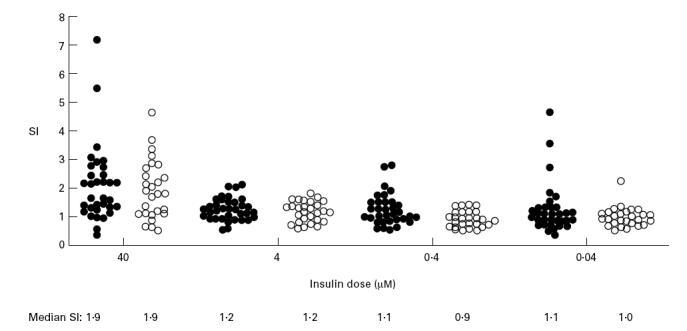
CD8+ depleted T cell responses to Zn-insulin. •, Patients with new onset type 1 diabetes, n = 35; ○, control subjects, n = 27.
Table 1.
Relationship between T cell proliferation against insulin or tetanus toxoid, insulin autoantibodies (IAA), and age in patients with new onset type 1 diabetes and healthy control subjects
Humoral responses
Elevated levels of IAA were detected in 21 (40%) of 53 patients and no control subjects (P = 0.0001). All patients with IAA were aged ≤ 20 years and 18 were aged ≤ 15 years.
Relationship between humoral and cell-mediated responses
No relationship was observed between humoral and cellular responses against insulin. Patients with elevated IAA levels showed T cell responses to 40 μm Zn-insulin (median SI 1.9) similar to patients without IAA (median SI 1.6, P = NS) and control subjects (median SI 1.9, P = NS). Moreover, no differences between patients with and without IAA were observed when patients were subdivided by age. No differences in T cell reactivity against tetanus toxoid were observed between patients with and without increased IAA (median SI 6.5 versus 10.4, P = NS) (Table 1).
Relationship between humoral and cell-mediated responses to insulin and HLA-DR or INS VNTR- IDDM2 locus alleles
HLA typing was obtained in 49 patients. Of the 19 patients with IAA in whom HLA-DR typing was available, 17 (89%) had the HLA-DR4 allele, compared with 13 (45%) of 29 without IAA (P = 0.002). No differences in cellular responses were observed between HLA-DR4 or non-DR4 patients or control subjects, and elevated responses were observed in patients with each of these alleles (Table 2). A decreased response to tetanus toxoid was observed in patients with the HLA-DR3 allele (P = 0.006). No other differences with respect to HLA-DR were observed.
Table 2.
Cell-mediated and humoral responses to insulin and INS VNTR-IDDM2 locus alleles in patients with new onset type 1 diabetes
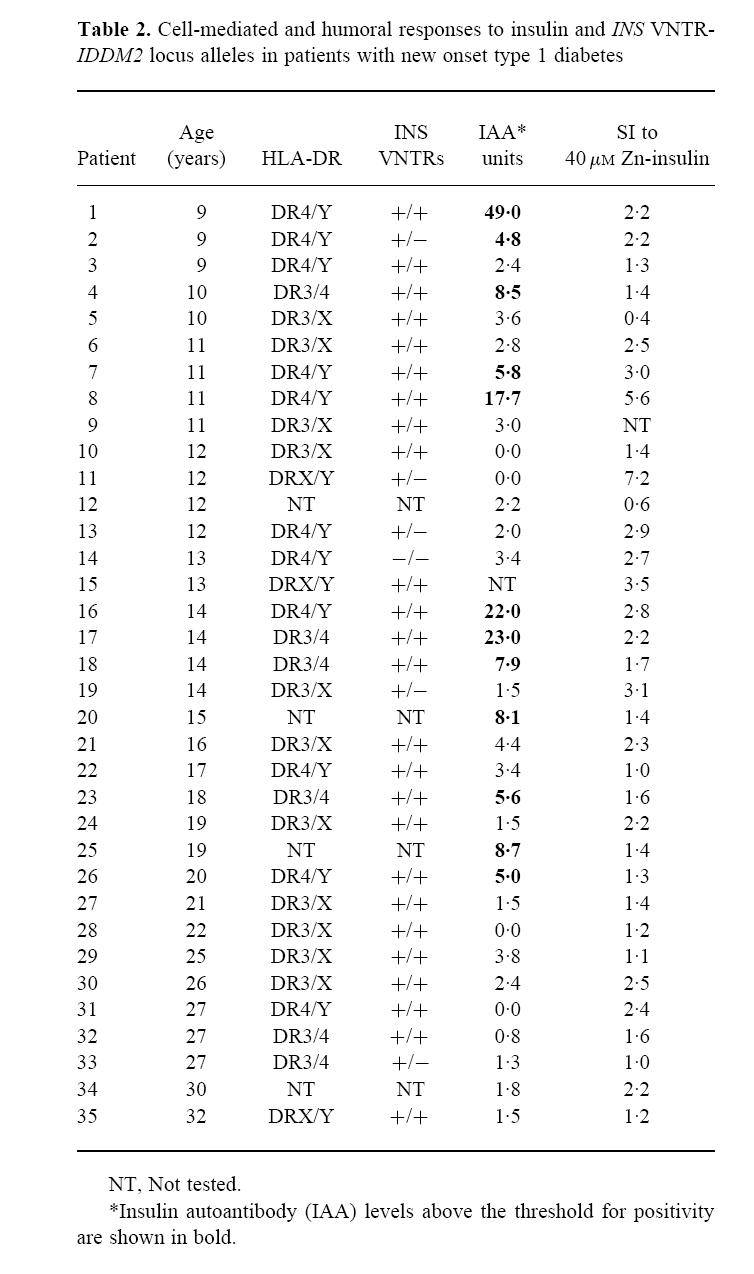
The type 1 diabetes-susceptible HphI +/+ homozygous INS VNTR-IDDM2 genotype was found in 39 (78%) of 50 patients and in 15 (58%) of 26 control subjects. Ten patients and nine control subjects had the HphI +/− heterozygous genotype, and one patient and two control subjects had the HphI −/− homozygous genotype (P = 0.05). No relationship between T cell proliferation against insulin and INS VNTR-IDDM2 alleles was observed. Elevated T cell proliferative responses to insulin were found in patients and control subjects who had either the diabetes-susceptible or diabetes-protective INS VNTR-IDDM2 genotypes. In particular, the highest proliferative response was seen in a patient with the protective HphI +/− genotype (Table 2), and similarly, the highest response found in control subjects was in an individual with the protective HphI +/− genotype (data not shown). Of the 21 patients with IAA, 16 had the susceptible INS VNTR-IDDM2 genotype, three a protective INS VNTR-IDDM2 genotype and in two IDDM2 typing was unavailable. Several patients with the susceptible INS VNTR-IDDM2 genotype did not have IAA, and no significant differences in IAA prevalences or levels were observed between those with and without the susceptible INS VNTR-IDDM2 genotype.
DISCUSSION
Insulin is an early target of humoral and cell-mediated autoimmunity in type 1 diabetes. Previous studies have shown that the antibody response is most pronounced in young patients, and occurs several years prior to diabetes onset [3,5]. Cell-mediated responses have also been detected in peripheral blood from patients at and prior to onset of disease [6–13], but appear less specific than antibody responses, as they were also found in control subjects in most of these studies. In our study we observed that proliferative T lymphocyte responses to insulin are low, and are similar in both diabetic patients and control subjects, in accordance with some [9,11,13] but not all previous studies [6–8,10,12].
The detection of proliferation to autoantigens in control subjects is not unique to insulin or type 1 diabetes [32]. This suggests that the presence of circulating T cells autoreactive to insulin and other autoantigens is not specific to disease but is a common occurrence. The detection of T cell responses to insulin in both young new onset type 1 diabetic patients and control subjects, and of IAA only in patients suggests that the measurement of autoantibodies will be more useful for disease prediction.
We found that cell-mediated responses are more pronounced in young subjects, confirming also for cellular responses to insulin the age relationship previously reported for autoantibodies against the hormone [4,5]. This age relationship was not found for the recall antigen tetanus toxoid, and suggests that sensitization to insulin is limited to childhood. Such sensitization is intriguing since exposure to insulin in breast milk [33] or in the peripheral circulation is ongoing from very early in life. Pugliese et al. [25] and Vafiadis et al. [26] speculated that sensitization to insulin may result early in life from ineffective tolerance induction by the decreased expression of insulin in the thymic epithelium of individuals with the type 1 diabetes-susceptible IDDM2 class I alleles. Thus, autoimmune responses to insulin would probably be found in both diabetic patients and control subjects having the HphI +/+ class I homozygous INS VNTR genotype. We could not, however, provide evidence for an association of PBMC proliferative responses to insulin with the INS VNTR-IDDM2 locus alleles. Proliferation was seen in patients and control subjects with either susceptible or protective genotypes. Furthermore, although in patients autoantibodies to insulin were mostly found in those with the susceptible class I homozygous INS VNTR-IDDM2 genotype, such a genotype was also present in a large number of patients without detectable IAA, and there were no significant differences in IAA prevalences or levels between patients with and without the susceptible genotype. Based upon the numbers analysed and genotype frequencies observed, this study has 50% power, with α = 0.05, to detect a 33% difference in IAA prevalence between patients with different genotypes. It should be considered therefore that weaker relationships between IAA and the susceptible class I homozygous INS VNTR-IDDM2 genotype may be missed.
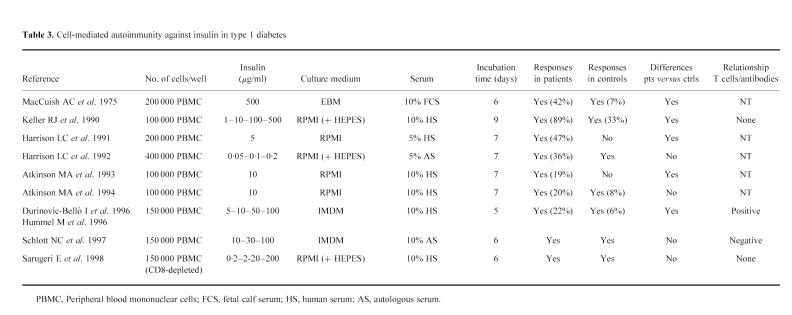
Another aspect of this study is the relationship between cell-mediated and humoral autoimmunity to insulin. Previous studies of responses to the islet autoantigen glutamic acid decarboxylase (GAD) and the putative antigen ICA69 have shown inverse relationships between peripheral blood T cell stimulation indices to antigen and autoantibody levels and have proposed that this may reflect a balance in TH1 and TH2 immunity to individual autoantigens [34,35]. Moreover, for GAD it was proposed that detection of T cell reactivity may be a stronger predictor of progression to disease than detection of autoantibodies [34]. This is surprising in view of the detection of GAD autoantibodies in most patients at and prior to disease onset. Also in the case of T cell reactivity to insulin, an inverse relationship with autoantibodies has been described [13,36], although others showed either no relation [7] or indeed higher T cell stimulation indices to insulin in patients with IAA [12,37]. In the current study we found no relationship between cellular and humoral responses against insulin, in that no differences in cellular responses to the hormone were observed between patients with or without increased levels of IAA. The contrasting relationships between T cell responses and IAA observed in these studies suggest marked differences in the ability of the assays to measure antigen-induced T cell proliferation. There are several methodological differences between assays, and the general heterogeneity of results for T cell proliferation against islet autoantigens probably reflects the lack of standardization and difficulty of interpretation of assays measuring T cell proliferation against autoantigens (Table 3). A potentially important difference between the methods in these studies is the use of autologous serum in the study by Schloot et al. [13] versus IAA-negative pooled human serum in our study and those by Durinovic-Bellòet al. [12] and by Hummel et al. [37]. The effect of the presence of antibodies reactive with test antigen in the T cell culture is variable [38–40], but may alter proliferation in younger patients, in whom IAA are more prevalent. Other factors, such as metabolic control, may also affect proliferative responses in patients.
Table 3.
Cell-mediated autoimmunity against insulin in type 1 diabetes
In conclusion, our study confirms the ability to detect a cell-mediated response to insulin in the peripheral blood of both patients with type 1 diabetes and control subjects. We show that this response is more prevalent in young subjects but is not related to IAA, and can be found in patients and control subjects regardless of their INS VNTR-IDDM2 locus alleles.
Acknowledgments
We thank Maria Pia Protti for her help and advice in the measurement of T cell proliferative responses and Vito Lampasona for his technical advice in INS VNTR-IDDM2 typing.
References
- 1.Bonifacio E, Bingley PJ. Islet autoantibodies and their use in predicting insulin-dependent diabetes. Acta Diabetol. 1997;34:185–93. doi: 10.1007/s005920050072. [DOI] [PubMed] [Google Scholar]
- 2.Roep B. T-cell responses to autoantigens in IDDM. Diabetes. 1996;45:1147–56. doi: 10.2337/diab.45.9.1147. [DOI] [PubMed] [Google Scholar]
- 3.Gorsuch AN, Spencer KM, Lister J, McNally JM, Dean BM, Bottazzo GF, Cudworth AG. Evidence for a long pre diabetic period in type 1 (insulin-dependent) diabetes mellitus. Lancet. 1981;2:1363–5. doi: 10.1016/s0140-6736(81)92795-1. [DOI] [PubMed] [Google Scholar]
- 4.Roll U, Ziegler AG. Combined antibody screening for improved prediction of IDDM—modern strategies. Exp Clin Endocrinol Diabetes. 1997;105:1–14. doi: 10.1055/s-0029-1211721. [DOI] [PubMed] [Google Scholar]
- 5.Vardi P, Ziegler AG, Mathews JH, et al. Concentration of insulin autoantibodies at onset of type 1 diabetes—inverse log-linear correlation with age. Diabetes Care. 1988;11:736–9. doi: 10.2337/diacare.11.9.736. [DOI] [PubMed] [Google Scholar]
- 6.MacCuish AC, Jordan J, Campbell CJ, Duncan LPJ, Irvine WJ. Cell-mediated immunity in diabetes mellitus. Lymphocyte transformation by insulin and insulin fragments in insulin-treated and newly-diagnosed diabetics. Diabetes. 1975;24:36–43. doi: 10.2337/diab.24.1.36. [DOI] [PubMed] [Google Scholar]
- 7.Keller RJ. Cellular immunity to human insulin in individuals at high risk for the development of type 1 diabetes mellitus. J Autoimmun. 1990;3:321–7. doi: 10.1016/0896-8411(90)90150-q. [DOI] [PubMed] [Google Scholar]
- 8.Harrison LC, DeAizpurua H, Loudovaris T, Campbell IL, Cebon JS, Tait BD, Colman PG. Reactivity to human islets and fetal pig proislets by peripheral blood mononuclear cells from subjects with preclinical and clinical insulin-dependent diabetes. Diabetes. 1991;40:1128–33. doi: 10.2337/diab.40.9.1128. [DOI] [PubMed] [Google Scholar]
- 9.Harrison LC, Chu SX, DeAizpurua HJ, Graham M, Honeyman MC, Colman PG. Islet-reactive T cells are a marker of preclinical insulin-dependent diabetes. J Clin Invest. 1992;89:1161–5. doi: 10.1172/JCI115698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Atkinson MA, Bowman MA, Kao KJ, Campbell L, Dush PJ, Shah SC, Simell O, Maclaren NK. Lack of immune responsiveness to bovine serum albumin in insulin-dependent diabetes. N Engl J Med. 1993;329:1853–8. doi: 10.1056/NEJM199312163292505. [DOI] [PubMed] [Google Scholar]
- 11.Atkinson MA, Bowman MA, Campbell L, Darrow BL, Kaufman DL, Maclaren NK. Cellular immunity to a determinant common to glutamate decarboxylase and coxsackie virus in insulin-dependent diabetes. J Clin Invest. 1994;94:2125–9. doi: 10.1172/JCI117567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Durinovic-Bellò I, Hummel M, Ziegler AG. Cellular immune response to diverse islet cell antigens in IDDM. Diabetes. 1996;45:795–800. doi: 10.2337/diab.45.6.795. [DOI] [PubMed] [Google Scholar]
- 13.Schloot NC, Roep BO, Wegmann D, Yu L, Chase HP, Wang T, Eisenbarth GS. Altered immune response to insulin in newly diagnosed compared to insulin-treated diabetic patients and healthy control subjects. Diabetologia. 1997;40:564–72. doi: 10.1007/s001250050716. [DOI] [PubMed] [Google Scholar]
- 14.Daniel D, Gill RG, Schloot N, Wegmann D. Epitope specificity, cytokine production profile and diabetogenic activity of insulin-specific T cell clones isolated from NOD mice. Eur J Immunol. 1995;25:1056–62. doi: 10.1002/eji.1830250430. [DOI] [PubMed] [Google Scholar]
- 15.Atkinson MA, Maclaren NK, Luchetta R. Insulitis and diabetes in NOD mice reduced by prophylactic insulin therapy. Diabetes. 1990;39:933–7. doi: 10.2337/diab.39.8.933. [DOI] [PubMed] [Google Scholar]
- 16.Thivolet CH, Goillot E, Bedossa P, Durand A, Bonnard M, Orgiazzi J. Insulin prevents adoptive cell transfer of diabetes in the autoimmune non-obese diabetic mouse. Diabetologia. 1991;34:314–9. doi: 10.1007/BF00405002. [DOI] [PubMed] [Google Scholar]
- 17.Zhang ZJ, Davidson L, Eisenbarth G, Weiner HL. Suppression of diabetes in nonobese diabetic mice by oral administration of porcine insulin. Proc Natl Acad Sci USA. 1991;88:10252–6. doi: 10.1073/pnas.88.22.10252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harrison LC, Dempsey-Collier M, Kramer DR, Takahashi K. Aerosol insulin induces regulatory CD8 γδ T cells that prevent murine insulin-dependent diabetes. J Exp Med. 1996;184:2167–74. doi: 10.1084/jem.184.6.2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kobayashi T, Nakanishi K, Murase T, Kosaka K. Small doses of subcutaneous insulin as a strategy for preventing slowly progressive β-cell failure in islet cell antibody-positive patients with clinical features of NIDDM. Diabetes. 1996;45:622–6. doi: 10.2337/diab.45.5.622. [DOI] [PubMed] [Google Scholar]
- 20.Harrison LC. Antigen-specific therapy for autoimmune disease: prospects for the prevention of insulin-dependent diabetes. Mol Med. 1995;1:722–7. [PMC free article] [PubMed] [Google Scholar]
- 21.Roll U, Christie MR, Füchtenbusch M, Payton MA, Hawkes CJ, Ziegler AG. Perinatal autoimmunity in offspring of diabetic parents. The German multicenter BABY-DIAB study: detection of humoral immune responses to islet antigens in early childhood. Diabetes. 1996;45:967–73. doi: 10.2337/diab.45.7.967. [DOI] [PubMed] [Google Scholar]
- 22.Ziegler AG, Standl E, Albert E, Mehnert H. HLA-associated insulin autoantibody formation in newly diagnosed type 1 diabetic patients. Diabetes. 1991;40:1146–9. doi: 10.2337/diab.40.9.1146. [DOI] [PubMed] [Google Scholar]
- 23.Bell GI, Horita S, Karam JH. A polymorphic locus near the human insulin gene is associated with insulin-dependent diabetes mellitus. Diabetes. 1984;33:176–83. doi: 10.2337/diab.33.2.176. [DOI] [PubMed] [Google Scholar]
- 24.Bennett ST, Lucassen AM, Gough SCL, et al. Susceptibility to human type 1 diabetes at IDDM2 is determined by tandem repeat variation at the insulin gene minisatellite locus. Nature Genet. 1995;9:284–92. doi: 10.1038/ng0395-284. [DOI] [PubMed] [Google Scholar]
- 25.Pugliese A, Zeller M, Fernandez A, et al. The insulin gene is transcribed in the human thymus and transcription levels correlate with allelic variation at the INS VNTR-IDDM2 susceptibility locus for type 1 diabetes. Nature Genet. 1997;15:293–7. doi: 10.1038/ng0397-293. [DOI] [PubMed] [Google Scholar]
- 26.Vafiadis P, Bennett ST, Todd JA, et al. Insulin expression in human thymus is modulated by INS VNTR alleles at the IDDM2 locus. Nature Genet. 1997;15:289–92. doi: 10.1038/ng0397-289. [DOI] [PubMed] [Google Scholar]
- 27.Williams AJK, Bingley PJ, Bonifacio E, Palmer JP, Gale EAM. A novel micro-assay for insulin-autoantibodies. J Autoimmun. 1997;10:473–8. doi: 10.1006/jaut.1997.0154. [DOI] [PubMed] [Google Scholar]
- 28.Naserke HE, Dozio N, Ziegler AG, Bonifacio E. Comparison of a novel micro-assay for insulin autoantibodies with the conventional radiobinding assay. Diabetologia. 1998;41:681–3. doi: 10.1007/s001250050968. [DOI] [PubMed] [Google Scholar]
- 29.Bonifacio E, Bingley PJ, Shattock M, Dean BM, Dunger D, Gale EAM, Bottazzo GF. Quantification of islet-cell antibodies and prediction of insulin-dependent diabetes. Lancet. 1990;335:147–9. doi: 10.1016/0140-6736(90)90013-u. [DOI] [PubMed] [Google Scholar]
- 30.Olerup O, Zetterquist H. HLA-DR typing by PCR amplification with sequence specific primers (PCR-SSP) in 2 hours: an alternative to serological DR typing in clinical practice including donor-recipient matching in cadaveric transplantations. Tissue Antigens. 1992;39:225–35. doi: 10.1111/j.1399-0039.1992.tb01940.x. [DOI] [PubMed] [Google Scholar]
- 31.Bein G, Glaser R, Kirchner H. Rapid HLA-DRB1 genotyping by nested PCR amplification. Tissue Antigens. 1992;39:68–73. doi: 10.1111/j.1399-0039.1992.tb01909.x. [DOI] [PubMed] [Google Scholar]
- 32.Zhang J, Weiner HL, Hafler DA. Autoreactive T-cells in multiple sclerosis. Int Rev Immunol. 1992;9:183–201. doi: 10.3109/08830189209061790. [DOI] [PubMed] [Google Scholar]
- 33.Kotanko P. Type I diabetes and the ‘milk hypothesis’. Diabetes Care. 1997;20:233–4. doi: 10.2337/diacare.20.2.233b. [DOI] [PubMed] [Google Scholar]
- 34.Harrison LC, Honeyman MC, DeAizpurua HJ, Schmidli RS, Colman PG, Tait BD, Cram DS. Inverse relation between humoral and cellular immunity to glutamic acid decarboxylase in subjects at risk of insulin-dependent diabetes. Lancet. 1993;341:1365–9. doi: 10.1016/0140-6736(93)90940-i. [DOI] [PubMed] [Google Scholar]
- 35.Roep BO, Duinkerken G, Schreuder GMT, Kolb H, de Vries RRP, Martin S. HLA-associated inverse correlation between T cell and antibody responsiveness to islet autoantigen in recent-onset insulin-dependent diabetes mellitus. Eur J Immunol. 1996;26:1285–9. doi: 10.1002/eji.1830260616. [DOI] [PubMed] [Google Scholar]
- 36.Ellis TM, Darrow B, Campbell L, Atkinson MA. Inverse relationship between humoral and cellular immune responses to glutamate decarboxylase 65 (GAD) and insulin in IDDM. Diabetes. 1995;44s:52A. [Google Scholar]
- 37.Hummel M, Durinovic-Bellò I, Ziegler AG. Relation between cellular and humoral immunity to islet cell antigens in type 1 diabetes. J Autoimmun. 1996;9:427–30. doi: 10.1006/jaut.1996.0059. [DOI] [PubMed] [Google Scholar]
- 38.Manca F, Fenoglio D, Li Pira G, Kunkl A, Celada F. Effect of antigen/antibody ratio on macrophage uptake, processing and presentation to T cells of antigen complexed with polyclonal antibodies. J Exp Med. 1991;173:37–48. doi: 10.1084/jem.173.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Watts C, Lanzavecchia A. Suppressive effect of antibody on processing of T cell epitopes. J Exp Med. 1993;178:1459–63. doi: 10.1084/jem.178.4.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Simitsek PD, Campbell DG, Lanzavecchia A, Fairweather N, Watts C. Modulation of antigen processing by bound antibodies can boost or suppress class II Major Histocompatibility Complex presentation of different T cell determinants. J Exp Med. 1995;181:1957–63. doi: 10.1084/jem.181.6.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]