Abstract
Cytokines produced from intestinal epithelial cells may function as signals to neighbouring immune cells. In the present study we analysed the effects of colonic epithelial cell lines (HT-29, Caco-2, HCT-116, Colo-320) and freshly isolated intestinal epithelial cells on IL-8 expression in the SV-40T transfected human microvascular endothelial cell line (HMEC-1). Epithelial cell-conditioned media and transwells preventing physical contact between epithelial and endothelial cells were used. TGF-β1 and IL-8 levels were determined by ELISA and Northern blot analysis. Increasing concentrations of IL-1β led to increasing production of IL-8. The addition of epithelial cell-conditioned medium or epithelial cells to HMEC-1 cells in a two-compartment co-culture system resulted in a strong decrease in IL-8 at the protein and mRNA level. Decrease of IL-8 was markedly stronger when epithelial cells were co-cultured in contact with HMEC-1 cells, indicating that not only soluble factor(s) play a role in the induction of IL-8 suppression in HMEC-1 cells. MoAbs against TGF-β1 partially inhibited down-regulation of endothelial IL-8 expression. In further studies, IL-8 expression in freshly isolated human intestinal microvascular endothelial cells (HIMEC) was also down-regulated by intestinal epithelial cells. Our results demonstrate that intestinal epithelial cells down-regulate IL-8 expression in HMEC-1 cells. TGF-β1 is a candidate factor of epithelial–endothelial communication in the colonic mucosa.
Keywords: epithelial cells, endothelial cells, TGF-β1, IL-8
INTRODUCTION
Mucosal recruitment of neutrophils involves sequential adhesion and transmigration across endothelial, lamina propria and epithelial compartments [1–3]. IL-8, the major chemotactic and activating peptide for neutrophils, activates the motile apparatus of neutrophils, induces a direct migration, the expression of surface adhesion molecules, the release of enzymes, and the production of reactive oxygen resulting in increased levels of intracellular cAMP [4–10]. IL-8 can be produced by mononuclear phagocytes, fibroblasts, various colonic epithelial cell types and endothelial cells [6,10,11]. Expression of the IL-8 gene can be enhanced by lipopolysaccharide (LPS), IL-1β and tumour necrosis factor-alpha (TNF-α) and is differentially regulated in various cell types. For example, IL-10, IL-4 and TGF-β, which can down-regulate IL-8 production in macrophages, had no effect on IL-8 generation by epithelial cells [12,13].
The endothelial cell production of IL-8 is of special interest because this chemokine secreted by the endothelium can appear immediately at the tissue–blood interface, where it can exert its effects on leucocyte emigration [4]. Recent studies have shown that TGF-β1 inhibits E-selectin and IL-8 expression on endothelial cells, thus inhibiting the migration of neutrophils across the endothelium [9,14]. Conversely, TGF-β1 directly stimulates neutrophil and monocyte chemotaxis [9], a situation that is important in leucocyte movement in tissues. While substantial progress has been made in the understanding of direct epithelial–neutrophil interactions in the gastrointestinal tract [1,2,15–19], no information is available concerning the participation of intestinal epithelial cells in regulation of the immune response at the endothelial cell wall. Epithelial cells produce substantial amounts of a specific array of soluble mediators, e.g. IL-8 and TGF-β, either constitutive or during the course of inflammation, and upon degranulation at sites of injury release these cytokines into the surrounding tissue [2,8,12]. Based on these findings, we hypothesized that human microvascular endothelial cells, apart from their vascular functions, may get signals from epithelial cells in the complex network of the intestine. This interaction may be biologically relevant, as epithelial cells are in a unique position in functioning as an early host-signalling system, thus implicating other neighbouring cells located in the underlying mucosa [12], e.g. endothelial cells.
In the present study we demonstrate down-regulation of IL-8 production and mRNA expression in the SV-40T-transfected human microvascular endothelial cell line (HMEC-1) model system [20] upon co-culture with either different human colonic epithelial cell lines or freshly obtained human intestinal epithelial cells. By using TGF-β-blocking antibodies we found that TGF-β1 released by colonic epithelial cells is a principal inhibitory agent. Taken together, our studies indicate that intestinal epithelial cells should be included in the cytokine regulatory network for IL-8 expression in endothelial cells, thus helping to evaluate pathophysiological events occurring under in vivo situations in the microenvironment of the endothelial wall.
MATERIALS AND METHODS
Cell lines and cell culture
The colonic carcinoma cell lines Colo-320 (ATCC, Rockville, MD), Caco-2, HCT-116 and HT-29 (German Collection of Microorganisms and Cell Cultures, Department of Human and Animal Cell Cultures, Braunschweig, Germany) were cultured in different media as follows: HT-29 (RPMI 1640; Gibco, Eggenstein, Germany), Caco-2 (minimal essential medium (MEM); Gibco), Colo-320 (RPMI 1640) and HCT-116 (McCoy 5a Medium; Gibco). All media contained 1% penicillin/streptomycin and 1 mml-glutamine. Cells were cultured with or without 10% AB serum. The human microvascular endothelial cell line HMEC-1 [20] was obtained from Professor T. A. Luger (Department of Dermatology, University of Münster, Germany). Experiments performed with the endothelial cell line used cells at passage levels 8–17. The cultures were mycoplasma-free and maintained with twice weekly passage. Cells were seeded into 24-well plates and grown until formation of confluent monolayers (3 days). Monolayers were then incubated for 24 h in fresh medium containing stimuli as indicated. To determine the constitutive release of TGF-β1, cells were plated at a density of 1 × 106 cells/ml in six-well plates (Greiner, Solingen, Germany) and cultured for 24–96 h serum-free or with 10% AB serum at 37°C in a 5% CO2 tissue incubator. The supernatants were collected, cleared by centrifugation and passaged through 0.22-μm sterile filters and kept at −70°C until evaluation by ELISA. Cell number and viability were examined by trypan blue exclusion.
Isolation of human intestinal epithelial cells
Epithelial cells were isolated from histologically normal areas of surgically resected colonic specimens from patients with tumour bowel resections as described by our group [21]. In three cases, histologically normal tissues from the small bowel (duodenum) of patients who underwent pancreatoduodenectomy were available for investigations. Cryosections of the surgical specimens were examined by an independent pathologist to ensure that only histologically normal areas were used for cell isolation. No patient was included in the study who had been on corticosteroids or any other immunosuppressive drug. The study protocol had been approved by the Human Studies Committee of the University of Münster.
In brief, small pieces of resected tissue were incubated in 1 mm/l dithiothreitol (DTT) for 15 min at room temperature and then washed twice in RPMI medium. Subsequently, the tissue was washed for 30 min in 5 mm/l EDTA for four times to remove epithelial cells. Supernatants were then centrifuged for 10 min at 400 g. The resultant pellet was dissolved in Hank's balanced salt solution (HBSS) and centrifuged at 500 g for 5 min. Epithelial cells were separated from the remaining tissue by passage through a 100-μm nylon mesh. Epithelial cells were then purified by density gradient centrifugation on a Percoll gradient. From the interface at the top of the Percoll layer intestinal epithelial cells were collected and washed twice with serum-free RPMI medium. Epithelial cell preparations were free of contaminating B cells, T cells and monocytes/macrophages as assessed by flow cytometry using CD19/CD20, anti-CD3 and CD33/CD14 markers. As a marker for intestinal epithelial cells we used FITC-labelled MoAbs against cytokeratin-18 and cytokeratin-20 (Progen, Heidelberg, Germany) by immunocytochemistry on cytospin preparations. Purity of epithelial cells was > 85%. Viability of the freshly isolated epithelial cells was assessed by trypan blue exclusion and staining with propidium iodide and was > 95% immediately after isolation, 75–85% after 72 h, and 73–83% after 96 h in culture. Epithelial cells were then cultured in RPMI medium with 10% AB serum and antibiotics on 24-well plates, which had been covered with a collagen I (2 μg/ml) layer. Under these conditions, epithelial cells could be held in culture for 3–4 weeks. Before stimulation, epithelial cells were held in culture for 3–4 days [21].
Isolation of human intestinal microvascular endothelial cells
Human intestinal microvascular endothelial cells were isolated from normal areas of surgically resected colonic specimens as previously described [22]. Briefly, the gut segments were opened along the antimesenteric side and rinsed extensively in sterile saline. The tissue was next incubated in 1 mm DTT in isotonic Ca2+- and Mg2+-free PBS pH 7.5 for 15 min at 20°C and subsequently washed in PBS. Mucus was carefully wiped away. Epithelial cells were removed by four subsequent incubations in 5 mm EDTA/PBS (30 min, 37°C) and intervening PBS rinses with mild surface scraping. After dissociation by collagenase/dispase/DNase (Boehringer Mannheim, Mannheim, Germany) of mucosal and submucosal tissue, cell suspension was sequentially passed through 200- and 90-μm stainless steel meshes and finally separated on Lymphoprep density gradient to remove granulocytes and erythrocytes. Cells were plated at a seeding density of 1 × 106/cm2 and cultured to subconfluence in endothelial serum-free medium containing 10% AB serum, hydrocortisone, and 02-dibutyryladenosine cyclic monophosphate (db-cAMP). Primary cultures were trypsinized and endothelial cells were isolated by paramagnetic beads armed with MoAb to CD31 (Dianova, Hamburg, Germany). For the paramagnetic bead separation of CD31+ cells a single-cell suspension of the primary subconfluent cell cultures was obtained by applying trypsin (0.5 mg/ml, 1:250) and EDTA (0.2 mg/ml) in PBS for 4 min at 37°C. Paramagnetic sheep anti-mouse IgG-coated monodisperse beads were armed according to the manufacturer's instructions with anti-human CD31 (0.2 μg antibody/mg beads). A small fraction of cells was mixed with armed beads and briefly centrifuged (400 g, 1 min, 4°C) and the percentage of rosetted cells was estimated in a haemocytometer. The rosettes were extracted by a magnetic particle concentrator for 2 min, after which non-rosetted cells were removed. Rosetted cells were washed six times, resuspended in normal growth medium, and plated into culture flasks. The paramagnetic bead separation of CD31+ cells usually resulted in 15–30% rosettes that were replated. Contaminating fibroblastoid cells (as judged by phase contrast microscopy) were rarely seen and, when present, were easily eliminated by an additional bead separation. Contamination of the cultures by other CD31+ cells was excluded by lack of staining for the leucocyte common antigen CD45 as well as the macrophage restricted form of CD68, whereas contamination of Ulex europaeus lectin I binding epithelial cells was excluded by lack of staining for cytokeratin. Contamination with fibroblasts was further excluded by using the clone 5B5 (isotype, IgG1, κ; Dako, Hamburg, Germany).
Co-culture of intestinal epithelial cells and HMEC-1 cells
Confluent HMEC-1 cells (5 × 105 cells/well) in 24-well plates were washed, overlaid with epithelial cell suspensions (2.5 × 105 cells/well, 2:1 ratio endothelial: epithelial cells) and cultured with and without 10% AB serum for the times stated. In some experiments, epithelial cells were separated from endothelial cells by Millipore filters (0.45 μm; Transwell). In other experiments, endothelial cells were cultured with supernatants harvested from transformed colonic epithelial cell lines and freshly isolated intestinal epithelial cells.
Functional inhibition experiments for absorption of TGF-β1 were performed as described previously [23]. In short, 1 ml epithelial cell conditioned medium was preincubated in 24-well plates that have been coated with an affinity-purified anti-TGF-β1 (100 μg/ml supernatant; Genzyme, Cambridge, MA). Plates were coated with antibody by adding concentrated antibody preparations to wells, incubating overnight at 4°C, and washing three times with PBS. Cytokine-absorbed supernatants (500 μl) were collected, added to endothelial cells, and incubated for optimal culture times after stimulation with IL-1β. Supernatants were collected for IL-8 measurement. An isotype-matched MoAb (mouse anticytokeratin-18; Sigma, Deisenhofen, Germany) was used as negative control. Free residual antibody was not detected in cytokine-absorbed supernatants, as determined by immunodiffusion tests (data not shown).
ELISA for IL-8 and TGF-β1
IL-8 was determined in culture supernatants using a sandwich ELISA assay developed by Laboserv (Giessen, Germany). The sensitivity of the IL-8 ELISA was 50 pg/ml. TGF-β1 was determined using an ELISA assay developed by Genzyme with a sensitivity of 50 pg/ml. Culture supernatants were all tested in triplicate. In all tests a standard cytokine preparation (recombinant cytokine at defined concentration) was used as internal control. All assays were tested for cross-reaction by adding several cytokines and soluble receptors to the different cytokine standards. No significant cross-reaction could be observed using the following cytokines (except for the recombinant protein which was determined in the assay): human IL-1β (Promega, Mannheim, Germany), IL-12, p55 and p75 form of the TNF receptor (R&D Systems, Minneapolis, MN), IFN-γ, IL-2, IL-4, IL-6, IL-8, IL-10, IL-13, IL-15, granulocyte colony-stimulating factor (G-CSF), granulocyte-macrophage colony-stimulating factor (GM-CSF) and TNF-α (Pepro Tech EC Ltd, London, UK).
Reverse transcriptase-polymerase chain reaction and Southern blot analysis
RNA was extracted with guanidinium thiocyanate/phenol/chloroform [24] and quantified by UV absorption spectrophotometry at 260 nm. Complementary DNA (cDNA) was prepared from 1 μg unfractionated RNA in 20 μl reverse transcription buffer (Gibco) containing 10 mmol/l Tris pH 8.3, 50 mmol/l KCl, 5 mmol/l MgCl2, 1 mmol/l each of deoxyadenosine triphosphate, deoxycytidine triphosphate, deoxyguanosine triphosphate, thymidine triphosphate, 20 U human placenta RNase inhibitor (Pharmacia, Freiburg, Germany), 0.1 μg oligo (dT)14 and 200 U reversed transcriptase (BRL; Superscript) at 37°C for 60 min. The reaction mixture was heat-inactivated for 10 min at 95°C, and 80 μl of 10 mmol/l Tris pH 8.3, 50 mmol/l KCl, 1.25 mmol/l MgCl2 containing 25 pmol each of the 5′ and 3′ primer were added. The reaction mixture was heated to 95°C for 5 min and subsequently kept at 85°C for 7 min, during which 1.25 U of Supertaq polymerase (HT Biotechnology, Cambridge, UK) was added. The cycling conditions for the polymerase chain reaction (PCR) reactions consisted of 1 min denaturation at 95°C and 2.5 min annealing and extension at 72°C (IL-10) or 65°C (IL-4, TGF-β1). Primers for IL-4, IL-10, TGF-β1 and β-actin were purchased from Clontech (Heidelberg, Germany). Details of the PCR have been published [12,25]. A 10-μl aliquot of the amplified DNA reaction mixture was subjected 1.7% agarose gel electrophoresis, and the amplified product was visualized by UV fluorescence after staining with ethidium bromide. PCR products were blotted onto nylon membrane and hybridized with 32P-labelled cDNA probes.
Northern blotting
Northern blot analysis was conducted according to standard procedures. Total cellular RNA from endothelial cells was prepared according to the method of Chomczynski & Sacchi [24], electrophoresed in 1% agarose-formaldehyde gel, and blotted onto nylon filters (Pharmacia; Genebind 45). The cDNA for IL-8 was labelled with a 32P-cCTP by random priming, using a commercially available multiprime DNA labelling kit (Amersham) to a specific activity of > 106 ct/min per ng DNA. Filters were prehybridized at 42°C for 4 h and then hybridized at 42°C for 18 h in a 50% formamide solution containing 5× SSC, 5× Denhardt's solution, 1% SDS, 0.05% pyrophosphate, and 0.25 mg/ml salmon sperm DNA. They were then washed by one rinse followed by three washes for 30 min each with 1× SSC/1% SDS at 60°C, and then exposed to a phosphor storage screen for a second hybridization by stripping of the probe DNA by washing in 1% SDS at 80°C for 1 h. Complete probe removal was confirmed by overnight exposure to the phosphor screen. A labelled oligonucleotide for β-actin served as a control. The phosphor screen was scanned using the ImageQuant PhosphorImager and software (Molecular Dynamics, Sunnyvale, CA), and the digitized file was analysed. The intensity of the IL-8 band was expressed as a ratio of the intensity of the bands for β-actin.
Statistical analysis
In the statistical calculations the data were treated as non-parametric, and results were analysed by the two-tailed Wilcoxon's signed rank test. Results are presented as median ± 95% confidence interval, unless stated otherwise. P < 0.05 was considered significant.
RESULTS
Expression of TGF-β, IL-10 and IL-4 mRNA in intestinal epithelial cells
We were first interested to qualitatively determine constitutive expression of the anti-inflammatory cytokines TGF-β, IL-10 and IL-4 in colonic epithelial cell lines and freshly obtained intestinal epithelial cells. By performing Southern blot analysis of reverse transcriptase (RT)-PCR products we showed that both transformed colonic epithelial cell lines and freshly obtained colonic and duodenal epithelial cells constitutively expressed detectable levels of mRNA for TGF-β1 (Fig. 1). Caco-2 contained detectable levels of mRNA for IL-10. mRNA for IL-10 and IL-4 was undetectable in any of the other cell lines. Finally, freshly isolated duodenal and colonic epithelial cells were found to have no mRNA for IL-10 and IL-4. In summary, both colonic epithelial cell lines and freshly obtained duodenal and colonic epithelial cells expressed mRNA for TGF-β. As positive controls cDNAs from cells known to express the specific cytokine mRNA were used (not shown).
Fig. 1.
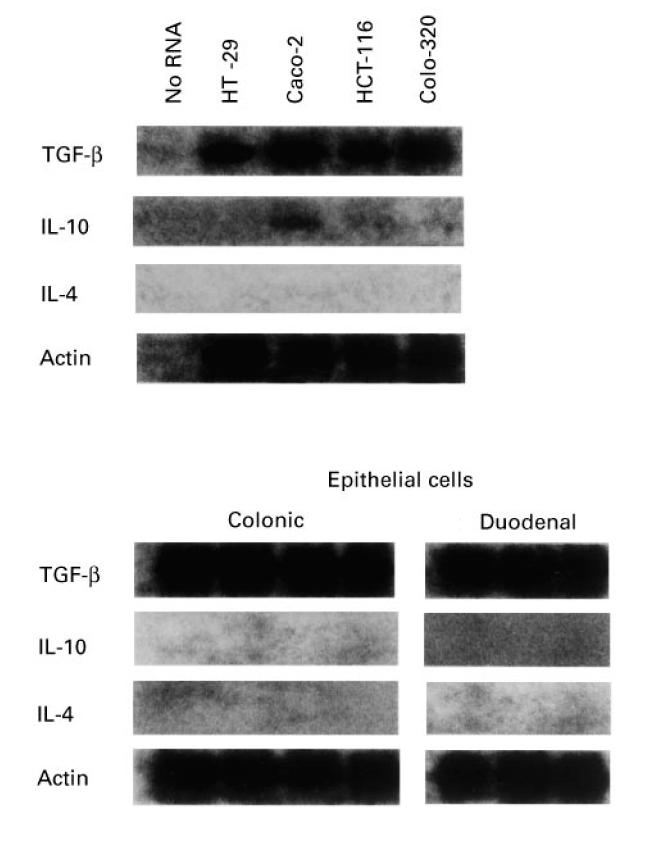
Expression of TGF-β, IL-10 and IL-4 mRNA in four human intestinal epithelial cell lines and freshly isolated intestinal epithelial cells demonstrated by Southern blot analysis of reverse transcriptase-polymerase chain reaction (RT-PCR) products. The β-actin mRNA was also amplified as control. Representative results from one of three experiments are shown.
TGF-β1 secretion by intestinal epithelial cells
In a recent study we have shown that colonic epithelial cell lines secrete substantial amounts of TGF-β1 [23]. We were therefore interested in time course kinetics of TGF-β1 secretion by freshly isolated duodenal and colonic epithelial cells. Epithelial cells were seeded into six-well plates at a density of 1 × 106 cells/ml and cultured for 24–96 h with 10% AB serum at 37°C in a 5% CO2 tissue incubator. The supernatants were collected, cleared by centrifugation and passaged through 0.22-μm sterile filters and kept at −70°C until evaluation by ELISA. Figure 2 shows that TGF-β1 secretion by freshly isolated intestinal epithelial cells progressively increased during the culture period in a time-dependent manner, reaching a peak at 96 h. This indicates that TGF-β1 is actively produced by intestinal epithelial cells.
Fig. 2.
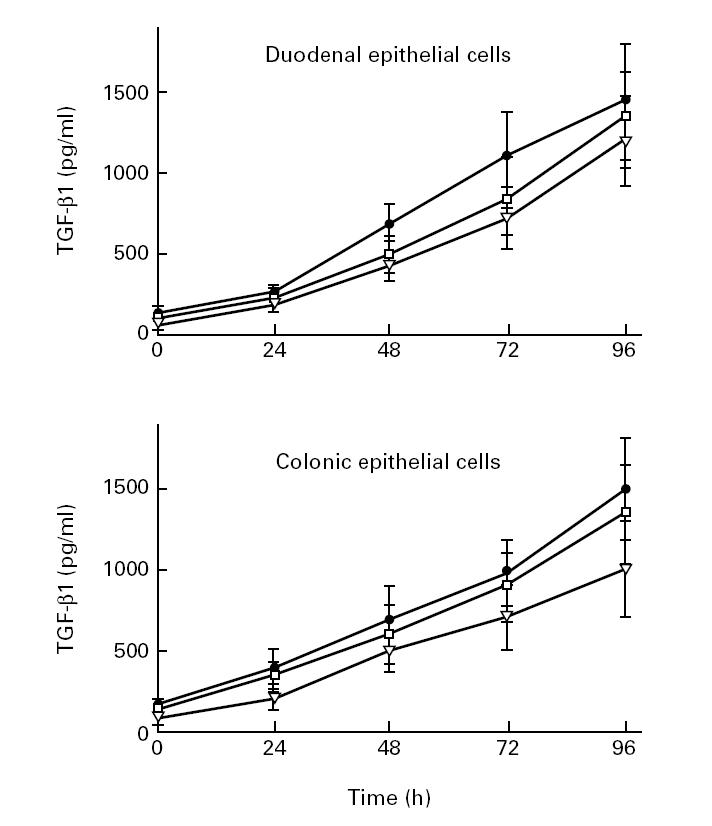
Time course analysis of protein levels of TGF-β1 in freshly isolated intestinal epithelial cells (three duodenal, three colonic). Confluent monolayers of epithelial cells were cultured without added stimuli. At various time points, TGF-β1 concentrations in the supernatants were determined by ELISA. Each results is the mean ± s.d. of triplicate cultures in one of three independent experiments that had similar results.
TGF-β1 and epithelial cell line-conditioned medium inhibit IL-8 production by HMEC-1
We turned our attention to the question of whether intestinal epithelial cells would influence IL-8 production and expression in the human microvascular endothelial cell line HMEC-1. Recent studies have shown that TGF-β1 is capable of inhibiting IL-8 secretion and mRNA expression in endothelial cells [9,14]. Therefore, HMEC-1 cells were cultured in 24-well plastic plates at a concentration of 5 × 105 cells/ml. Cells were pretreated for 20 h with epithelial cell-conditioned media or exogenous recombinant TGF-β1, and then stimulated with IL-1β or medium alone. Supernatants were collected after 4 h, and the accumulated IL-8 produced over this time was measured by ELISA. Increasing concentrations of IL-1β from 1 ng to 50 ng/ml led to increasing production of IL-8. Addition of TGF-β1 (0.5–2 ng) to HMEC-1 cells 20 h before IL-1β stimulation inhibited the secretion of IL-8 (Fig. 3a). In our experiments, co-culture of HMEC-1 cells with epithelial cell-conditioned media also down-regulated endothelial IL-8 expression (Fig. 3b). No significant inhibition of IL-8 concentrations from unstimulated HMEC-1 was found.
Fig. 3.
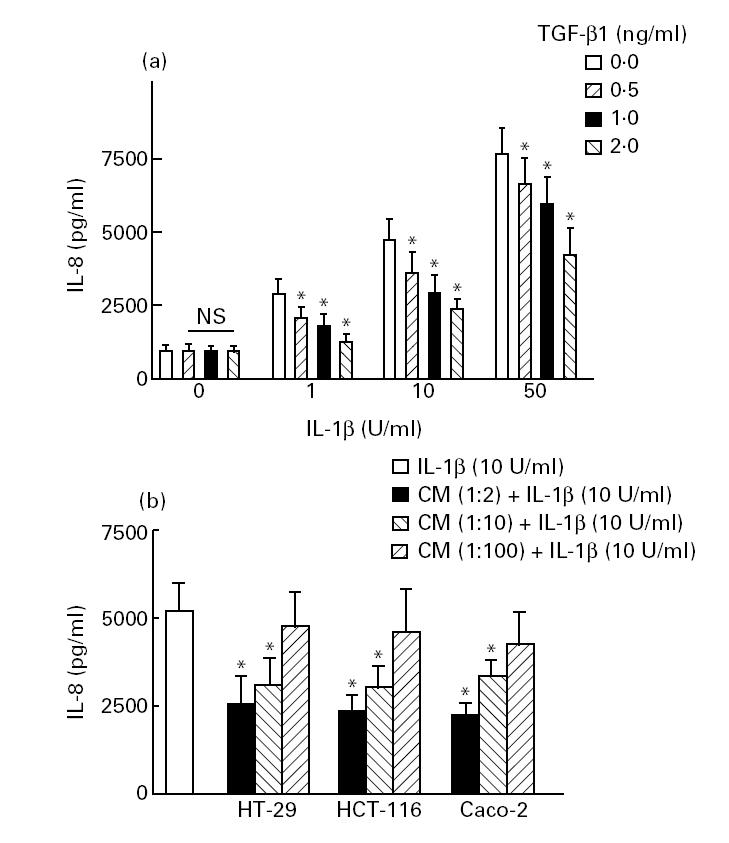
Effects of exogenous recombinant TGF-β1 and epithelial cell-conditioned media on IL-8 secretion by human microvascular endothelial cell line (HMEC-1) cells. HMEC-1 cells (5 × 105) were plated on Costar plates in either the absence or the presence of 0.5–2 ng/ml TGF-β (a) or epithelial cell-conditioned media (b). After 20 h, some wells were stimulated with varying concentrations of IL-1β and incubated for 4 h. The supernatants were collected after 4 h, and immunoreactive IL-8 was measured by ELISA. Each result is the median ± 95% confidence interval (CI) of triplicate cultures in one of three independent experiments that had similar results. *P < 0.05 compared with IL-1β-activated HMEC incubated without TGF-β1 or epithelial cell-conditioned media.
IL-8 regulation in HMEC-1 cells during epithelial–endothelial interaction in single and separated growth compartments
The role of soluble factors in the down-regulation of IL-8 in epithelial–endothelial co-cultures was further assessed by using separated growth compartments that allowed free diffusion of small molecules. This experimental setting also led to a down-regulation of IL-8 production in HMEC-1 cells comparable to that observed with epithelial cell-conditioned media (Fig. 4).
Fig. 4.
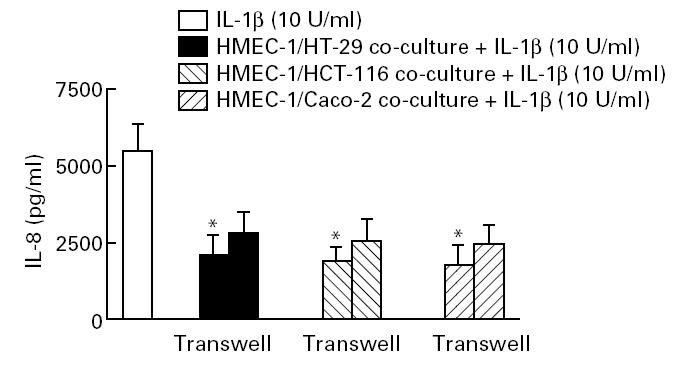
Role of cell contact versus soluble factors in epithelial cell-regulated IL-8 secretion in human microvascular endothelial cell line (HMEC-1) cells. Epithelial cells (2.5 × 105) were separated from HMEC-1 cells by a 0.45-μm filter, or cultured in contact with HMEC-1 for 20 h. After 20 h, some wells were stimulated with 10 U/ml IL-1β and incubated for 4 h. The supernatants were collected after 4 h, and immunoreactive IL-8 was measured by ELISA. Each result is the median ± 95% confidence interval (CI) of triplicate cultures in one of three independent experiments that had similar results. *P < 0.05 compared with co-culture experiments performed in transwells.
From co-culture experiments, where pericytes or smooth muscle cells were grown together with aortic endothelial cells, it was found that, while each cell type alone only produced predominantly latent TGF-β, the combination of both cell types led to in situ activation of the latent form [26]. In our study, a variety of epithelial cells cultured in a single compartment with endothelial cells revealed a significantly stronger inhibitory effect on endothelial IL-8 production compared with conditioned media or co-culture experiments performed in Transwell tissue culture plates (Fig. 4). We conclude that other factors, such as those which are cell/cell contact dependent, are important for the stronger induction of IL-8 suppression in HMEC-1 cells. Cell enumeration studies showed no significant change in cell number over the time frame of the co-culture experiments performed in our study (data not shown).
TGF-β1 and colonic epithelial cell line-conditioned medium inhibit IL-8 mRNA expression by HMEC-1
Total RNA was isolated from primary HMEC-1 cells, pretreated with medium, epithelial cell-conditioned media or exogenous recombinant TGF-β1 for 18 h, and then stimulated with IL-1β or medium alone for 4 h. For Northern blot analysis, RNA from four primary lines was pooled to obtain sufficient quantities of RNA and the filter probed for IL-8 and β-actin mRNA. Unstimulated HMEC-1 cells produced some IL-8 mRNA, which was clearly up-regulated by IL-1β. Pretreatment of HMEC-1 with recombinant TGF-β1 or conditioned media from both colonic epithelial cell lines and freshly obtained intestinal epithelial cells reduced the intensity of the IL-8 mRNA in IL-1β-activated HMEC-1 cells (Fig. 5). IL-8:β-actin intensity ratios were calculated from the quantified bands.
Fig. 5.
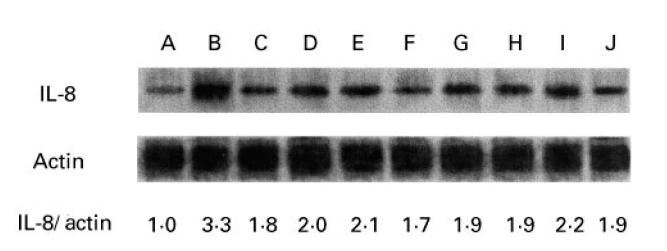
Northern blot analysis of the effects of conditioned media of colonic epithelial cell lines and freshly obtained intestinal epithelial cells on IL-8 mRNA expression in human microvascular endothelial cell line (HMEC-1) cells. HMEC-1 cells were plated into 24-well trays and were incubated with TGF-β1 (1.0 ng/ml) or epithelial cell-conditioned media, incubated overnight for 18 h, then left either unstimulated or stimulated with 10 U/ml IL-1β for 4 h. Total RNA was prepared and pooled from four separate lines. Total RNA (7 μg per lane) was loaded, and the mRNAs for IL-8 and β-actin were probed sequentially by Northern blotting. Lane A, no stimulation; lane B, 10 U/ml IL-1β; lane C, TGF-β1 and IL-1β; further lanes: CM-HT-29 and IL-1β (lane D), CM-HCT-116 and IL-1β (lane E), and CM-Caco-2 and IL-1β (lane F). Lanes G–J show the effects of conditioned media of freshly obtained intestinal epithelial cells (two colonic, two duodenal) on IL-8 expression in HMEC-1 cells. Representative results from one of three experiments are shown.
Effects of administration of anti-TGF-β1 on endothelial IL-8 production
Neutralization of TGF-β1 with anti-TGF-β MoAbs affected the ability of epithelial cell-conditioned media to inhibit IL-8 secretion from endothelial cells. As shown in Fig. 6, reduced endothelial IL-8 production was significantly restored by treatment with neutralizing antibodies to TGF-β1. These results confirmed that TGF-β is a major, but probably not the only inhibitor of endothelial IL-8 production during epithelial–endothelial cell interaction, as even at very high concentrations of TGF-β-blocking antibodies IL-8 synthesis was not fully restored (not shown).
Fig. 6.
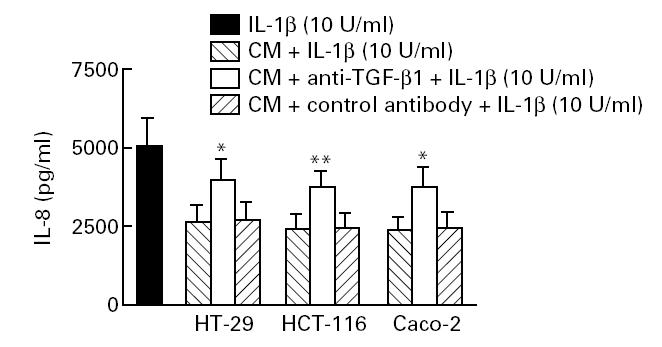
Pretreatment with anti-TGF-β antibody partially reversed endothelial IL-8 down-regulation induced by epithelial cell-conditioned medium. Twenty-four-well plates were coated with an affinity-purified anti-TGF-β1 MoAb (100 μg/ml supernatant; Genzyme, Cambridge, MA), incubated overnight at 4°C and washed four times with PBS. Epithelial cell-conditioned medium (1 ml) was added to each well and incubated at 37°C for 1 h. Five hundred microlitre cytokine-absorbed supernatants were then collected, added to endothelial cells, and incubated for optimal culture times after stimulation with IL-1β. An isotype-matched MoAb (mouse anti-cytokeratin-18; Sigma) was used as negative control. Each result is the median ± 95% confidence interval (CI) of triplicate cultures in one of three independent experiments that had similar results. *P < 0.05; **P < 0.001 (compared with conditioned medium incubated with control antibody).
Effects of TGF-β1 and colonic epithelial cell-conditioned media on IL-8 secretion by human intestinal microvascular endothelial cells
To relate the responses by the cell lines to those occurring in vivo, we also examined responses of human intestinal microvascular endothelial cells (HIMEC) in co-culture with human intestinal epithelial cells. In our experiments, IL-8 secretion by HIMEC in response to IL-1β was similar to that obtained with the endothelial cell line HMEC-1, suggesting that they were fully responsive. Addition of epithelial cell-conditioned medium 20 h before IL-1β stimulation inhibited the secretion of IL-8 (Fig. 7a). MoAbs against TGF-β1 partially inhibited down-regulation of IL-8 secretion by HIMEC. Moreover, pretreatment of HIMEC with conditioned media from freshly obtained intestinal epithelial cells reduced the intensity of the IL-8 bands in IL-1β-activated HIMEC (Fig. 7b).
Fig. 7.
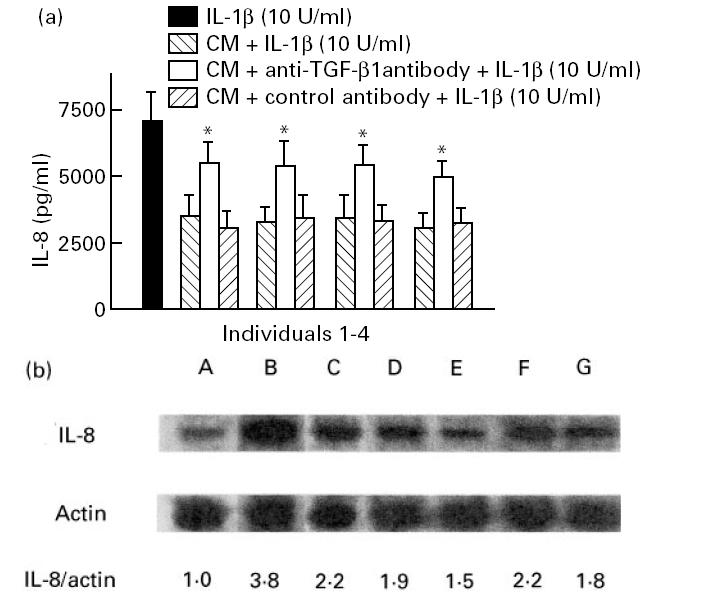
Effects of conditioned media from freshly isolated colonic epithelial cells (individuals 1–4) on IL-8 secretion (a) by human intestinal microvascular endothelial cells (HIMEC). HIMEC (5 × 105) were plated on Costar plates in either the absence or the presence of colonic epithelial cell-conditioned media. After 20 h, some wells were stimulated with 10 U/ml IL-1β and incubated for 4 h. The supernatants were collected after 4 h, and immunoreactive IL-8 was measured by ELISA. Each results is the median ± 95% confidence interval (CI) of triplicate cultures in one of five independent experiments that had similar results. *P < 0.05 compared with conditioned medium incubated with control antibody. (b) Representative Northern blot analysis of the effects of conditioned media of freshly obtained colonic epithelial cells on IL-8 mRNA expression in HIMEC. HIMEC were plated to 24-well trays and were incubated with epithelial cell-conditioned media, incubated overnight for 18 h, then left either unstimulated or stimulated with 10 U/ml IL-1β for 4 h. Total RNA (7 μg per lane) was loaded, and the mRNAs for IL-8 and β-actin were probed sequentially by Northern blotting. Lane A, no stimulation; lane B, 10 U/ml IL-1β; lane C, TGF-β1 and IL-1β. Lanes D–G show the effects of conditioned media of freshly obtained intestinal epithelial cells (two colonic, two duodenal) on IL-8 expression in HIMEC.
DISCUSSION
This study for the first time demonstrates down-regulation of IL-8 production and mRNA expression in HIMEC upon co-culture with intestinal epithelial cells and epithelial cell-conditioned media. Addition of neutralizing antibodies to TGF-β to the cultures partially abolishes inhibitory effects, depending on cell line and stimulus. On the basis of the data presented here, we conclude that colonic epithelial cells secrete biologically relevant quantities of activated TGF-β1, thereby acting specifically on IL-8 expression in endothelial cells.
In our experiments, epithelial cell-derived TGF-β1 secretion continued to increase up to 96 h after commencement of cultivation. The time course of TGF-β1 secretion for intestinal epithelial cell lines was similar to that obtained with freshly isolated intestinal epithelial cells. In our study, epithelial cell-derived TGF-β was found to be a major autocrine or paracrine inhibitor of endothelial IL-8 expression, as co-cultures treated with neutralizing antibodies to this growth factor showed a dose-dependent abolishment of the inhibitory effect. Anti-TGF-β MoAb was only able to inhibit part of the response, suggesting the presence of a TGF-β-independent mechanism. When epithelial cell supernatants were acid-treated (pH 2.3) at room temperature for 4 h, these supernatants retained the ability to suppress endothelial IL-8 production (data not shown). Aside from TGF-β most proteins would be denatured by acid treatment. Eckmann et al. [12] proposed that epithelial cells of the gastrointestinal tract would not play a major role in down-regulation of the acute inflammatory response through the production of TGF-β, as mRNA levels and secretion of this anti-inflammatory cytokine were not up-regulated in colon epithelial cells in response to TNF-α and IL-1β stimulation or Salmonella invasion [8,13,17]. Nonetheless, the extremely low concentration (picomolar) of TGF-β required to elicit a strong inhibition of endothelial IL-8 mRNA expression, as shown in the present study, suggests that TGF-β produced by intestinal epithelial cells may be functioning as a very potent signal for endothelial cells.
It is not yet clear whether the existing TGF-β in our co-culture supernatants represents a true constitutive expression, as the level and distribution of expression are undoubtedly governed by the local environment [26]. In vivo studies further indicate that expression of TGF-β receptors may be down-regulated during acute inflammation [27]. Most cultured cells secrete TGF-β in a latent form [28,29], although there are reports of the production of activated TGF-β by human cancer cells in vitro [30–32]. In our study, intestinal epithelial cell-conditioned media and epithelial cells work on IL-1β-induced release of IL-8 from endothelial cells, indicating that the inhibitory effect of bioactive TGF-β on endothelial IL-8 secretion would be inflammation-dependent.
TGF-β mRNA has been found in the non-transformed duodenal cell line IEC-6 [33]. A recent study of Pang et al. failed to demonstrate TGF-β message in various established duodenal cell lines, suggesting that there may be a regional difference in the expression and/or secretion of this cytokine along the gut [34]. In our experiments, unstimulated duodenal epithelial cells strongly down-regulated IL-8 expression in HIMEC and HMEC-1 cells and substantial amounts of duodenal TGF-β could be detected at the protein and mRNA level. Conflicting reports might be due to the different techniques used, which may reflect the multiple manipulations performed during isolation and culture processing of intestinal epithelial cells [12,34].
In our study, a variety of cytokine-activated endothelial cells cultured in a single compartment with intestinal epithelial cells revealed additional inhibitory effect on endothelial IL-8 production compared with conditioned media or co-culture experiments performed in Transwell tissue cultures plates. This might be explained by the high density of cells in this system, leading to a microenvironment with more metabolic acidification of the medium or to the accumulation of proteolytic enzymes that activate more latent TGF-β [26,29]. We found no inhibitory effect on endothelial IL-8 expression when HMEC-1 were incubated with crude cell membrane preparations from colonic epithelial cells, indicating that viable and metabolically active epithelial cells are necessary for the inhibitory effect on endothelial IL-8 expression (data not shown). In our co-culture experiments, endothelial cells were overlaid with only low concentrations of epithelial cells (2.5 × 105/well), thereby avoiding substantial production of IL-8 by the latter cells.
It has to be noted that the endothelium is somewhat distant from the epithelial cells under non-inflammatory conditions in vivo and that there may be intervening structures as well which may prevent epithelial cell-derived soluble mediators from reaching the endothelial cell. Whether or not in vivo contact or close proximity or a distinctly appropriate microenvironment between these two cell types is essential for the generation of activated TGF-β1 and certain other signals, awaits further investigation. The interaction between epithelial and endothelial cells may be biologically relevant during inflammatory reactions of the intestinal mucosa, where epithelial cells may come in close contact with endothelial cells.
In conclusion, intestinal epithelial cells may act through generating TGF-β under both physiological and inflammatory conditions, thereby inhibiting endothelial IL-8 production and limiting the cascade of inflammatory events. This may be relevant, as in the case of inflammation proinflammatory cytokines released by different neighbouring cell types, e.g. activated macrophages, can markedly increase IL-8 and E-selectin expression in endothelial cells [35]. A currently held concept for the function of intestinal epithelial cells that emerges from our studies is therefore that of a homeostatic regulator; this is supported by the observation that the first abnormality in TGF-β-null mice is leucocyte–endothelial adhesion and tissue infiltration [9]. It remains to be established under which conditions epithelial cells secrete TGF-β and under which circumstances this is a deleterious event or plays, conversely, a positive role in the resolution of inflammation in different in vivo locations.
Acknowledgments
We thank Dagmar Herbring and Elke Weber for excellent technical assistance. This work was supported by a grant from the Deuteche Forschungsgemeinschaft (Sonderforschungsbereich 293; Grant B6 to N.L.).
References
- 1.Meenan J, Mevissen M, Monajemi H, Radema SA, Soule HR, Moyle M, Tytgat GNJ, van Deventer SJH. Mechanisms underlying neutrophil adhesion to apical epithelial membranes. Gut. 1996;38:210–5. doi: 10.1136/gut.38.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang GTJ, Eckmann L, Savidge TC, Kagnoff MF. Infection of human intestinal epithelial cells with invasive bacteria upregulates apical intercellular adhesion molecule-1 (ICAM-1) expression and neutrophil adhesion. J Clin Invest. 1996;98:572–83. doi: 10.1172/JCI118825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Diacovo TG, Roth SJ, Buccola JM, Bainton DF, Springer TA. Neutrophil rolling, arrest, and transmigration across activated surface adherent platelets via sequential action of P-selectin and the β2-integrin CD11b/CD18. Blood. 1996;88:146–57. [PubMed] [Google Scholar]
- 4.Rot A, Hub E, Middleton J, et al. Some aspects of IL-8 pathophysiology III: chemokine interaction with endothelial cells. J Leuk Biol. 1996;59:39–44. doi: 10.1002/jlb.59.1.39. [DOI] [PubMed] [Google Scholar]
- 5.Grimm MC, Elsbury SKO, Pavli P, Doe WF. Interleukin-8: cells of origin in inflammatory bowel disease. Gut. 1996;38:90–98. doi: 10.1136/gut.38.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Daig R, Andus T, Aschenbrenner E, Falk W, Schölmerich J, Gross V. Increased interleukin-8 expression in the colon mucosa of patients with inflammatory bowel disease. Gut. 1996;38:216–22. doi: 10.1136/gut.38.2.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ueno A, Murakami K, Yamanouchi K, Watanabe M, Kondo T. Thrombin stimulates production of interleukin-8 in human umbilical vein endothelial cells. Immunology. 1996;88:76–81. doi: 10.1046/j.1365-2567.1996.d01-635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jung HC, Eckmann L, Yang SK, Panja A, Fierer J, Morzycka-Wroblewska E, Kagnoff MF. A distinct array of proinflammatory cytokines is expressed in human colon epithelial cells in response to bacterial invasion. J Clin Invest. 1995;95:55–65. doi: 10.1172/JCI117676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith WB, Noack L, Khew-Goodall Y, Isenmann S, Vadas MA, Gamble JR. Transforming growth factor-β1 inhibits the production of IL-8 and the transmigration of neutrophils through activated endothelium. J Immunol. 1996;157:360–8. [PubMed] [Google Scholar]
- 10.Kuijpers TW, Hakkert BC, Hart MHL, Roos D. Neutrophil migration across monolayers of cytokine prestimulated endothelial cells: a role for PAF and IL-8. J Cell Biol. 1992;117:123–9. doi: 10.1083/jcb.117.3.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lukacs NW, Strieter RM, Elner V, Evanoff HL, Burdick MD, Kunkel SL. Production of chemokines, interleukin-8 and monocyte chemoattractant protein-1, during monocyte:endothelial cell interactions. Blood. 1995;86:2767–73. [PubMed] [Google Scholar]
- 12.Eckmann L, Jung HC, Schuerer-Maly CC, Panja A, Morzycka-Wroblewska E, Kagnoff MF. Differential cytokine expression by human intestinal epithelial cell lines: regulated expression of interleukin-8. Gastroenterology. 1993;105:1689–97. doi: 10.1016/0016-5085(93)91064-o. [DOI] [PubMed] [Google Scholar]
- 13.Schuerer-Maly CC, Eckmann L, Kagnoff MF, Falco MT, Maly FE. Colonic epithelial cell lines as a source of interleukin-8; stimulation by inflammatory cytokines and bacterial lipopolysaccharide. Immunology. 1994;81:85–91. [PMC free article] [PubMed] [Google Scholar]
- 14.Gamble JR, Khew-Goodall Y, Vadas MA. Transforming growth factor-beta inhibits E-selectin expression on human endothelial cells. J Immunol. 1993;150:4494–503. [PubMed] [Google Scholar]
- 15.Colgan SP, Parkos CA, Delp C, Arnaout MA, Madara JL. Neutrophil migration across cultured intestinal epithelial monolayers is modulated by epithelial exposure to IFN gamma in a highly polarized fashion. J Cell Biol. 1993;120:785–98. doi: 10.1083/jcb.120.3.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parkos CA, Delp C, Arnaout MA, Madara JL. Neutrophil migration across a cultured intestinal epithelium; dependence on a CD11/CD18-mediated event and enhanced efficiency in physiological direction. J Clin Invest. 1991;88:1605–12. doi: 10.1172/JCI115473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCormick BA, Colgan SP, Delp-Archer C, Miller SI, Madara JL. Salmonella typhimurium attachment to human intestinal epithelial monolayers: transcellular signalling to subepithelial neutrophils. J Cell Biol. 1993;123:895–907. doi: 10.1083/jcb.123.4.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Madara JL. Migration of neutrophils through epithelial monolayers. Trends Cell Biol. 1994;4:4–7. doi: 10.1016/0962-8924(94)90026-4. [DOI] [PubMed] [Google Scholar]
- 19.Nash S, Stafford J, Madara JL. Effects of polymorphonuclear leukocyte transmigration on the barrier function of cultured intestinal epithelial monolayers. J Clin Invest. 1987;80:1104–13. doi: 10.1172/JCI113167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ades A, Candal FJ, Swerlick RA, George VG, Summers S, Bosse DC, Lawley TJ. HMEC-1: establishment of an immortalized human microvascular endothelial cell line. J Invest Dermatol. 1992;99:683–90. doi: 10.1111/1523-1747.ep12613748. [DOI] [PubMed] [Google Scholar]
- 21.Kucharzik T, Lügering N, Pauels HG, Domschke W, Stoll R. IL-4, IL-10 and IL-13 down-regulate monocyte-chemoattracting protein-1 (MCP-1) production in activated intestinal epithelial cells. Clin Exp Immunol. 1998;111:152–7. doi: 10.1046/j.1365-2249.1998.00481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haraldsen G, Rugtveit J, Kvale D, Scholz T, Muller WA, Hovig T, Brandtzaeg P. Isolation and longterm culture of human intestinal microvascular endothelial cells. Gut. 1995;37:225–34. doi: 10.1136/gut.37.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kucharzik T, Lügering N, Winde G, Domschke W, Stoll R. Colon carcinoma cell lines stimulate monocytes and lamina propria mononuclear cells to produce IL-10. Clin Exp Immunol. 1997;110:296–302. doi: 10.1111/j.1365-2249.1997.tb08331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–9. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 25.Lügering N, Kucharzik T, Fisahn M, Domschke W, Stoll R. Crohn's disease (CD) patients suffering from peripheral arthritis or ankylosing spondylitis reveal restricted T cell receptor Vβ regions in different temporal phases of disease. Clin Exp Immunol. 1996;105:278–84. doi: 10.1046/j.1365-2249.1996.d01-766.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lawrence DA. Transforming growth factor-beta: a general review. Eur Cytokine Netw. 1996;7:363–74. [PubMed] [Google Scholar]
- 27.Raqib R, Lindberg AA, Björk L, Bardhan PK, Wretlind B, Andersson U, Andersson J. Down-regulation of gamma interferon, tumor necrosis factor type I, interleukin 1 (IL-1) type I, IL-3, IL-4, and transforming growth factor type I receptors at the local site during the acute phase of shigella infection. Infect Immun. 1995;63:3079–83. doi: 10.1128/iai.63.8.3079-3087.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taipale J, Lohi J, Saarinen J, Kovanen PT, Keski-Oja J. Human mast cell chymase and leukocyte elastase release latent transforming growth factor-beta1 from the extracellular matrix of cultured human epithelial and endothelial cells. J Biol Chem. 1995;270:4689–96. doi: 10.1074/jbc.270.9.4689. [DOI] [PubMed] [Google Scholar]
- 29.Dignass AU, Podolsky DK. Cytokine modulation of intestinal epithelial cell restitution: central role of transforming growth factor beta. Gastroenterology. 1993;105:1323–32. doi: 10.1016/0016-5085(93)90136-z. [DOI] [PubMed] [Google Scholar]
- 30.Anzano MA, Rieman D, Prichett W, Bowen-Pope DF, Greig R. Growth factor production by human colon carcinoma cell lines. Cancer Res. 1989;49:2898–904. [PubMed] [Google Scholar]
- 31.Sun L, Wu S, Coleman K, Fields KC, Humphrey LE, Brattain MG. Autocrine transforming growth factor-β1 and β2 expression is increased by cell crowding and quiescence in colon carcinoma cells. Exp Cell Res. 1994;214:215–24. doi: 10.1006/excr.1994.1251. [DOI] [PubMed] [Google Scholar]
- 32.Coffey RJ, Shipley GD, Moses HL. Production of transforming growth factors by human colon cancer lines. Cancer Res. 1986;46:1164–9. [PubMed] [Google Scholar]
- 33.McGee DW, Bamberg T, Vitkus SJD, McGhee JR. A synergistic relationship between TNF-α, IL-1β, and TGF-β1 on IL-6 secretion by the IEC-6 intestinal epithelial cell line. Immunology. 1995;86:6–11. [PMC free article] [PubMed] [Google Scholar]
- 34.Pang G, Buret A, O'Loughlin E, Smith A, Batey R, Clancy R. Immunologic, functional and morphological characterization of three new human small intestinal epithelial cell lines. Gastroenterology. 1996;11:8–18. doi: 10.1053/gast.1996.v111.pm8698229. [DOI] [PubMed] [Google Scholar]
- 35.Noble KE, Panayiotidis P, Collins PW, Hoffbrand AV, Yong KL. Monocytes induce E-selectin gene expression in endothelial cells: role of CD11/CD18 and extracellular matrix proteins. Eur J Immunol. 1996;26:2944–51. doi: 10.1002/eji.1830261221. [DOI] [PubMed] [Google Scholar]


