Abstract
We previously reported that c-kit+ stem cells which give rise to extrathymic T cells are present in the liver of adult mice. Further characterization of extrathymic T cells in the liver of adult mice is conducted here. When mice with a liver shield were lethally (9.5 Gy) irradiated, all mice survived. All tested organs showed a distribution pattern of hepatic lymphocytes on day 7. The distribution pattern in the liver was characterized by an abundance of NK (CD3− IL-2Rβ+) and extrathymic T cells (CD3intIL-2Rβ+) before and after irradiation. To determine their function, post-irradiation allogeneic bone marrow transplantation (BMT) was performed in mice with or without a liver shield. Allogeneic BM cells were rejected in mice with a liver shield and specific activation of CD8+ CD3intIL-2Rβ+ cells was induced. At that time, potent cytotoxicity of liver mononuclear cells (MNC) against allogeneic thymocytes was induced. Both NK1.1+ and NK1.1− subsets of CD3int cells expanded in these mice. An in vivo elimination experiment of the subsets indicated that the NK1.1+ subset of CD3int cells (i.e. NK T cells) was much more associated with the rejection of allogeneic BM cells. However, even after the elimination of NK T cells, allogeneic BM cells were rejected. In this case, granulocytes expanded in parallel with NK1.1− subsets. Granulocytes may also be associated with the rejection of allogeneic BM cells. These results suggest that the liver is an important haematopoietic organ even in adult life.
Keywords: c-kit+ stem cells, extrathymic T cells, liver, NK cells, NK T cells
INTRODUCTION
Accumulating evidence from recent studies on lymphocytes in the digestive tract, including the liver [1–4] and intestine [5–8], has revealed that these lymphocytes are mainly either NK cells or extrathymic T cells, both of which might have developed earlier phylogenetically than conventional T cells. The liver comprises NK cells and intermediate T cell receptor (TCR) cells (TCRint cells), whereas the intestine comprises unique T cells such as γδ T cells and double-positive (DP) αβ T cells. Concerning the existence of c-kit+ stem cells in the liver [9] and intestine [10], these NK cells and extrathymic T cells may be generated in situ from their own stem cells. To investigate this possibility, we recently used the parabiotics of B6.Ly5.1 and B6.Ly5.2 mice which shared circulation until day 3 after parabiosis (submitted for publication). The origin of lymphocytes was determined by anti-Ly5.1 and anti-Ly5.2 MoAbs in conjunction with immunofluorescence tests. All lymphocytes in the blood, spleen, lymph nodes and intestinal Peyer's patches became a half-and-half mixture by day 14 after parabiosis. In sharp contrast, extrathymic T cells in the liver and intestine hardly mixed with partner cells by day 14 and thereafter. These results suggest that extrathymic T cells in the liver and intestine arise from their own stem cells (at least already pre-existing at birth), independent of those in the bone marrow. Among extrathymic T cells, those in the intestine tend to function at the sites where they are generated, while those in the liver sometimes migrate to other sites of inflammation.
A major objective of the present study was to characterize the function of NK cells and extrathymic T cells (including NK T cells) which are generated in the liver. In this study, a liver shield was employed to expand NK cells and extrathymic T cells of hepatic origin at generalized sites of the body. The maximal and selective expansion of NK and extrathymic T cells was induced when mice whose livers were shielded were lethally (9.5 Gy) irradiated (i.e. accompanying the maximal suppression of cells of both bone marrow and thymic origins). Using this experimental protocol, we were able to show some unique characteristics of NK cells and extrathymic T cells of hepatic origin; namely, in terms of their migration to other sites, the phenotype of NK cells and extrathymic T cells expanded at other sites. We especially emphasize here that NK T cells among TCRint cells have potent cytotoxicity against allogeneic lymphocytes (i.e. the disparity of polymorphic MHC antigens). In other words, the recognition of MHC by NK T cells is not confined to monomorphic mononuclear cell (MNC) antigens such as CD1 [11–15] and TL molecules [16,17]. The present study yielded new information on NK T cells and TCRint cells generated in the liver.
MATERIALS AND METHODS
Mice
Young male C57Bl/6 (B6) (H-2Kb) and C3H/HeN (H-2Kk) mice were purchased from Charles River Inc. (Atsugi, Japan). All mice were fed under specific pathogen-free conditions. Animal welfare was in accordance with the guidelines of Niigata University.
Irradiation
Mice aged 6–8 weeks were anaesthetized with i.p. injected Tribromoethanol (2,2,2-tribromoethanol; Aldrich Chemical Co., Milwaukee, WI) and positioned in an apparatus designed to provide whole-body irradiation (Fig. 1). The liver of experimental animals was shielded with a lead plate; control animals were unshielded. All irradiated mice received a single 9.5-Gy dose, delivered from a 60Co γ-ray model (Toshiba RCR-120-C5-111TBq) or a Toshiba LMR-15C 10-MV linear accelerator (Toshiba Co., Tokyo, Japan).
Fig. 1.
Position where the liver is protected by a lead plate. All unshielded mice irradiated with a lethal dose of 9.5 Gy died within 5 days. However, mice with a liver shield did not succumb.
Allogeneic bone marrow transplantation
After irradiation, 5 × 106 bone marrow (BM) cells of allogeneic origin were intravenously injected (e.g. B6 → C3H/He and C3H/He → B6).
Cell preparation
Mice were anaesthetized with ether and killed by total bleeding from the incised axillary artery and vein. The liver was removed, cut into small pieces with scissors, pressed through 200 G stainless steel mesh, and suspended in Eagle's minimal essential medium (MEM) containing 5 mm HEPES (Nissui Pharmaceutical Co., Tokyo, Japan) and 2% heat-inactivated newborn calf serum. Washed once, the cells were resuspended in 30–35% Percoll solution containing 100 U/ml heparin and centrifuged at 2000 rev/min for 15 min at room temperature [18]. The pellet which contained liver MNC was resuspended in erythrocyte lysis solution (155 mm NH4Cl, 10 mm KHCO3, 1 mm EDTA, 170 mm Tris, pH 7.3), then washed twice with medium.
Thymocytes were obtained by forcing the thymus through 200 G steel mesh. Splenocytes were obtained by pressing the spleen through the steel mesh, followed by pellet treatment with erythrocyte lysis solution. BM cells from femurs and tibias were obtained by cutting the ends of the bones and forcing medium through the bone shaft. BM cells were further separated from erythrocytes with erythrocyte lysis solution.
Immunofluorescence tests
Surface phenotypes of cells were identified by using MoAbs in conjunction with a two-colour immunofluorescence test [19]. The MoAbs used included FITC- or biotin-conjugated reagents of anti-CD3 (145-2C11), NK1.1 (PK136), TER119 (erythroid marker), anti-Gr-1 (RA3-8C5), anti-Mac-1 (M1/70), anti-H-2Kb, and anti-H-2Kk MoAbs, obtained from PharMingen (San Diego, CA). Biotin-conjugated anti-IL-2Rβ (TM-β1) MoAb, FITC- and PE-conjugated anti-CD4 (L3T4), and anti-CD8α (Lyt-2) MoAbs were purchased from Becton Dickinson (Mountain View, CA). Biotin-conjugated reagents were developed with PE-conjugated avidin (Caltag Labs, San Francisco, CA). The fluorescence-positive cells were analysed by a FACScan (Becton Dickinson). Dead cells were excluded by forward scatter, side scatter and PI gating. Fc receptor blocker (anti-CD16 antibody; PharMingen) was used if necessary.
Cytotoxicity assay
Target cells used in the cytotoxicity assays were syngeneic or allogeneic thymocytes, NK-sensitive YAC-1 cells and NK-resistant EL-4 cells [20,21]. Target cells (3 × 106 cells) were labelled with 100 μCi Na251CrO4 for 60 min at 37°C in RPMI 1640 medium containing 10% fetal calf serum (FCS), washed three times with the medium, and subjected to the cytotoxicity assay. Labelled targets (104/well) were incubated in a total volume of 200 μl with effector cells in the medium in 96-well round-bottomed microtitre plates. The plates were centrifuged after incubation for 4 h, after which the supernatant was harvested and counted with a gamma counter. The cytotoxicity was calculated as the percentage of releasable counts after subtraction of spontaneous release. Spontaneous release was < 15% of the maximum release.
RESULTS
Distribution of lymphocytes of hepatic origin to other organs when mice were lethally irradiated
When unshielded mice were lethally (9.5 Gy) irradiated, all mice died within 5 days (data not shown). When the liver of mice was shielded with a lead plate (Fig. 1), none of them succumbed. This might be due to the existence of c-kit+ stem cells in the liver.
We then examined how lymphocyte subsets repopulated various organs in these mice. To simultaneously identify NK cells (CD3−IL-2Rβ+), extrathymic T cells (CD3intIL-2Rβ+), and conventional T cells (CD3highIL-2Rβ−), two-colour staining for CD3 and IL-2Rβ was conducted (Fig. 2). In untreated control mice, NK and CD3int cells were abundant in the liver and spleen but were extremely rare in the BM (see top of Fig. 2). In this figure, only a B6 control is represented but a C3H/He control shows the similar staining pattern (data not shown). However, a unique staining pattern emerged on day 7 in both B6 and C3H/He irradiated mice protected with a liver shield. Namely, large proportions of NK and CD3int cells appeared in all tested organs. This might be due to the following reasons: (i) radiosensitive conventional T cells had decreased in proportion and had not yet expanded on day 7; and (ii) NK and CD3int cells of hepatic origin had expanded and had also begun to be generated in various organs. On day 21, NK and CD3int cells which once appeared in the spleen and BM again disappeared from these organs of both mice, possibly because NK and CD3int cells of hepatic origin did not have the receptors for homing in on these organs for a long period.
Fig. 2.
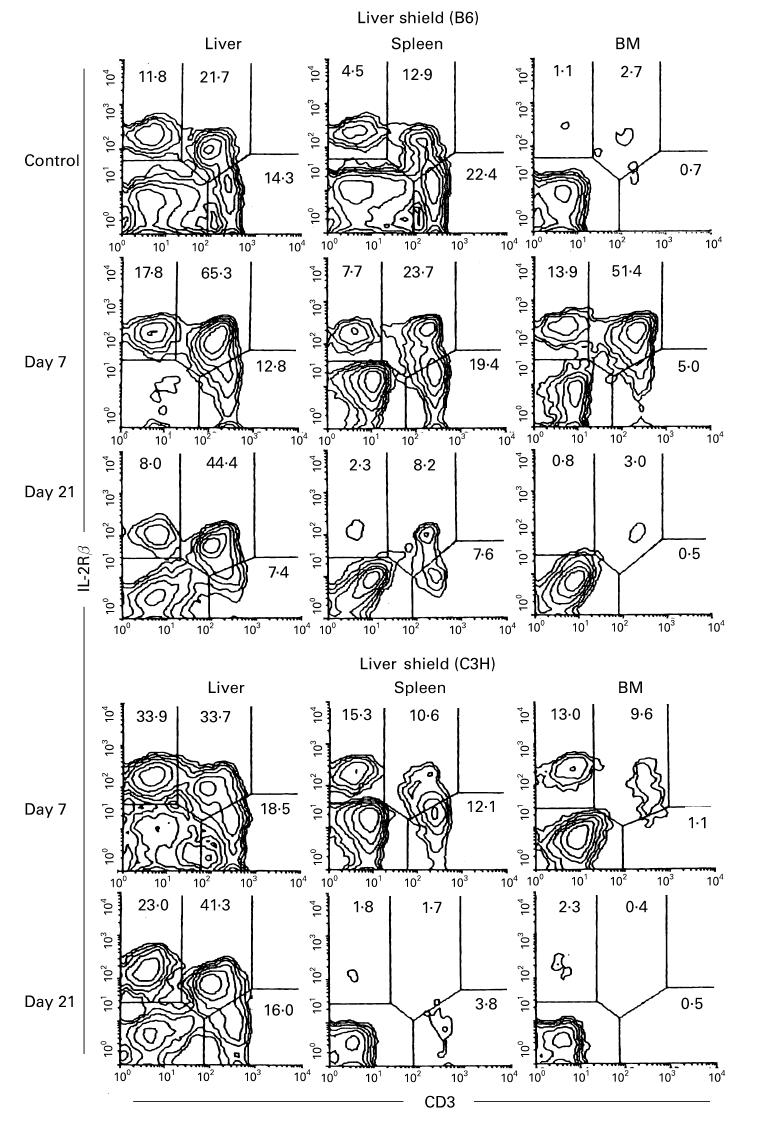
Migration of lymphocyte subsets in the liver to the spleen and bone marrow (BM) after irradiation in mice with a liver shield. B6 and C3H/He mice with a liver shield were irradiated (9.5 Gy) and phenotypic characterization was performed in various organs on days 7 and 21. Control indicates untreated B6 mice. Two-colour staining for CD3 and IL-2Rβ was conducted to identify NK cells (CD3− IL-2Rβ+), extrathymic T cells (CD3intIL-2Rβ+), and conventional T cells (CD3high IL-2Rβ−). Numbers indicate the proportion of fluorescence-positive cells in corresponding areas. Representative results of three experiments are depicted.
Complete elimination of allogeneic BM cells in irradiated mice with a liver shield
To examine the interaction of NK and extrathymic T cells (i.e. CD3int cells) (both of them remained when the liver was shielded) with allogeneic cells, irradiated C3H/He mice either with or without a liver shield were injected with 5 × 106 BM cells of B6 mice origin (Fig. 3). The origins of lymphocytes were first identified by anti-H-2Kb and anti-H-2Kk MoAbs (Fig. 3a). It was found that there was no expansion of donor lymphocytes in irradiated mice with a liver shield (see day 7). In sharp contrast, the majority of expanding lymphocytes in irradiated mice without a liver shield were of donor origin, especially on day 21.
Fig. 3.
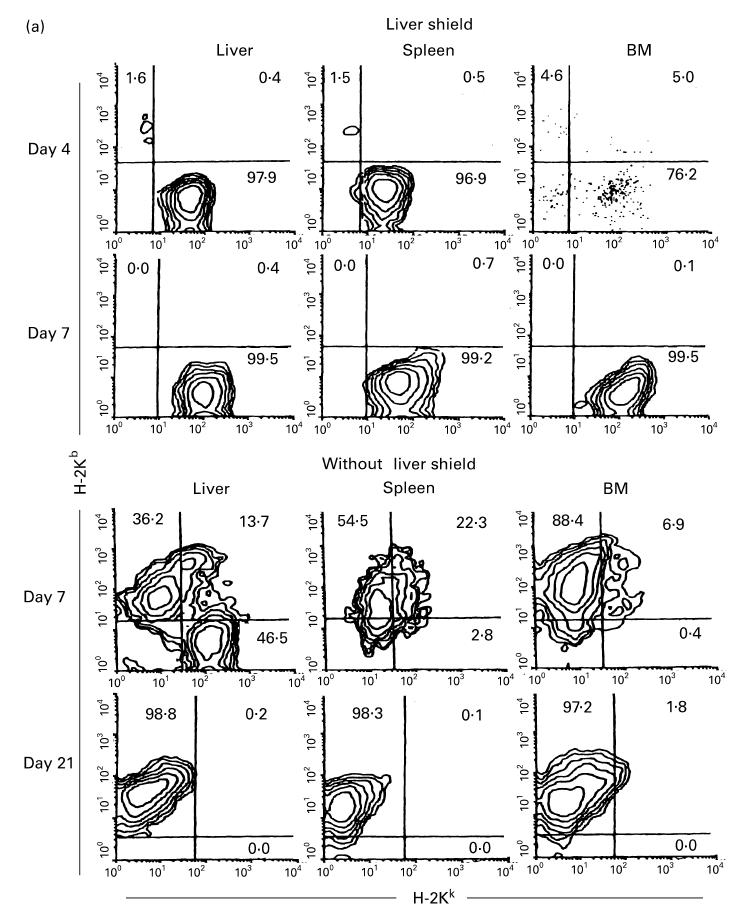
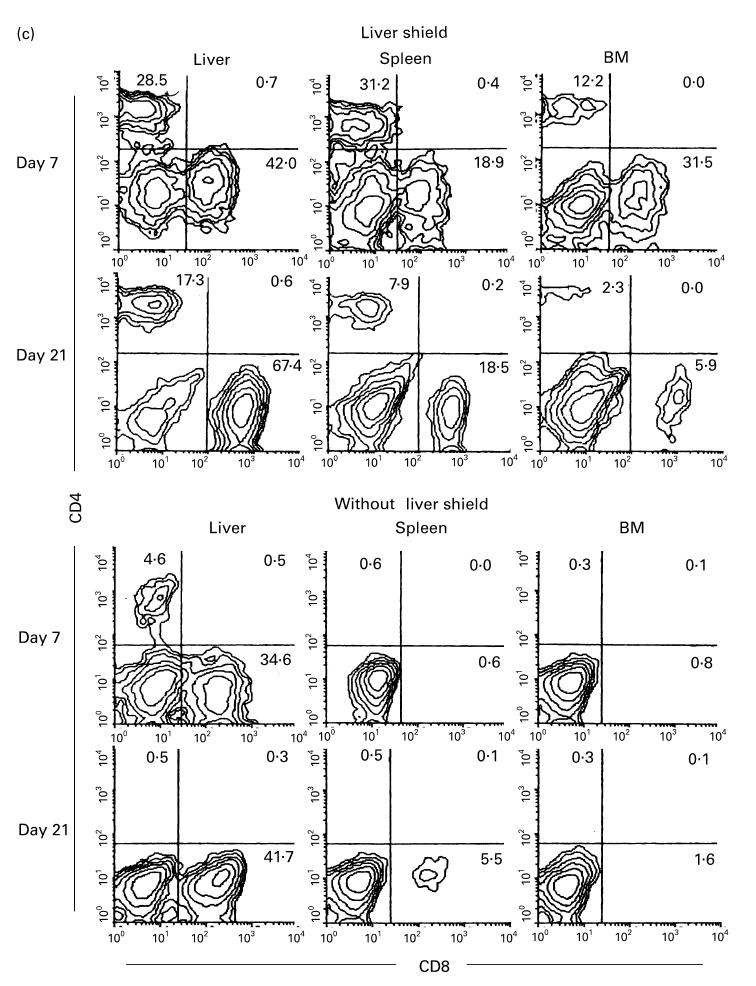
(see also pp 438 and 439.) Phenotypic characterization of lymphocytes in various organs of irradiated mice with or without a liver shield, which were subsequently subjected to allogeneic bone marrow transplantation (BMT). (a) Two-colour staining for H-2Kb and H-2Kk. (b) Two-colour staining for CD3 and IL-2Rβ. (c) Two-colour staining for CD4 and CD8. In this experiment, irradiated mice were C3H/He and mice subjected to BMT were B6 mice. Representative results of three experiments are depicted. In mice with a liver shield, lymphocytes (mainly CD3intCD8+) of recipient origin (H-2Kk) expanded. On the other hand, CD3intCD8+ lymphocytes of donor origin (H-2Kb) expanded in mice without a liver shield.
There was a possibility that NK and CD3int cells were somewhat activated during their interaction with allogeneic BM cells. With this in view, two-colour staining for CD3 and IL-2Rβ was conducted in irradiated mice which underwent bone marrow transplantation (BMT) (Fig. 3b). Such mice with or without a liver shield were examined. Surprisingly, the expansion of CD3int cells was extremely prominent in all tested organs. Compared with the pattern without BMT (see day 21 of B6 and C3H/He mice in Fig. 2), it was striking. The difference was seen in CD3int cells of all tested organs in irradiated mice with a liver shield which subsequently underwent BMT. Actual cell yields from various organs in these mice increased to a level more than three times that in the same mice without BMT. In other words, a major population which interacted with allogeneic cells might be CD3int cells.
Lethally irradiated mice with an unshielded liver which underwent subsequent BMT showed expansion of CD3int cells only in the liver. To determine further the phenotype of expanding CD3int cells, two-colour staining for CD4 and CD8 was conducted (Fig. 3c). Reflecting the recognition of allogeneic MHC antigens, most expanding T cells became CD8+ in irradiated mice with or without a liver shield which were subsequently subjected to BMT.
In a final part of these experiments, cell yields in irradiated mice with or without a liver shield which were subsequently subjected to BMT are represented (Fig. 4). A major difference was that the number of BM cells increased in irradiated mice with a liver shield, while the number of liver MNC increased in irradiated mice without a liver shield.
Fig. 4.
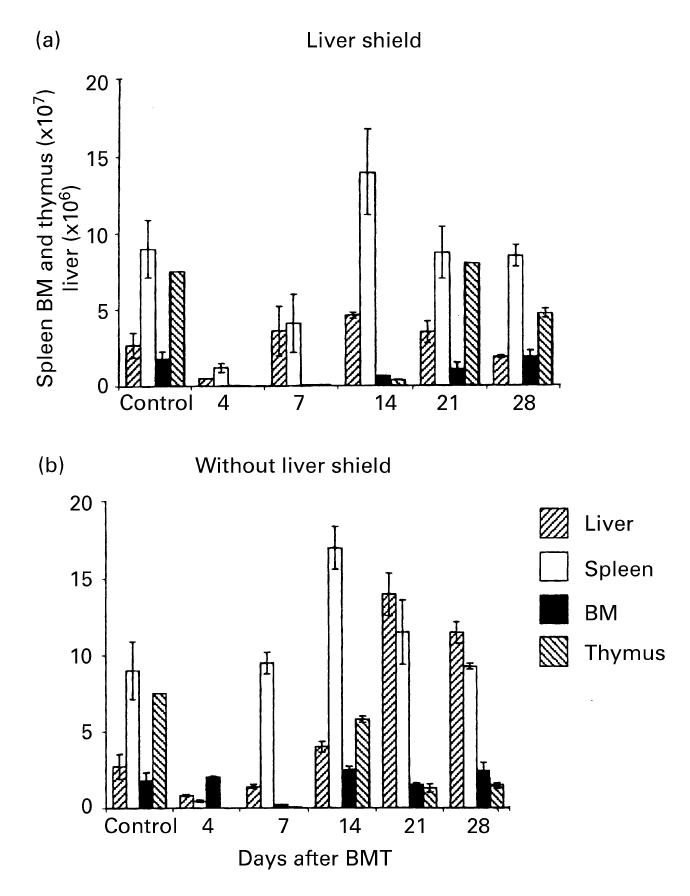
Time kinetics of the number of cells yielded in various organs of C3H/He mice, with or without a liver shield, which were subjected to allogeneic (B6) bone marrow transplantation (BMT). The major sites for expanding cells after treatment were the liver and spleen. The mean and 1 s.d. from five mice are represented.
Induction of cytotoxicity in the liver of irradiated mice with a liver shield
As shown previously [20,21], CD3int cells mediate autoreactivity against syngeneic thymocytes while NK cells mediate NK cytotoxicity against YAC-1 cells. Since many NK cells and CD3int cells were induced in the liver of irradiated mice with a liver shield which were subjected to allogeneic BMT, it was examined how these cytotoxicities were distributed (Fig. 5). Liver MNC were obtained from normal control C3H/He mice and irradiated C3H/He mice with a liver shield, which were subjected to allogeneic (B6 mice) BMT (day 7). Reflecting the expansion of CD3int cells in mice with a liver shield, liver MNC mediated autologous cytotoxicity against C3H/He thymocytes. More importantly, they also acquired allogeneic cytotoxicity against B6 thymocytes. Liver MNC in mice with a liver shield also exerted NK cytotoxicity against YAC-1 cells and some unique cytotoxicity against NK-resistant EL-4 cells. Liver MNC of control mice showed only a low level of cytotoxicity against syngeneic thymocytes and YAC-1 cells.
Fig. 5.
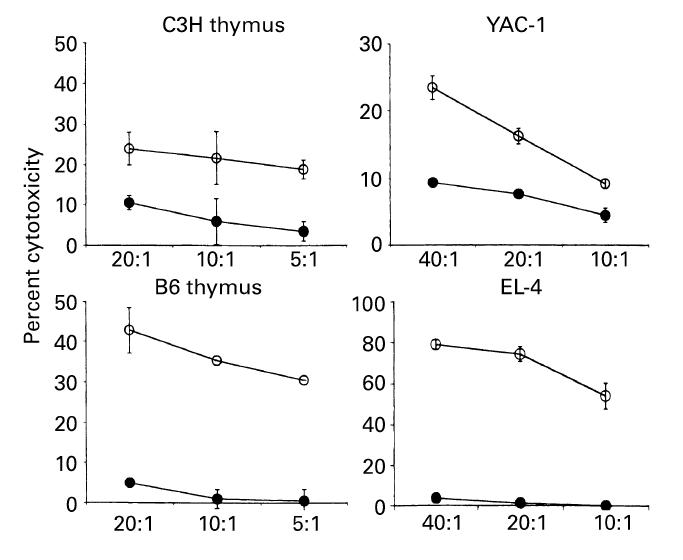
Induction of potent cytotoxicity against syngeneic thymocytes, allogeneic thymocytes, and other targets in mice with a liver shield and which underwent allogeneic bone marrow transplantation (BMT). Effector cells were isolated from the liver of control C3H/He mice (•) or irradiated C3H/He mice (○) with a liver shield and subjected to allogeneic (B6) BMT. The mean and 1 s.d. from four experiments are depicted. Possibly due to the increased proportions of NK cells and CD3int cells in the liver of those mice and the interaction of B6 cells, potent cytotoxicity against allogeneic thymocytes (B6), YAC-1 cells (NK-sensitive target), syngeneic thymocytes (C3H/He), and EL-4 (NK-resistant target) was induced.
Generation of erythroid and myeloid cells in the liver, spleen, and bone marrow
Attention was turned to the generation of erythroid and myeloid cells in irradiated mice with or without liver shield, which were subjected to BMT. Two-colour staining for CD3 and TER119 (erythroid cell marker) and that for Mac-1 and Gr-1 were conducted (Fig. 6). In mice with liver shield, the generation of erythroid cells occurred in the spleen and BM but not at all in the liver. In the case of myeloid cells, the generation of macrophages (Mac-1+ Gr-1−) occurred in the liver while the generation of granulocytes (Mac-1+ Gr-1+) occurred in all tested organs.
Fig. 6.
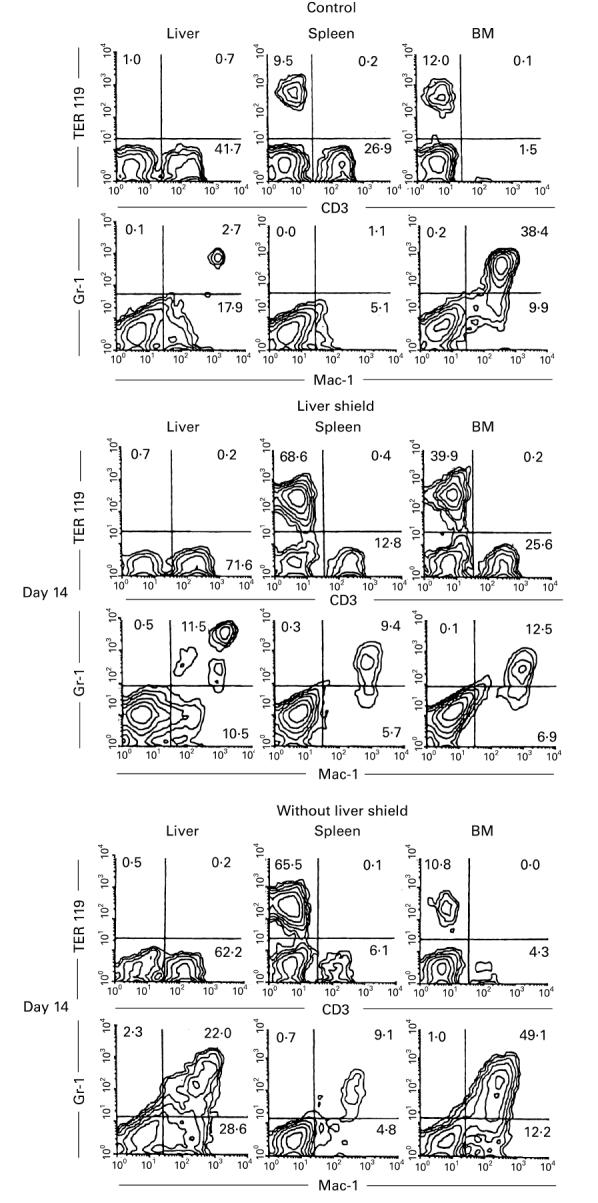
Prominent expansion of erythroid cells in the spleen and of myeloid cells in the liver of irradiated mice with a liver shield. Two-colour staining for TER119 and CD3 and that for Gr-1 and Mac-1 were conducted in control mice and irradiated mice with or without a liver shield (on day 14). Representative results of three experiments are depicted. Erythroid (TER119+) cells expanded prominently in the spleen of mice with a liver shield, whereas myeloid cells expanded in the liver of the same mice without a liver shield.
The generation of erythroid and myeloid cells in irradiated mice without liver shield, which were subjected to allogeneic BMT, was somewhat different from that in the above mentioned mice. Thus, the generation of erythroid cells (TER119+ CD3−) occurred mainly in the spleen. Instead, the generation of myelold cells, including both macrophages and granulocytes, occurred in the liver and bone marrow.
Induction of NK T cells in mice with a liver shield
To characterize further the properties of CD3int cells, the NK1.1+ subset of CD3int cells (i.e. NK T cells) was examined. Approximately 50% of CD3int cells in the liver are NK1.1+T cells in normal mice. In this experiment, B6 mice (NK1.1+ strain) were used as recipients and C3H/He mice (NK1.1− strain) were used as donors for BMT (Fig. 7). It was found that NK T cells also expanded in parallel with the expansion of CD3int cells (Fig. 7a). This was prominent in the liver but not in other tested organs.
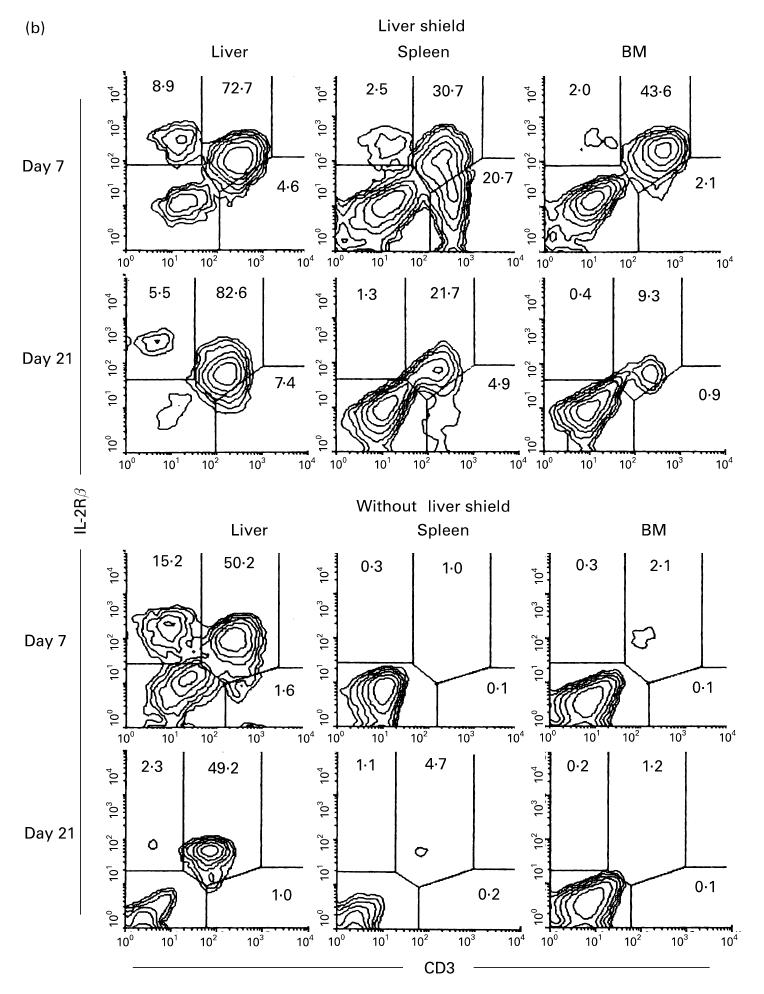
Fig. 7.
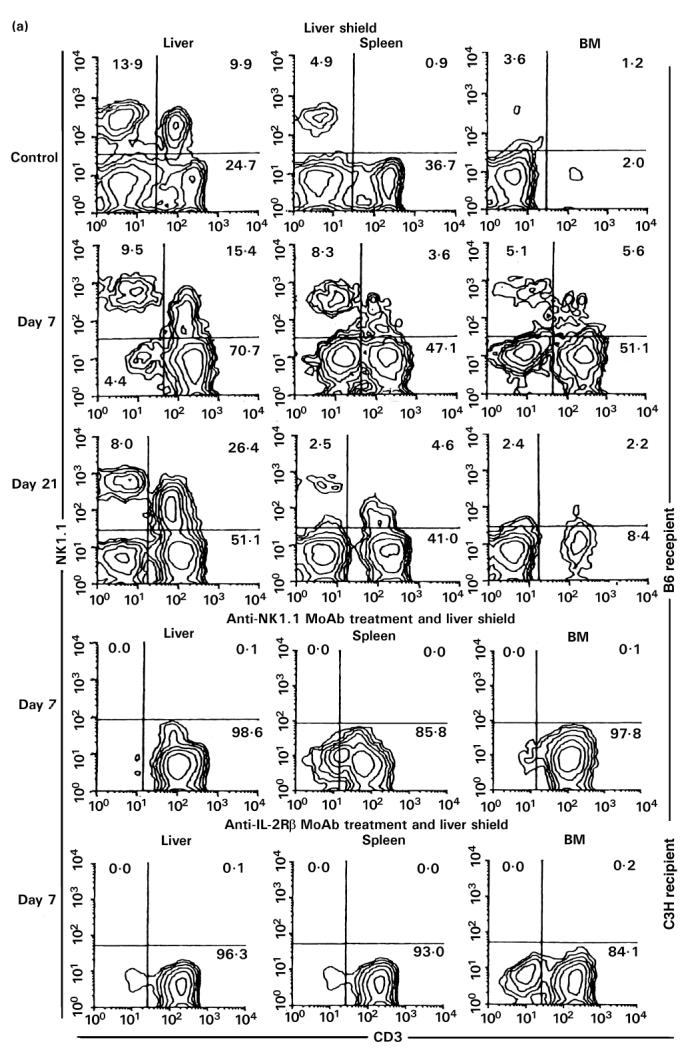
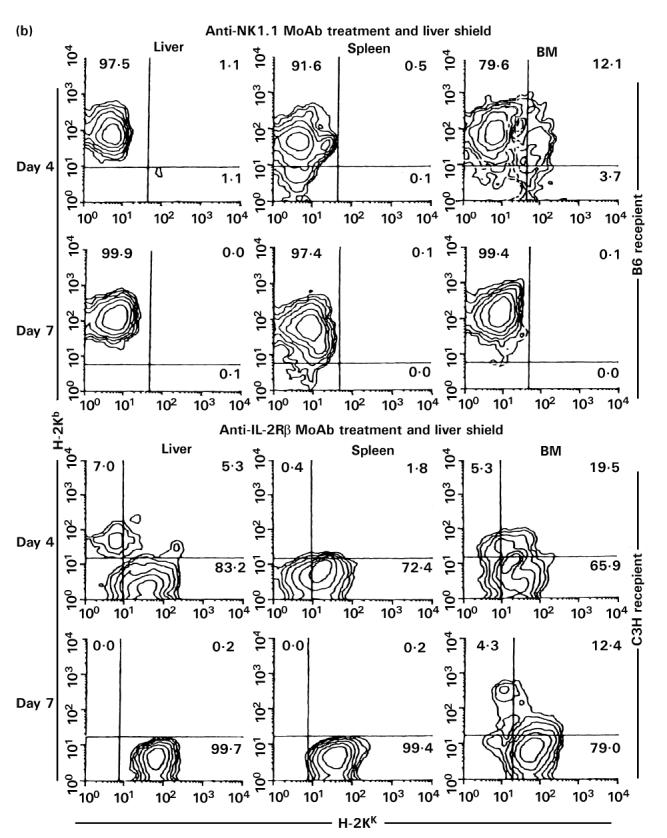
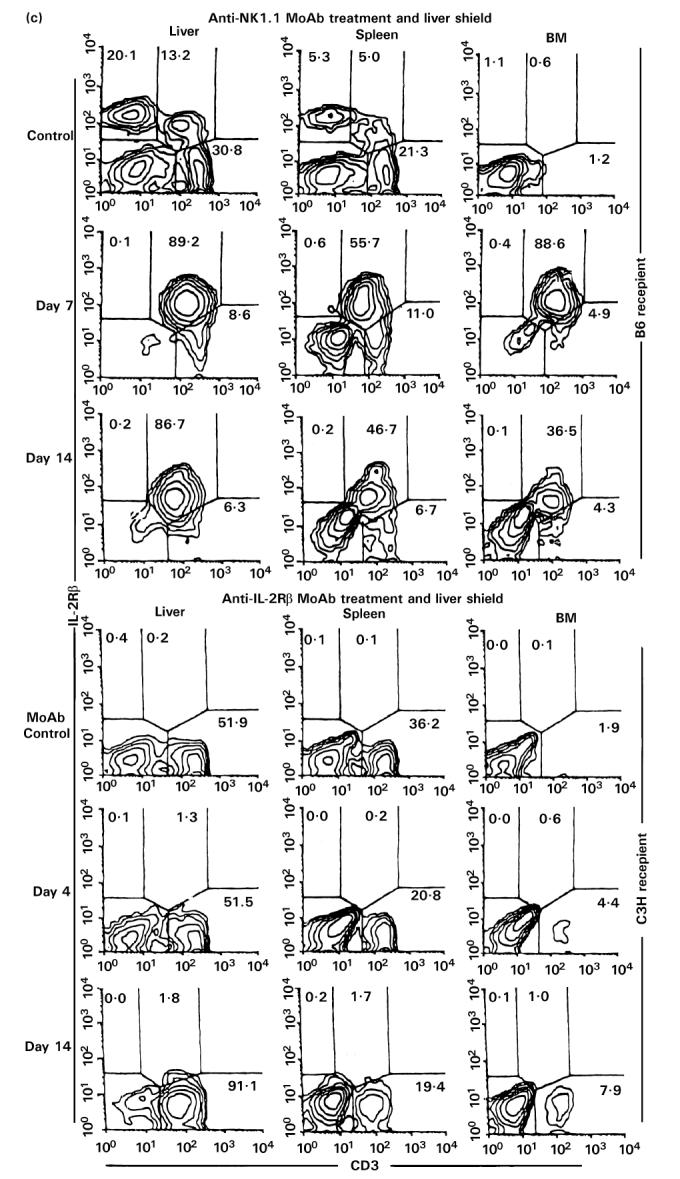
(See also pp 444 and 445.) Injection of anti-NK1.1 MoAb or anti-IL-2Rβ MoAb in vivo. (a) Two-colour staining for CD3 and NK1.1. (b) Two-colour staining for H-2Kb and H-2Kk. (c) Two-colour staining for CD3 and IL-2Rβ. To eliminate NK, NK T cells and TCRint cells, in vivo injection of anti-NK1.1 MoAb (200 μg/mouse) or anti-IL-2Rβ MoAb (500 μg/mouse) was performed, respectively. A single in vivo injection of anti-NK1.1 MoAb was first performed. Every 3 days after in vivo injection of anti-IL-2Rβ MoAb was then continued at least 21 days from the beginning. Representative data of three experiments are depicted. The elimination of NK and NK T cells (or CD3int cells) was complete.
To characterize further the function of NK T cells, in vivo elimination of NK T cells was conducted by the injection of anti-NK1.1 MoAb (see middle row in Fig. 7a). It was confirmed that this injection clearly eliminated NK and NK T cells in all tested organs. Allogeneic BMT was also done in the combination of C3H/He→B6. A similar result was produced by the injection of anti-IL-2Rβ MoAb. This treatment also eliminated NK and CD3int cells. Allogeneic BMT was also done in the combination of B6→C3H/He.
By using this elimination method for NK and NK T cells (or CD3int cells), irradiated mice with a liver shield were then examined as to how they rejected allogeneic BM cells (Fig. 7b). Some difference resulted from the treatments with anti-NK1.1 and anti-IL-2Rβ MoAbs. In the case of treatment with anti-NK1.1 MoAb, only lymphocytes of recipient origin expanded. These results suggest that the remaining NK1.1− subset of CD3int cells was associated with the rejection of allogeneic BMT. In contrast, some lymphocytes of donor origin expanded in mice treated with anti-IL-2Rβ MoAb, suggesting that IL-2Rβ−CD3int cells were still associated to some degree with the rejection of allogeneic BM cells (see Discussion).
Even after the elimination of NK and NK T cells, CD3int cells (namely the NK1.1− subset of CD3int cells) were confirmed to expand in these mice (top of Fig. 7c). Due to the elimination of IL-2Rβ+ cells, only IL-2Rβ−CD3+ cells expanded in mice treated with anti-IL-2Rβ MoAb (a low row of Fig. 7c).
Possible association of granulocytes with the rejection of allogeneic BM cells by NK1.1−CD3int cells
As already shown, NK1.1−CD3int cells almost entirely lack cytotoxicity against allogeneic cells. However, mice treated with anti-NK1.1 MoAb still rejected allogeneic BM cells. In this regard, we investigated whether granulocytes were activated in these mice (Fig. 8). This concept arises from the fact that NK1.1−CD3int cells have the ability to induce granulocytes at sites of inflammation (submitted for publication). Two-colour staining for Mac-1 and Gr-1 showed that many granulocytes appeared in all tested organs of these mice. These results suggest a possible association of granulocytes with the rejection of allogeneic BM cells by a NK1.1− subset of CD3int cells.
Fig. 8.
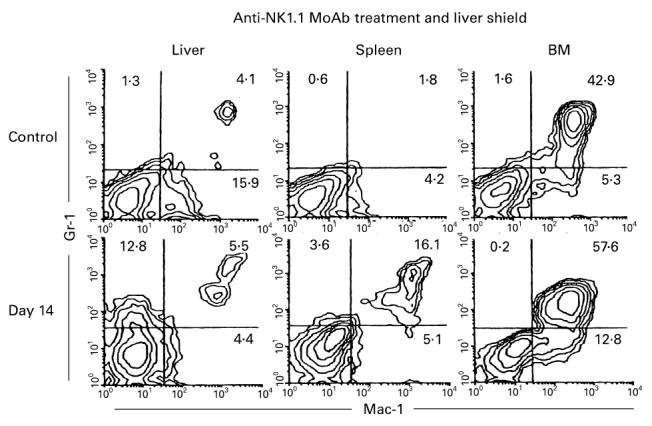
Expansion of granulocytes in irradiated mice with a liver shield, treatment with anti-NK1.1 MoAb, and subsequent allogeneic bone marrow transplantation (BMT). Two-colour staining for Gr-1 and Mac-1 was conducted. Representative results of three experiments are shown. Even after the elimination of NK and NK T cells, NK1.1−CD3int cells rejected allogeneic BM cells. In this case, granulocytes always expanded, especially in the BM.
DISCUSSION
In the present study, we demonstrate that a liver shield prevented lethally (9.5 Gy) irradiated mice from succumbing and that lymphocyte subsets existing in the liver replaced those in the spleen and bone marrow. Such lymphocyte subsets included NK cells (CD3−IL-2Rβ+) and extrathymic T cells (CD3intIL-2Rβ+). In addition to the migration of lymphocyte subsets from the liver to other organs, the generation of erythroid cells was induced in the spleen and the generation of myeloid cells was induced in the bone marrow. These results suggest that cells which survived due to the liver shield contained not only mature lymphocytes but also pluripotent stem cells in the liver of these mice. In other words, stem cells in the liver usually give rise to NK cells and extrathymic T cells, but if they migrate to other organs they begin to induce a potential as pluripotent stem cells. Until recently, many investigators believed that the bone marrow is only the site where pluripotent stem cells exist. However, we reveal in this study that the adult liver also comprises pluripotent stem cells.
It is well known that the liver is a haematopoietic organ at fetal stages [22,23]. Even after birth, this organ carries pluripotent stem cells [16,24]. As shown in our previous study [16], c-kit+ stem cells exist in the parenchymal space of the adult liver and many NK cells and extrathymic T cells are generated in situ. In addition, we found that parabiotics of B6.Ly5.1 and B6.Ly5.2 mice which shared circulation were not replaced by partner cells in CD3intIL-2Rβ+ cells or NK1.1+ T cells in the liver (submitted for publication). It is therefore concluded that stem cells which exist in the liver do not arise from those in the BM, at least after birth.
The migration of NK cells and extrathymic T cells into the spleen and BM was found to be always a temporal event around day 7 after irradiation. On day 21, almost all NK cells and extrathymic T cells disappeared from these organs. As shown previously [25–27], NK cells and extrathymic T cells have CD44+ l-selectin− while conventional T cells have CD44−l-selectin+. This inverted expression of adhesion molecules between these lymphocytes might be associated only with their temporal migration, as shown in this study. In other words, it is not appropriate for the adhesion molecules on NK cells and extrathymic T cells to stay longer in the spleen and BM.
To determine the functional characteristics of NK cells and extrathymic T cells in the liver, we applied allogeneic BMT in irradiated mice with or without a liver shield. The result was very striking; irradiated mice with a liver shield completely rejected allogeneic BM cells, while irradiated mice without a liver shield accepted allogeneic BM cells. As shown in the cytotoxicity assay, liver MNC which remained in mice with a liver shield showed potent cytotoxicity against not only syngeneic thymocytes but also allogeneic thymocytes. Many investigators believe that TCRint cells or NK T cells recognize some self-antigens in the context with monomorphic CD1 [11–15] or TL antigens [16,17]. However, this result suggests that TCRint cells in the liver may recognize allogeneic MHC antigens as well. Reflecting the recognition of allogeneic MHC antigen, a major population of TCRint cells became CD8+.
Among TCRint cells, there are two subsets, namely, NK1.1+ and NK1.1− cells. The former are called NK T cells or natural T cells [12–15]. However, we are also concerned with the existence of an NK1.1− subset among TCRint cells [4]. Indeed, mice with a liver shield which rejected allogeneic BM cells showed the expansion of the NK1.1− subset as well as the NK1.1+ subset. Mice treated with anti-NK1.1 MoAb were found to be still associated with the rejection of allogeneic BM cells. Since the NK1.1− subset of TCRint cells almost entirely lacks allogeneic cytotoxicity [21], some other effector cells might be responsible for this phenomenon. Granulocytes are one such candidate. At the sites of T cell responses, granulocytes have begun to be known as cooperative effector cells in graft-versus-host disease (GVHD) responses and virus-induced inflammations (submitted for publication).
In the experiment using anti-IL-2Rβ MoAb, it was found that the remaining IL-2Rβ−CD3int cells were still associated with the rejection of allogeneic BM cells, if it was incomplete. In this experimental protocol, all expanding T cells were estimated to be CD3int cells (or NK cells) (see Fig. 3b). In this regard, these IL-2Rβ−cells were CD3int cells.
Finally, it should be emphasized that the liver is also an important organ which possess its own stem cells. Usually, they do not give rise to erythroid and myeloid cells at this site. However, the present study has revealed that they may give rise to erythroid and myeloid cells, depending on the microenvironments to which they migrate.
Acknowledgments
We wish to thank Mrs Yuko Kaneko for preparation of the manuscript.
References
- 1.Abo T, Ohteki T, Seki S, Koyamada N, Yoshikai Y, Masuda T, Rikiishi H, Kumagai K. The appearance of T cells bearing self-reactive T cell receptor in the livers of mice injected with bacteria. J Exp Med. 1991;174:417–24. doi: 10.1084/jem.174.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seki S, Abo T, Ohteki T, Sugiura K, Kumagai K. Unusual αβ T cells expanded in autoimmune lpr mice are probably a counterpart of normal T cell in the liver. J Immunol. 1991;147:1214–21. [PubMed] [Google Scholar]
- 3.Sato K, Ohteki T, Hasegawa K, et al. Evidence for extrathymic generation of intermediate TCR cells in the liver revealed in thymectomized, irradiated mice subjected to bone marrow transplantation. J Exp Med. 1995;182:759–67. doi: 10.1084/jem.182.3.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Watanabe H, Miyaji C, Kawachi Y, Iiai T, Ohtsuka K, lwanaga T, Takahashi-Iwanaga H, Abo T. Relationships between intermediate TCR cells and NK1.1+ T cells in various immune organs. NK1.1+ T cells are present within a population of intermediate TCR cells. J Immunol. 1995;155:2972–83. [PubMed] [Google Scholar]
- 5.De Geus B, Van Den Enden M, Coolen C, Nagelkerken L, Van Der Heijden P, Rozing H. Phenotype of intraepithelial lymphocytes in euthymic and athymic mice: implications for differentiation of cells bearing a CD3-associated γδ T cell receptor. Eur J Immunol. 1990;20:291–8. doi: 10.1002/eji.1830200210. [DOI] [PubMed] [Google Scholar]
- 6.Guy-Grand D, Cerf-Benusussan NB, Malissen B, Malassis-Seris M, Briottet C, Vassalli P. Two gut intraepithelia CD8+ lymphocyte populations with different T cell receptors: a role for the gut epithelium in T cell differentiation. J Exp Med. 1991;173:471–81. doi: 10.1084/jem.173.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bandeira A, Itohara S, Bonneville M, Burlen-Defranoux O, Mota-Santos T, Coutinho A, Tonegawa S. Extrathymic origin of intestinal intraepithelial lymphocytes bearing T-cell antigen receptor γδ. Proc Natl Acad Sci USA. 1991;88:43–47. doi: 10.1073/pnas.88.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rocha B, Vassalli P, Guy-Grand D. The Vβ repertoire of mouse gut homodimeric α CD8+ intraepithelial T cell receptor αβ lymphocytes reveals a major extrathymic pathway of T cell differentiation. J Exp Med. 1991;173:483–6. doi: 10.1084/jem.173.2.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Watanabe H, Miyaji C, Seki S, Abo T. C-kit+ stem cells and thymocyte precursors in the livers of adult mice. J Exp Med. 1996;184:687–93. doi: 10.1084/jem.184.2.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kanamori Y, Ishimaru K, Nanno M, Maki K, Ikuta K, Nariuch H, Ishikawa H. Identification of novel lymphoid tissues in murine intestinal mucosa where clusters develop. J Exp Med. 1996;184:1449–59. doi: 10.1084/jem.184.4.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Porcelli S, Morita CT, Brenner MB. CD1b restricts the response of human CD4−CD8− T lymphocytes to a microbial antigen. Nature. 1992;360:593–7. doi: 10.1038/360593a0. [DOI] [PubMed] [Google Scholar]
- 12.Bendelac A. Positive selection of mouse NK1+ T cells by CD1-expressing cortical thymocytes. J Exp Med. 1995;182:2091–6. doi: 10.1084/jem.182.6.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Porcelli SA, Modlin RL. CD1 and the expanding universe of T cell antigens. J Immunol. 1995;155:3709–10. [PubMed] [Google Scholar]
- 14.MacDonald HR. NK1.1+ T cell receptor-α/β+ cells: new clues to their origin, specificity, and function. J Exp Med. 1995;182:633–8. doi: 10.1084/jem.182.3.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vicari AP, Zlotnik A. Mouse NK1.1+ T cells: a new family of T cells. Immunol Today. 1996;17:71–76. doi: 10.1016/0167-5699(96)80582-2. [DOI] [PubMed] [Google Scholar]
- 16.Watanabe H, Ohtsuka K, Obata Y, et al. Generalized expansion of extrathymic T cells in various immune organs of TL-transgenic mice. Biomed Res. 1993;14:273–88. [Google Scholar]
- 17.Joyce S, Negishi I, Boesteanu A, DeSilva AD, Sharma P, Chorney MJ, Loh DY, Van Kaer L. Expansion of natural (NK1+) T cells that express αβ T cell receptors in transporters associated with antigen presentation-1 null and thymus leukemia antigen positive mice. J Exp Med. 1996;184:1579–84. doi: 10.1084/jem.184.4.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goossens PL, Jouin H, Marchal G, Milon G. Isolation and flow cytometric analysis of the free lymph myeloid cells present in murine liver. J Immunol Methods. 1990;132:137–44. doi: 10.1016/0022-1759(90)90407-m. [DOI] [PubMed] [Google Scholar]
- 19.Iiai T, Kimura M, Kawachi Y, Hirokawa K, Watanabe H, Hatakeyama Y, Abo T. Characterization of intermediate TCR cells expanding in the liver, thymus and other organs in autoimmune lpr mice: parallel analysis with their normal counterparts. Immunology. 1995;84:601–8. [PMC free article] [PubMed] [Google Scholar]
- 20.Kawamura T, Kawachi Y, Moroda T, Weerasinghe A, Iiai T, Seki S, Takada G, Abo T. Cytotoxic activity against tumour cells mediated by intermediate TCR cells in the liver and spleen. Immunology. 1996;89:68–75. doi: 10.1046/j.1365-2567.1996.d01-719.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moroda TI, Iiai T, Suzuki S, et al. Autologous killing by a population of intermediate TCR cells and its NK1.1+ and NK1.1− subsets, using Fas ligand/Fas molecules. Immunology. 1997;26:219–26. doi: 10.1046/j.1365-2567.1997.00240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kawamura T, Toyabe S, Moroda T, et al. Neonatal granulocytosis is a postpartum event which is seen in the liver as well as in the blood. Hepatology. 1997;26:1567–72. doi: 10.1053/jhep.1997.v26.pm0009397999. [DOI] [PubMed] [Google Scholar]
- 23.Zanjani ED, Ascensao JL, Tavassoli M. Liver-derived fetal hematopoietic stem cells selectively and preferentially home to the fetal bone marrow. Blood. 1993;81:399–404. [PubMed] [Google Scholar]
- 24.Taniguchi H, Toyoshima T, Fukao K, Nakauchi H. Presence of hematopoietic stem cells in the adult liver. Nature Med. 1996;2:198–203. doi: 10.1038/nm0296-198. [DOI] [PubMed] [Google Scholar]
- 25.Ohtsuka K, Iiai T, Watanabe H, Tanaka T, Miyasaka M, Sato K, Asakura H, Abo T. Similarities and differences between extrathymic T cells residing in mouse liver and intestine. Cell Immunol. 1994;153:52–66. doi: 10.1006/cimm.1994.1005. [DOI] [PubMed] [Google Scholar]
- 26.Arai K, Iiai T, Nakayama M, et al. Adhesion molecules on intermediate TCR cells. 1. Unique expression of adhesion molecules, CD44+ L-selectin, on intermediate TCR cells in the liver and the modulation of their adhesion by hyaluronic acid. Immunology. 1995;84:64–7 1. [PMC free article] [PubMed] [Google Scholar]
- 27.Nakayama M, Arai K, Hasegawa K, et al. Adhesion molecules on intermediate TCR cells. II. Hepatoprotective effects of hyaluronic acid on acute liver injury. Cell Immunol. 1995;166:275–85. doi: 10.1006/cimm.1995.9970. [DOI] [PubMed] [Google Scholar]


