Abstract
Individuals with properdin, C3 or late complement component deficiency (LCCD) frequently develop meningococcal disease. Vaccination of these persons has been recommended, although reports on efficacy are scarce and not conclusive. We immunized 53 complement-deficient persons, of whom 19 had properdin deficiency, seven a C3 deficiency syndrome and 27 had LCCD with the tetravalent (ACYW) meningococcal capsular polysaccharide vaccine. Serological studies were performed in 43 of them. As controls 25 non-complement-deficient relatives of the complement-deficient vaccinees and 21 healthy non-related controls were vaccinated. Post-vaccination, complement-deficient individuals and controls developed a significant immunoglobulin-specific antibody response to capsular polysaccharides group A, C, Y, W135, but a great individual variation was noticed. Also, the proportion of vaccinees of the various vaccinated groups with a significant increase in bactericidal titre (assayed with heterologous complement) was similar. Opsonization of meningococci A and W135 with sera of the 20 LCCD individuals yielded in 11 (55%) and eight (40%) sera a significant increase of phagocytic activity after vaccination, respectively. Despite vaccination, four complement-deficient patients experienced six episodes of meningococcal disease in the 6 years post-vaccination. Four episodes were due to serogroup B, not included in the vaccine. Despite good response to serogroup Y upon vaccination, disease due to serogroup Y occurred in two C8β-deficient patients, 3.5 and 5 years post-vaccination. These results support the recommendation to vaccinate complement-deficient individuals and to revaccinate them every 3 years.
Keywords: meningococci, Neisseria meningitidis, complement, vaccination, phagocytosis, properdin
INTRODUCTION
The annual incidence of meningococcal disease in the general population is in the range of 1–3 per 100 000 in European countries and the USA [1]. Patients with a complement deficiency either of an alternative pathway component (e.g. properdin), C3 or a late complement component (C5, C6, C7, C8, C9) have a 5000–10 000-fold greater risk of meningococcal disease than normal individuals [2,3]. Recurrent meningococcal disease occurs in 6% of properdin-deficient individuals because they have the capacity to develop antibody-mediated defence against meningococci via the intact classical pathway [4–6]. In contrast, approx. 50–60% of the late complement component-deficient (LCCD) persons experience recurrent episodes of meningococcal disease [3,7]. This may be due to a decreased immune response to meningococcal antigens in LCCD persons compared with normal individuals, or due to the lack of an intact terminal pathway, required to provide protection by antibodies. Antibody-mediated meningococcal phagocytic killing is the only way LCCD patients can be protected against recurrent episodes of meningococcal disease.
Because of the high risk of (recurrent) meningococcal disease, it has been recommended to vaccinate complement-deficient individuals against meningococcal disease [2–4]. The meningococcal vaccine available, consisting of polysaccharides from serogroups A, C, W135 and Y, provides protection for a restricted period of about 3 years in complement-sufficient persons, since it is unable to activate T helper cells and to induce memory [8–13]. A polysaccharide vaccine against meningococcus serogroup B, the most frequent isolated meningococcal pathogen in Europe and the USA, is not available. Capsular polysaccharide B is poorly immunogenic in humans [14]. Since complement-deficient persons contract meningococcal disease rather frequently due to the uncommon serogroups W135 and Y [2–4], the tetravalent (A, C, W135 and Y) polysaccharide vaccine is an appropriate vaccine to provide protection, assuming that effective antibodies are developed, by which serum bactericidal activity and/or opsonophagocytosis are enhanced.
Due to the low frequency of complement deficiencies, being 0.03% in the general population in the Western world [3], the immune response of complement-deficient persons upon vaccination with the tetravalent vaccine has been assessed in only eight properdin-deficient persons [15–17], three persons with C3 deficiency syndrome and 39 LCCD persons [18–20]. Most vaccinees in these reports had already experienced meningococcal disease and the protective effect of vaccination was only evaluated during follow-up periods too short to record vaccine failures. We studied the induction of antibodies upon vaccination with the tetravalent meningococcal polysaccharide vaccine in 43 persons with various complement deficiencies, of whom 15 were without previous meningococcal disease. Their specific antibody levels and serum bactericidal activity were compared with those developed in 25 complement-sufficient relatives of the complement-deficient persons and in 21 healthy unrelated controls. To assess whether LCCD persons may develop protection against recurrent meningococcal disease by an increase of antibody-mediated phagocytic killing, opsonophagocytic activity of pre-vaccination and post-vaccination sera of LCCD patients was studied. After vaccination, 99 vaccinees were contacted regularly and interviewed by telephone to report episodes of meningococcal disease.
STUDY POPULATION, MATERIALS AND METHODS
Study population
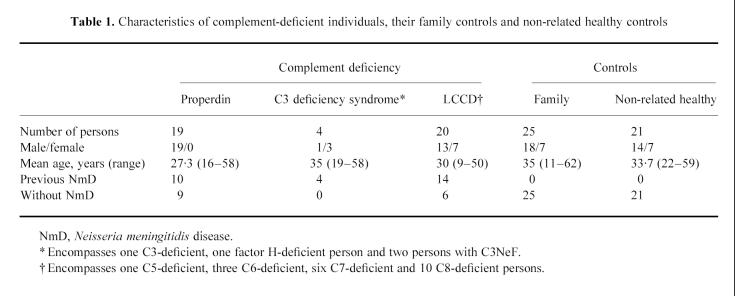
Complement-deficient patients were identified because of previous meningococcal disease as described elsewhere [21]. Their complement-deficient relatives with or without previous meningococcal disease were identified by pedigree studies [22]. In total, 53 complement-deficient persons were vaccinated, of whom 19 persons had properdin deficiency, seven a C3 deficiency syndrome and 27 a LCCD. After vaccination, serological and serum bactericidal studies were performed in 43 of them (Table 1). Among the 19 properdin-deficient persons, five had had meningococcal disease episodes due to serogroup W135, four due to serogroup Y, and one due to serogroup C. All properdin-deficient patients were males, because the properdin gene is on the X-chromosome. Of the four patients with C3 deficiency syndrome, three had previous meningococcal disease due to serogroup W135 and one had two episodes due to serogroup C and B. Of 20 LCCD patients, 14 had suffered from 16 episodes of culture-proven meningococcal disease. These episodes were due to serogroups C in three, to W135 in three, to Y in two, to B in two, to X in two, to A in one, to Z in one and non-groupable meningococci in two. All complement-deficient persons were healthy and over the age of 9 years. Vaccination was done in 1991–1992. Complement-deficient persons with previous meningococcal disease were vaccinated at least 0.5 years (range 0.5–30 years) after their last meningococcal disease episode. None of them had had a meningococcal vaccination previously, except one C6-deficient patient who had been vaccinated with a meningococcal AC vaccine 5 years earlier [23].
Table 1.
Characteristics of complement-deficient individuals, their family controls and non-related healthy controls
Two control groups were included in the study (Table 1). One control group consisted of 25 healthy relatives of the complement-deficient persons and the other control group of 21 healthy non-related volunteers. Complement-deficient individuals and controls were similar in number (except for four patients with C3 deficiency syndrome) and mean age, but were different in gender and varied in age. All controls had normal complement function as measured by CH50 and AP50 and had never been vaccinated against meningococci. They were vaccinated with the same lot of the tetravalent polysaccharide vaccine as the complement-deficient persons. Controls had no episodes of previous meningococcal disease. Permission for vaccination was granted by Commission ex article 14 of the Netherlands Department of Public Health, the Hague, and informed consent was given by all participants.
Materials
Vaccine
The meningococcal polysaccharide A, C, Y, W135 vaccine (MencevaxACYW), lot N134A13L, was kindly provided by SmithKline Beecham (Rixemsart, Belgium). A single dose with 0.5 ml of the vaccine containing 50 μg of each polysaccharide was injected subcutaneously in the deltoid region once.
Meningococcal test strains
Meningococcal serogroup A-strain M1027 and serogroup C-strain C11 (used for preparation of the purified capsular polysaccharides A and C) were donated by the manufacturer of the vaccine (SmithKline Beecham). The serogroup W135-strain 6338 of the vaccine was kindly provided by the Walter Reed Army Institute (Washington, DC). The vaccine serogroup Y-strain 6306 was unsuitable, and replaced by a less serum-sensitive serogroup Y strain obtained from the Netherlands Reference Laboratory for Bacterial Meningitis (AMC, University of Amsterdam, Amsterdam and National Institute for Public Health and Environmental Protection, Bilthoven, The Netherlands).
Serum samples
Serum samples from vaccinees were collected immediately prior to vaccination and 1, 2 and 6 months after vaccination. Serum samples were flash frozen and stored in aliquots at −80°C.
Complement source for bactericidal assay
For each meningococcal serogroup (A, C, Y, W135) tested in the bactericidal assay, serum was used from a healthy individual without serum bactericidal activity. These sera had no blocking activity when they were added to a meningococcal bactericidal assay test with homologous complement, after heat inactivation. The sera, used as complement source, had normal haemolytic complement activity (as determined by CH50 and AP50).
Preparation of polymorphonuclear leucocytes
Polymorphonuclear leucocytes (PMN) from two healthy volunteers (one heterozygous and one homozygous for the FcγRIIa/H131), known to have a high affinity for IgG1, IgG2 and IgG3, were used as effectors in the phagocytic assays. PMN were isolated from heparinized blood and separated from platelets and mononuclear cells by density gradient centrifugation over 1.076 g/ml Percoll (Pharmacia, Uppsala, Sweden) as described previously [24]. Erythrocytes were removed by isotonic lysis at 0°C. Purified PMN were resuspended in calcium-free HEPES (Merck, Schuchardt, Germany) containing 20 mm HEPES, 132 mm NaCl, 6 mm KCl and 1.2 mm KH2PO4.
Methods
Meningococcal capsular antibody quantification
An ELISA was used to measure specific IgG, IgM and IgA against the capsular polysaccharides. The capsular polysaccharides of serogroups A, C, Y, W135 were coated unto the ELISA plates with methylated albumin, as described previously [25–27]. The purified polysaccharides used as antigen in this ELISA were provided by SmithKline Beecham. A pool of serum from adults vaccinated with a tetravalent meningococcal vaccine (reference serum CDC 1992) was kindly provided by Dr G. M. Carlone (CDC, Atlanta, GA) and was used in all assays as a standard [28]. IgA, IgM and IgG antibody levels in this standard serum against each polysaccharide (C, Y and W135) were defined as 1000 U/ml each. For capsular polysaccharide A the IgG, IgM and IgA levels of the reference serum were defined as 4000 U/ml, because in the reference serum the antibody levels against capsular polysaccharide of serogroup A were four-fold higher than against capsular polysaccharide of serogroup C [28]. The specific serum IgG and IgM antibodies against the various meningococcal capsular polysaccharides could be completely inhibited by competitive inhibition assay [25]. No cross-inhibition was observed by the various polysaccharides.
Bactericidal assay
A modification of the assay procedure as proposed by the CDC was used [12]. After overnight culture on gonococcal agar base (GC; Difco, Detroit, MI) medium, meningococci were grown until mid-log phase in rotating tryptic soy broth (TSB; Difco), washed and resuspended in Gey's balanced salt solution (Life Technologies Gibco BRL, Gaithersburg, MD). The number of colony-forming units (CFU) was adjusted to 2 × 104/ml. Of this bacterial suspension, 25 μl (0.5 × 103 CFU) were added to 50 μl of serial dilutions of heat-inactivated serum (56°C, 30 min). Heterologous complement (17% final volume) was added. Each well of a 96-well microtitre plate (Greiner Labortechnik, Langerthal, Germany) contained 150 μl of the mixture. One serum with known bactericidal activity was included in each experiment and used as an internal standard. Controls for killing by buffer, complement or heat-inactivated serum alone were also included in each experiment. Incubation was done at 37°C for 60 min. At time 0 and 60 min 7-μl samples were dripped and by tilting run over a GC plate. After incubation for 18 h at 37°C, the number of CFU was counted. Bactericidal titre was defined as the serum dilution at which > 50% of the initial bacterial inoculum was killed. The assay showed maximal intra- and interassay difference of one dilution step.
Phagocytosis assay
Meningococci of serogroup A and W135 were grown in TSB to mid-log phase, washed in RPMI 1640 (Life Technologies Gibco BRL) + 25 mm HEPES (Merck, Darmstad, Germany) supplemented with 0.2% human serum albumin (Central Laboratory of the Netherlands Red Cross Blood Transfusion Service (CLB), Amsterdam, The Netherlands) and resuspended in RPMI–HEPES plus albumin to a concentration of 7 × 103 CFU/ml. The bacterial suspension (30 μl) was added to 60 μl undiluted serum and 30 μl PMN (7 × 106 cells/ml) in calcium-free HEPES to a total volume of 120 μl. Aliquots (20 μl) were taken after 0, 90 and 240 min incubation at 37°C, and were plated onto chocolate agar plates. Colonies were counted after overnight incubation. All tests were done in duplicate. Controls were bacteria suspended in serum/calcium-free HEPES and bacteria suspended in calcium-free HEPES with PMN (7 × 106 cells/ml). Pre- and post-vaccination sera were always tested simultaneously in one assay.
Colony counts obtained from the bacterial suspension–serum–PMN mixture and from the controls, after incubation for 240 min, were used to calculate the percentage phagocytic killing. A significant increase of phagocytic activity induced by vaccination was defined as an increase of > 50% killing.
Statistical analysis
Geometric means of antibody levels, correlations between antibody levels, bactericidal and phagocytic activity, and Mann–Whitney rank sum tests of the differences in antibody levels between persons with various complement deficiencies and controls were calculated with Statgraph, version 3.0. Correlation of the increase in serum bactericidal titre and the increase in IgG and IgM anticapsular polysaccharide antibody titres was calculated as the correlation of (2log bactericidal titre at 1 month after vaccination minus 2log titre prior to vaccination) and (log antibody titre at 1 month after vaccination minus log titre prior to vaccination).
RESULTS
Development of IgG, IgM and IgA serum anticapsular antibodies
Pre-vaccination serum levels
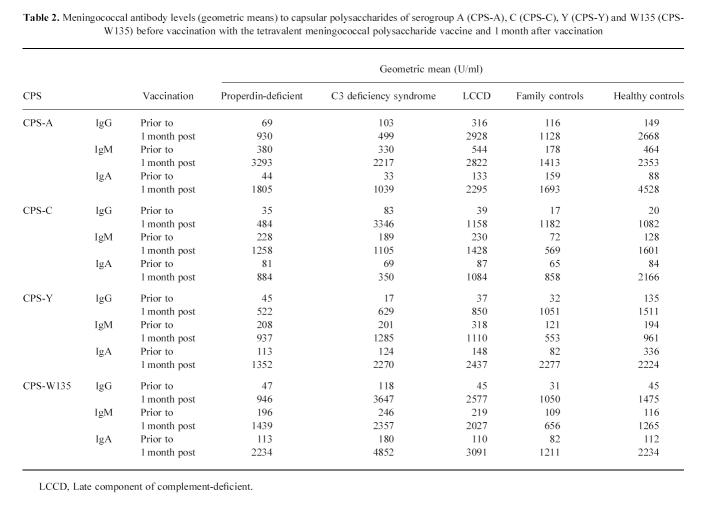
Serum IgG, IgM and IgA levels (U/ml) against the capsular polysaccharides of meningococci A, C, Y and W135 of persons with various complement deficiencies and the complement-sufficient controls were low and showed no significant differences (Table 2). Additionally, levels of anticapsular antibodies did not differ significantly between males and females.
Table 2.
Meningococcal antibody levels (geometric means) to capsular polysaccharides of serogroup A (CPS-A), C (CPS-C), Y (CPS-Y) and W135 (CPS-W135) before vaccination with the tetravalent meningococcal polysaccharide vaccine and 1 month after vaccination
Significantly higher levels of IgG, IgM and IgA against capsular polysaccharide of serogroup W135 were found in eight complement-deficient persons with previous meningococcal disease due to serogroups W135 than in the 15 complement-deficient persons without a previous meningococcal disease (Mann–Whitney, P < 0.05). Also the IgM levels against the C polysaccharide in four patients with previous meningococcal serogroup C disease were higher than in the 15 non-diseased complement-deficient persons, although not yet significantly (Mann–Whitney, P = 0.08).
Post-vaccination serum levels
All vaccinees responded to the four serogroups of the vaccine, except two unrelated properdin-deficient persons who did not respond to any of the four polysaccharides. In addition, two other unrelated properdin-deficient persons and one factor H-deficient person only responded against two of the four vaccine polysaccharides. Geometric means of the anticapsular polysaccharide antibody levels of vaccinees from either of the four groups increased significantly 1 month post-vaccination (Fig. 1), reaching levels equal to those of the (post-vaccination) reference serum. The induced levels of the various immunoglobulin classes against the four serogroups upon vaccination showed a wide range. Patients with C3 deficiency syndrome had the highest geometric means of the antibody levels. Responses in males and females were similar. In the different complement-deficient groups, the percentage of complement-deficient individuals with a four-fold or greater increase of the IgG, IgA and IgM antibody titre varied between 60% and 100%. Such percentages were also found for persons of both control groups. No difference in response to the vaccination was observed between those with and those without previous meningococcal disease. Maximum levels of anticapsular polysaccharide IgG were reached 2 months after vaccination (Fig. 1). Six months after vaccination, IgG levels were still significantly higher than those in the pre-vaccination sera (Fig. 1). The geometric mean IgG level against serogroups Y and W135 in the C3 deficiency syndrome group declined significantly compared with that in properdin-deficient individuals, LCCD individuals, the family controls and the healthy non-related controls. In more detail, it appeared that this decline was due to the strong decrease of IgG level in one C3NeF patient of the four persons with C3 deficiency syndrome.
Fig. 1.
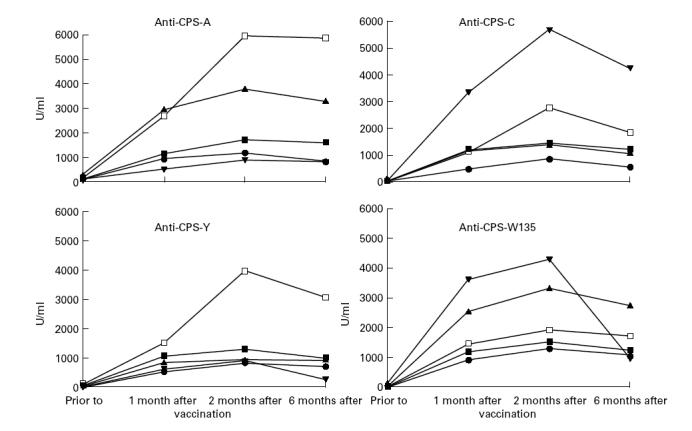
IgG levels (geometric means) to capsular polysaccharides of serogroup A (CPS-A), C (CPS-C), Y (CPS-Y) and W135 (CPS-W135) in late complement component-deficient (LCCD; ▴), properdin-deficient (PD; •), C3 deficiency syndrome (C3D; ▾), family controls (▪) and healthy non-related controls (□) before vaccination and 1, 2 and 6 months after vaccination with the tetravalent meningococcal polysaccharide vaccine.
Serum bactericidal activity
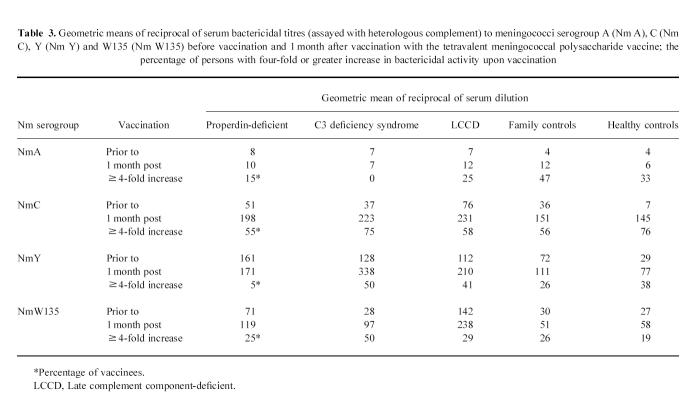
Serum bactericidal activity in all sera was tested in the presence of heterologous complement. Complement-mediated serum bactericidal activity was present in pre-vaccination serum from 17 (89%) of 19 properdin-deficient individuals, 16 (64%) of 25 family controls and in 10 (47%) of 21 healthy controls against all four serogroups. Pre-vaccination serum bactericidal activity against all four serogroups was found in 14 (70%) of 20 LCCD individuals and in all four persons with C3 deficiency syndrome. Post-vaccination bactericidal titres in sera from complement-deficient persons, against all four serogroups, were significantly (Mann–Whitney rank sum test, P < 0.003) higher than in post-vaccination sera from their complement-normal relatives and the healthy controls (Table 3). The same observation was made for the pre-vaccination sera. All sera had a weak bactericidal activity against serogroup A. The complement-deficient persons with previous meningococcal disease had similar serum bactericidal activity to those without previous meningococcal disease. The percentage of persons with a four-fold or greater increase of bactericidal activity 1 month after vaccination ranged widely (Table 3). The increase in bactericidal titre remained stable until 6 months after vaccination.
Table 3.
Geometric means of reciprocal of serum bactericidal titres (assayed with heterologous complement) to meningococci serogroup A (Nm A), C (Nm C), Y (Nm Y) and W135 (Nm W135) before vaccination and 1 month after vaccination with the tetravalent meningococcal polysaccharide vaccine; the percentage of persons with four-fold or greater increase in bactericidal activity upon vaccination
Correlation between the increase in anticapsular antibodies and serum bactericidal activity in response to vaccination
Post-vaccination bactericidal activity and post-vaccination increase of IgG levels (1 month post-vaccination) correlated only in the properdin-deficient patients for serogroup C (r = 0.7, P = 0.008). For IgG and IgM only in LCCD persons such a correlation was present against W135 (r = 0.44, P = 0.05; r = 0.52, P = 0.017). For controls a correlation between serum bactericidal activity and increase of IgG and IgM levels post-vaccination was found for serogroups Y (r = 0.3, P = 0.04; r = 0.29, P = 0.046) and C (r = 0.35, P = 0.017; r = 0.46, P = 0.0012). Bactericidal activity against serogroup A correlated only with post-vaccination IgG and IgM levels (r = 0.3, P = 0.024).
Opsonophagocytic activity of LCCD sera against serogroup A and W135 strains
Phagocytic killing by PMN from healthy controls of meningococci opsonized with the pre-vaccination sera of the 14 LCCD patients with previous episodes of meningococcal disease and pre-vaccination sera of six LCCD persons without previous meningococcal disease were not different. Compared with the pre-vaccination sera, phagocytic killing of serogroup A meningococci was significantly enhanced by post-vaccination sera from 11 (55%) of the 20 LCCD individuals (Fig. 2). Killing of serogroup W135 was significantly increased in post-vaccination serum from eight (40%) of the 20 individuals. Enhancement of killing by PMN of both serogroup A and W135 occurred in sera from nine (47%) LCCD individuals. Results of phagocytic killing assay were similar in sera from C5-, C6-, C7-, C8-deficient persons. No association was found between IgA, IgG and IgM anticapsular antibody levels as measured by ELISA and an enhanced phagocytic killing of meningococcal A or W135 strains, except between the IgM level and killing of serogroup A (r = 0.6, P = 0.005). Opsonophagocytic activity of the serum after vaccination was strongly impaired for serogroup A in the C5-deficient patient and for serogroup W135 in two unrelated C7-deficient patients.
Fig. 2.
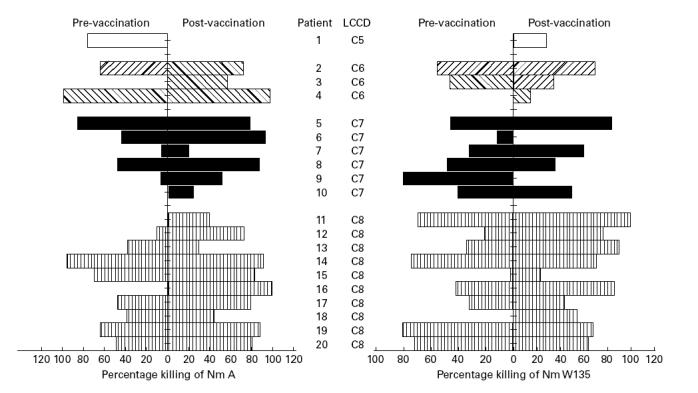
Percentages of meningococci serogroups A and W135 killed by test polymorphonuclear leucocytes (PMN) in the presence of sera taken before and 6 months after vaccination of 20 late complement component-deficient (LCCD) patients (each set of bars represents the results obtained with serum of one individual).
Assessment of meningococcal disease after vaccination
After vaccination all 99 vaccinees were asked to report subsequent episodes of meningococcal disease and were interviewed by telephone in November 1997. The interval between vaccination and the final assessment whether disease had occurred ranged between 4 and 6 years. In total, six episodes of meningococcal disease occurred in four of the 53 complement-deficient vaccinees. No meningococcal disease occurred in the controls. Three of them had experienced meningococcal disease prior to vaccination. One was a 24-year-old C3-deficient female, with previous meningococcal disease due to serogroup B. She developed 2 years after vaccination a serogroup B meningococcal disease. A C8β-deficient male, aged 24 years, with previously meningococcal disease by a non-groupable meningococcus, developed meningococcal sepsis due to meningococcus Y, 3.5 years after vaccination. This patient had high antibody levels (IgG 800 U/ml, IgM 2900 U/ml and IgA 1890 U/ml) against polysaccharide Y 6 months after vaccination. Another C8β-deficient patient, a 23-year-old woman, had previous meningococcal disease episodes due to serogroups Y and C. One year after vaccination she developed a serogroup B meningitis, and 2 years later she had clinically meningococcal septicaemia with petechia (culture results were not available). Five years after vaccination she had an episode of meningitis due to meningococcal serogroup Y. She also had a good response upon vaccination against capsular polysaccharide of serogroup Y. Antibody levels were IgG 200 U/ml, IgM 7200 U/ml and IgA 1700 U/ml 1 month after vaccination. The fourth vaccinee with meningococcal disease after vaccination was a 19-year-old C8β-deficient woman. She contracted meningococcal disease due to serogroup B 2 years after vaccination.
DISCUSSION
Complement-deficient persons have a highly increased risk of contracting meningococcal disease [2–4]. Vaccination in them has been recommended, although large controlled studies of the efficacy of the vaccination are not available [3,4]. We studied the occurrence of meningococcal disease after vaccination with the tetravalent meningococcal polysaccharide vaccine in 53 complement-deficient patients and 46 complement-sufficient controls. The response to this vaccine is supposed to be effective in vaccinees > 2 years old [13]. In our study all vaccinees were > 9 years old. Vaccine failures, defined as the occurrence of meningococcal disease due to a meningococcal serogroup included in the vaccine within 3 years after vaccination, were not found. This contrasts with the observation of Platonov et al., who found that two of 18 vaccinated LCCD patients developed meningococcal meningitis within 1 year after vaccination [18]. Since the strains from these two episodes were not serogrouped, it remains unknown whether these episodes represent real vaccine failures. In our study, four complement-deficient vaccinees developed six recurrences of meningococcal disease, four with serogroup B and two with serogroup Y. Both episodes due to serogroup Y occurred in C8β-deficient patients, 3.5 and 5 years after vaccination. They had a good antibody response against the capsular Y polysaccharide after vaccination. The results indicate that protection of complement-deficient persons at risk of meningococcal disease is provided by vaccination, but that they require re-vaccination each 3–3.5 years like complement-sufficient individuals.
Pre-vaccination serum antibody levels against meningococcal capsular polysaccharides in complement-deficient vaccinees and in the healthy control groups were similar. The levels of pre-vaccination anticapsular antibodies among complement-deficient persons with previous meningococcal disease were higher towards the disease-causing serogroup than among complement-deficient persons without previous meningococcal disease. Platonov et al. found that there was no significant difference in pre-vaccination antibody levels between LCCD patients with previous meningococcal disease and controls [18], but they did not test anticapsular polysaccharide antibody levels against the particular serogroup having caused disease prior to vaccination.
Serum bactericidal activity against meningococci in pre-vaccination sera and post-vaccination sera of complement-deficient individuals was higher than that in sera of controls. Sera of both complement-deficient individuals and controls had weak serum bactericidal activity against the serogroup A strain. This strain was rather serum-resistant, but was used in the tests because its polysaccharide is included in the vaccine. Although the complement-deficient persons and controls developed rather similar antibody levels against anticapsular polysaccharides, the post-vaccination serum bactericidal activity of controls was significantly lower. In addition, the increase of serum bactericidal activity did not correlate with the increase of serum anticapsular polysaccharide antibodies. Identical observations were made in properdin-deficient patients who were vaccinated with the tetravalent meningococcal vaccine [15]. Apparently, serum bactericidal activity is also exerted by antibodies elicited against non-capsular polysaccharide bacterial antigens. This assumption is supported by the observation that antibody levels against the capsular polysaccharides were significantly higher in sera of complement-deficient persons with previous meningococcal disease than in sera of complement-deficient persons without meningococcal disease, whilst bactericidal activity in sera of both groups was similar. Previously, Figueroa & Densen [3] suggested that particularly antibodies directed against the meningococcal lipopolysaccharides (LPS) are strongly induced in complement-deficient persons who have experienced meningococcal disease. In properdin-deficient patients such antibodies, together with the anticapsular polysaccharide antibodies, may exert serum bactericidal activity, mediated by intact classical and terminal pathway of complement activation. Indeed, properdin-deficient patients have a lower recurrence rate of meningococcal disease than LCCD patients [3,4]. Four properdin-deficient patients from four different families did not respond or only to two of the four vaccine polysaccharides. However, their properdin-deficient relatives showed a good response to all four vaccine polysaccharides upon vaccination, offering no clue to an hereditary factor influencing the vaccine response.
Protection against meningococcal disease in persons with LCCD is only due to antibody-mediated opsonophagocytic killing [3,19,29]. If PMN require late complement components for phagocytic killing, vaccination is not useful in LCCD individuals. We found that phagocytic killing of serogroups A and W135 was enhanced in post-vaccination sera from nine (47%) of the 20 LCCD persons. The results of two earlier studies demonstrated an efficient phagocytic killing of meningococci opsonized with serum from LCCD patients [19,29]. We found that anticapsular IgG antibody levels and opsonophagocytic activity did not coincide. A similar observation was reported by Schlesinger et al. [29]. After vaccination, their three C7-deficient individuals had a significantly increased phagocytic activity despite the increase of specific anti-meningococcal antibody levels being rather moderate. Andreoni et al. [19] hypothesized that antibodies against subcapsular antigens promote phagocytic killing more efficiently than antibodies against capsular antigens. However, their results indicated that the anticapsular polysaccharide antibodies are most efficacious for phagocytic killing. The response upon vaccination may be diverse, obscuring the correlation between the anticapsular polysaccharide antibody levels and opsonophagocytic activity. Indeed, opsonophagocytosis of serogroups A and W135 was completely blocked in sera from one and two LCCD patients, respectively. We hypothesize that the impaired opsonophagocytic activity of these sera is caused by the high anticapsular polysaccharide IgM levels by which subcapsular epitopes may be shielded. Although these three sera had high IgM antibody levels after vaccination, opsonophagocytosis in other high level IgM sera was adequate. Therefore, the mechanisms by which vaccination decreases opsonophagocytic activity in a number of LCCD persons have to be elucidated.
In conclusion, vaccination in complement-deficient persons induced similar antibody responses and bactericidal activity to those in complement-normal subjects. Meningococcal disease due to serogroup A, C, Y and W135 did not occur in vaccinees within 3.5 years after vaccination. In the 25 years of exposure before vaccination the LCCD patients had suffered from nine proven infections relevant to this study, or about one infection in 2.8 years. So, whether the 3.5 years without relevant infection represents protection by vaccination or mere change can not be determined. Although prior to vaccination only three of the 40 meningococcal episodes in the complement-deficient patients were due to serogroup B, all three infections within 2 years after vaccination were due to serogroup B. To obtain additional data demonstrating that vaccination is protective we have recently re-vaccinated the complement-deficient persons and we continue the study.
Acknowledgments
This study was supported by the Praeventiefonds, grant number 28-1873. We thank all general practitioners, medical specialists and patients for their cooperation in the study; Ilse Schuurman for technical assistance, and Department of Infectious Diseases of the GG and GD, Amsterdam, for providing the vaccination controls. The assistance of SmithKline Beecham is much appreciated for providing the meningococcal polysaccharide vaccine and the purified capsular polysaccharides for the ELISA.
References
- 1.Jones D. Epidemiology of meningococcal disease in Europe and the USA. In: Cartwright K, editor. Meningococcal disease. London: John Wiley and Sons; 1995. pp. 147–58. [Google Scholar]
- 2.Ross SC, Densen P. Complement deficiency states and infection. Med (Baltimore) 1984;63:243–73. [PubMed] [Google Scholar]
- 3.Figueroa JE, Densen P. Infectious disease associated with complement deficiencies. Clin Microbiol Rev. 1991;4:359–95. doi: 10.1128/cmr.4.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sjöholm AG. Inherited complement deficiency states: implications for immunity and immunological disease. APMIS. 1990;98:861–74. doi: 10.1111/j.1699-0463.1990.tb05008.x. [DOI] [PubMed] [Google Scholar]
- 5.Cunliffe NA, Snowden N, Dunbar EM, Haeney MR. Recurrent meningococcal septicemia and properdin deficiency. J Infect Dis. 1995;31:67–68. doi: 10.1016/s0163-4453(95)91550-8. [DOI] [PubMed] [Google Scholar]
- 6.Nielsen HE, Koch C, Mansa B, Magnussen P, Bergmann O. Complement and immune globulin studies in 15 cases of chronic meningococcaemia: properdin deficiency and hypoimmunoglobulinaemia. Scan J Infect Dis. 1990;22:31–36. doi: 10.3109/00365549009023116. [DOI] [PubMed] [Google Scholar]
- 7.Platonov AE, Beloborodov VB, Vershinina IV. Meningococcal disease in patients with late complement component deficiency: studies in the USSR. Medicine. 1993;72:374–92. doi: 10.1097/00005792-199311000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Frasch CE. Meningococcal vaccines: past, present and future. In: Cartwright K, editor. Meningococcal disease. London: John Wiley and Sons; 1995. pp. 245–85. [Google Scholar]
- 9.Artenstein MS, Gold R, Zimmerly JG, Wyle FA, Schneider H, Harkins C. Prevention of meningococcal disease by group C polysaccharide vaccine. N Engl J Med. 1970;282:417–20. doi: 10.1056/NEJM197002192820803. [DOI] [PubMed] [Google Scholar]
- 10.Gotschlich EC, Goldschneider I, Artenstein MS. Human immunity to the meningococcus. IV. Immunogenicity of group A and C meningococcal polysaccharides in human volunteers. J Exp Med. 1969;129:1367–84. doi: 10.1084/jem.129.6.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Griffiss JM, Brandt BL, Broud DD. Human immune response to various doses of Y and W135 meningococcal polysaccharide vaccines. Infect Immun. 1982;37:205–8. doi: 10.1128/iai.37.1.205-208.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zangwill KM, Stout RW, Carlone GM, et al. Duration of the antibody response after meningococcal polysaccharide vaccination in US Air Force personnel. J Infect Dis. 1994;169:847–52. doi: 10.1093/infdis/169.4.847. [DOI] [PubMed] [Google Scholar]
- 13.King WJ, MacDonald NE, Wells G, et al. Total and functional antibody response to a quadrivalent meningococcal polysaccharide vaccine among children. J Pediatrics. 1996;128:196–202. doi: 10.1016/s0022-3476(96)70389-x. [DOI] [PubMed] [Google Scholar]
- 14.Diaz Romero J, Outschoorn IM. Current status of meningococcal group B vaccine candidates: capsular and noncapsular. Clin Microbiol Rev. 1994;7:559–75. doi: 10.1128/cmr.7.4.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Söderström C, Braconier JH, Käythy H, Sjöholm AG, Thuresson B. Immune response to tetravalent meningococcal vaccine: opsonic and bactericidal functions of normal and properdin deficient sera. Eur J Clin Microbiol Infect Dis. 1989;8:220–4. doi: 10.1007/BF01965264. [DOI] [PubMed] [Google Scholar]
- 16.Braconier J, Sjöholm AG, Söderström C. Fulminant meningococcal infections in a family with inherited deficiency of properdin. Scand J Infect Disv. 1983;15:339–45. doi: 10.3109/inf.1983.15.issue-4.04. [DOI] [PubMed] [Google Scholar]
- 17.Densen P, Weiler JM, McLeod Griffiss J, Hoffmann LG. Familial properdin deficiency and fatal meningococcaemia; correction of the bactericidal defect by vaccination. N Engl J Med. 1987;316:922–6. doi: 10.1056/NEJM198704093161506. [DOI] [PubMed] [Google Scholar]
- 18.Platonov AE, Beloradov VB, Vershinina IV, Käythy H. Vaccination of patients deficient in late complement component with tetravalent meningococcal capsular polysaccharide vaccine. Clin Exp Immunol. 1995;100:32–39. doi: 10.1111/j.1365-2249.1995.tb03600.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Andreoni J, Käythy H, Densen P. Vaccination and the role of capsular polysaccharide antibody in prevention of recurrent meningococcal disease in late complement component-deficient individuals. J Infect Dis. 1993;168:227–31. doi: 10.1093/infdis/168.1.227. [DOI] [PubMed] [Google Scholar]
- 20.Biselli R, Casapollo I, d'Amelio R, Salvato S, Matricardi PM, Braï M. Antibody response to meningococcal polysaccharides A and C in patients with complement defects. Scand J Immunol. 1993;37:644–50. doi: 10.1111/j.1365-3083.1993.tb01677.x. [DOI] [PubMed] [Google Scholar]
- 21.Fijen CAP, Kuijper EJ, Hannema AJ, Sjöholm AG, van Putten JPM. Complement deficiencies in patients over ten years old with meningococcal disease due to uncommon serogroups. Lancet. 1989;ii:585–8. doi: 10.1016/s0140-6736(89)90712-5. [DOI] [PubMed] [Google Scholar]
- 22.Fijen CAP. Amsterdam: University of Amsterdam; 1995. Meningococcal disease and complement deficiency in the Netherlands. Thesis. [Google Scholar]
- 23.Daha MR, Bertina RM, Thompson J, Kauffmann RH, Nicholson-Weller A, Veltkamp JJ, Briët E. Combined hereditary deficiency of the sixth component of complement and factor VIII coagulant activity in a Dutch family. Clin Exp Med. 1982;48:733–8. [PMC free article] [PubMed] [Google Scholar]
- 24.Kuypers TW, Eckmann CM, Weening RS, Roos D. A rapid turbidometric assay of phagocytosis and serum opsonizing capacity. J Immunol Methods. 1989;124:85–94. doi: 10.1016/0022-1759(89)90189-0. [DOI] [PubMed] [Google Scholar]
- 25.Carlone GM, Frasch CE, Siber GR, et al. Multicentre comparison of levels of antibody to the Neisseria meningitidis group A capsular polysaccharide measured by using an enzyme-linked immunosorbent assay. J Clin Microbiol. 1992;30:154–9. doi: 10.1128/jcm.30.1.154-159.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gheesling LL, Carlone GM, Pais LB, et al. Multicenter comparison of Neisseria meningitidis serogroup C anti-capsular polysaccharide antibody levels measured by a standardized enzyme-linked immunosorbent assay. J Clin Microbiol. 1994;32:1475–82. doi: 10.1128/jcm.32.6.1475-1482.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Akinwolere OAO, Kumararatne DS, Bartlett R, Goodall DM, Catty D. Two enzyme linked immunosorbent assays for detecting antibodies against meningococcal capsular polysaccharides A and C. J Clin Pathol. 1994;47:405–10. doi: 10.1136/jcp.47.5.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holder PK, Maslanka SE, Pais LB, Dykes J, Plikaytis BD, Carlone GM. Assignment of Neisseria meningitidis serogroup A and C class-specific anticapsular antibody concentrations to the new standard reference serum CDC 1992. Clin Diagnost Lab Immunol. 1995;2:132–7. doi: 10.1128/cdli.2.2.132-137.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schlesinger M, Greenberg R, Levy J, Käythy H, Levy R. Killing of meningococci by neutrophils: effect of vaccination on patients with complement deficiency. J Infect Dis. 1994;170:449–53. doi: 10.1093/infdis/170.2.449. [DOI] [PubMed] [Google Scholar]