Abstract
Sodium antimony gluconate (SAG) is the mainstay of treatment for visceral leishmaniasis (VL) or kala-azar. In view of the increasing incidence of refractoriness to SAG in India, we compared the levels of parasite-specific IgG and IgG subclasses in 20 longitudinally followed up kala-azar patients. In both SAG-responsive (n = 10) and unresponsive patients (n = 10), the levels of total IgG, IgG1, IgG2, IgG3 and IgG4 were increased, the rank order being IgG1 > IgG2 > IgG3 = IgG4. Following treatment, a significant decrease in total IgG and the four subclasses occurred in the SAG-responsive group, whereas in the SAG-unresponsive group these levels were unchanged or slightly increased. Therefore, monitoring of IgG1 and IgG2 levels in Indian kala-azar patients is a good serologic alternative to monitoring the disease status.
Keywords: IgG subclasses, antimonial unresponsiveness, kala-azar
INTRODUCTION
Protozoan parasites of the genus Leishmania are obligate intracellular protozoan parasites that reside in mononuclear phagocytes and cause a wide spectrum of clinical manifestations causing substantial morbidity and mortality in an estimated 12 million people world-wide [1]. The major clinical presentations depend upon the causative species and immunological state of the host. These range from a simple cutaneous lesion through to the disfiguring mucocutaneous leishmaniasis, and finally to the visceralized form or kala-azar, which is fatal if left untreated [2].
Research on the immunopathology of parasitic diseases has led to the development of highly pertinent models for understanding several aspects of regulation in the immune system. The outcome of leishmanial infections is broadly determined by functionally distinct T-helper (Th) cell populations, Th1 and Th2 which secrete different patterns of cytokines [3,4]. Infection with Leishmania major, an agent for human cutaneous leishmaniasis, is the best documented example of the differential activation of the Th1 and Th2 subsets [5]. Uncontrolled non-healing infections, i.e. susceptibility to disease, is associated with proliferation of Th2 cells and production of IL-4, IL-5 and IL-10, resulting in an ineffective anti-parasite response. On the other hand, healing responses, i.e. resistance to disease, are dependent on the expansion of interferon-gamma (IFN-γ)-producing CD4+ Thl helper cells, which control the spread of primary infection by activating macrophages [6].
In visceral leishmaniasis (VL) or kala-azar, the immunological mechanisms underlying the susceptibility or resistance to disseminated visceral parasitism remain far less defined. It is generally accepted that the acute phase of the disease is associated with a marked impairment of cellular responsiveness to leishmanial antigens and mitogens [7–9]. It is accompanied by the inability to generate IL-2 and IFN-γ production, accounting for macrophage deactivation which allows parasite persistence [10]. An accompanying increase in IL-4 and IL-10 levels points towards an initial Th2 response [11]. However, recovery from active disease is associated with a restoration of lymphocyte response to leishmanial antigens and rise in IFN-γ levels, indicating a shift towards a Th1 response [12]. This analogy, however, does not strictly hold true for Indian kala-azar, as the immune response in the active stage of the disease is not a strictly polarized Th2 response. Measurement of splenic and bone marrow cytokine mRNA levels has shown increased levels of both IL-10 (Th2) and IFN-γ (Th1), suggesting an initial mixed Th1–Th2 response [13,14]; resolution of infection showed a simultaneous decrease in both IL-10 and IFN-γ levels, indicating that both Th1 and Th2 components of the immune system regress [14].
Human B lymphocyte differentiation and proliferation are directly regulated by T cells and soluble lymphokines secreted by T cells. Therefore, measurement of individual IgG subclass concentrations may indirectly determine polarization of the immune response, thereby serving as a surrogate marker of T cell responsiveness. This relationship between antibody isotypes and clinical manifestations has been reported in several diseases, including cutaneous leishmaniasis [15], filariasis [16], schistosomiasis [17], onchocerciasis [18] and leprosy [19].
Serologic testing in VL is a useful diagnostic tool but unsatisfactory for assessment of parasite clearance or monitoring chemotherapeutic response, as it does not distinguish between remote and recent infection [20]. However, studies regarding variations in IgG subclass distribution before and after effective sodium antimony gluconate (SAG) treatment in Sudanese VL patients suggest that individual IgG1 and IgG3 subclasses are suitable serological markers of parasite clearance [21].
In the light of an alarming increase in the incidence of antimonial unresponsiveness in Indian kala-azar in the present epidemic [22], we have evaluated the parasite-specific IgG subclass distribution of longitudinally followed kala-azar patients before and after SAG treatment with a view to understanding its correlation with drug (un)responsiveness. The monitoring of IgG1 and IgG2 levels before and after a single course of SAG may be potentially useful for monitoring individual chemotherapeutic response and allow early prediction of SAG unresponsiveness.
PATIENTS AND METHODS
Study population
The study population consisted of 20 parasitologically proven VL patients recruited from kala-azar-endemic regions of Bihar and West Bengal. A total of 32 individuals served as controls, consisting of patients presenting with co-endemic diseases such as malaria (n = 10), tuberculosis (n = 10) and healthy volunteers (n = 12).
Study design
Coded peripheral blood samples (1 ml) of biopsy-proven cases of kala-azar were sent to the Indian Institute of Chemical Biology, Calcutta, for serodiagnosis by ELISA, whereas Giemsa-stained biopsy smears were reviewed independently in the School of Tropical Medicine according to WHO recommendations [23]. The diagnosis was documented by demonstration of amastigotes in Giemsa-stained smears of splenic or bone marrow aspirates.
Blood samples were collected on admission and on completion of a single course of SAG treatment (20 mg/kg body weight for 4–6 weeks), their chemotherapeutic response was clinically and parasitologically assessed and they were classified as SAG-responsive (remission of fever, regression of liver and spleen and absence of parasites in Giemsa-stained tissue smears) or SAG-non-responsive (persistence of fever and hepatosplenomegaly along with the presence of parasites in Giemsa-stained tissue smears).
Bone marrow or splenic aspirations are part of the routine investigations of suspected kala-azar patients admitted to the School of Tropical Medicine, Calcutta. Informed consent was obtained for collection of peripheral blood.
Antigen
Crude Leishmania antigen was prepared from a L. donovani strain MHOM/IN/83/Ag83 [24]. Promastigotes were harvested with PBS and the cell pellet resuspended in lysis buffer (20 mm Tris–HCl, 40 mm NaCl, pH 7.4) containing 2 mm PMSF, 1 mg/ml leupeptin, 5 mm EDTA and 5 mm iodoacetamide [25].
ELISA
Immobilized crude Leishmania antigen was used to coat 96-well flat-bottomed microtitre plates (5 μg/ml, 50 μl/well in 0.02 m phosphate buffer pH 7.8). Following overnight incubation, the wells were washed three times with 0.1% Tween-20 in PBS (PBS–T) pH 7.2, washing buffer. The wells were then blocked with 2% fetal calf serum (FCS) in PBS for 8 h at 4°C. Patient serum, diluted 1:500 for total IgG ELISA and 1:50 for isotype ELISA, was incubated overnight at 4°C and washed with PBS–T. For isotype analysis, wells were further incubated with either mouse anti-human IgG1, IgG2, IgG3 or IgG4 (diluted 1:2000; Sigma, St Louis, MO) overnight at 4°C and washed with PBS–T. Binding was assayed colorimetrically using either horseradish peroxidase (HRP) conjugated to anti-human IgG (1:5000; Cappel, Malvern, PA) for IgG ELISA or to anti-mouse IgG (1: 5000; Sigma) for isotype ELISA followed by 100 μl of 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) ammonium salt (ABTS) as the substrate [26]. Optical density (OD) was measured at 405 nm. Negative controls, asymptomatic individuals, were included on each plate.
Statistical analysis
Statistical significance of results was compared by Student's t-test (paired and independent). Values < 0.05 were considered significant.
RESULTS
Clinical features
Detailed clinical data are presented in Table 1. All 20 kala-azar patients presented with typical features of the disease, mainly fever, weight loss and hepatosplenomegaly along with typical laboratory abnormalities including hypergammaglobulinaemia and pancytopenia [2]. At the end of a single course of SAG, the chemotherapeutic response of these patients was defined clinically and parasitologically and they were classified as SAG-responsive or SAG-non-responsive.
Table 1.
Clinical data for kala-azar patients included in this study
The mean duration of illness, based on the duration of fever, was comparable in both drug-responsive and non-responsive groups (151 days; range 15–270 days versus 156 days; 60–360 days). As expected, the degree of splenomegaly was greater than the extent of hepatomegaly in both groups. The degree of hepatomegaly was significantly higher in the drug-unresponsive group (2.4 ± 1.6 cm versus 4.2 ± 1.4 cm; P < 0.05). Similarly, the degree of splenomegaly was 1.3-fold higher in the drug-unresponsive group (8.4 ± 2.6 cm versus 10.8 ± 3.4 cm; P < 0.05). Following a single course of SAG, the hepatomegaly decreased 1.4-fold in both groups, whereas the spleen regressed 2.4-fold in the SAG-responsive group compared with 1.4-fold in the SAG-unresponsive group.
Microscopical examination
Bone marrow or splenic aspirates were taken prior to SAG administration where the diagnosis of kala-azar was documented by the presence of amastigotes in Giemsa-stained tissue smears. On completion of a single course of SAG, a repeat tissue biopsy of these patients showed the parasite burden had disappeared completely in the SAG-responsive patients, whereas the parasite persisted in SAG-resistant cases.
Leishmania-specific IgG levels in kala-azar patients and control groups
Using a crude L. donovani lysate as the coating antigen, the presence of high anti-leishmanial antibody levels was detected by ELISA in 20 biopsy-proven VL patients irrespective of their chemotherapeutic response (Table 2,Fig. 1). Reactivity was significantly higher in kala-azar patients compared with patients with malaria, tuberculosis and healthy controls (P < 0.01); sensitivity and specificity were 100%.
Table 2.
Mean ± s.d. (optical density (OD) 405 nm) of IgG antibody levels against Leishmania donovani antigen in different studied groups
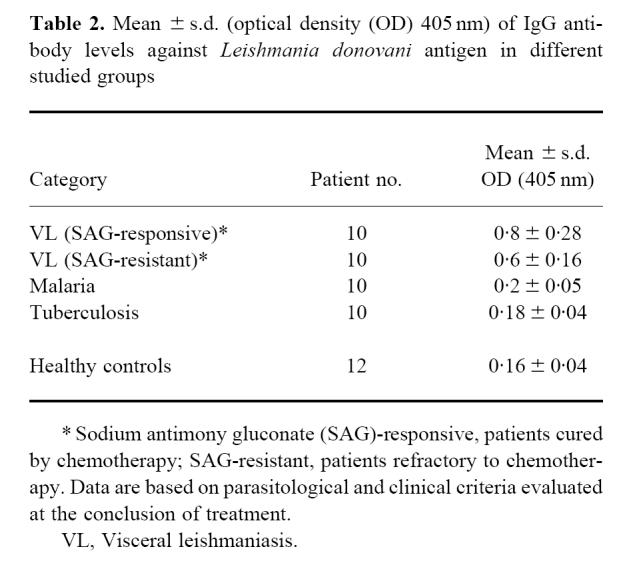
Fig. 1.
Antibody reactivity of visceral leishmaniasis (VL) patients before and after a single course of sodium antimony gluconate (SAG) treatment. Group A, SAG-responsive patients before (○) and after (•) treatment; group B, SAG-resistant patients before (Δ) and after (▴) treatment. Sera was diluted 1:500 and assayed by ELISA as described in Patients and Methods.
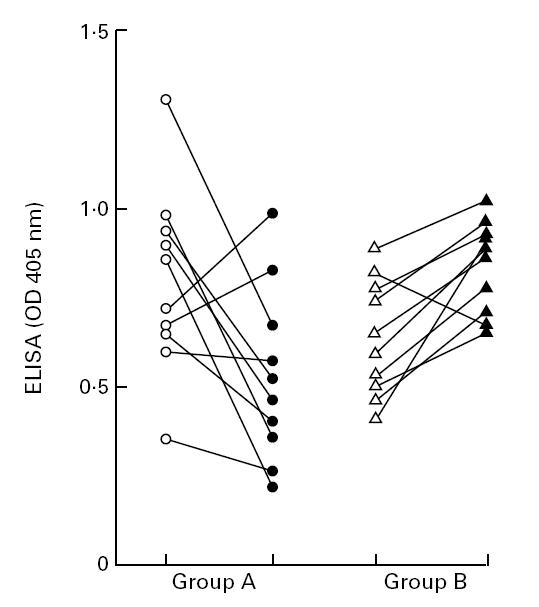
Following reduction of parasite burden in the drug-responsive group (n = 10), a significant decrease in the mean OD ± s.d. of total IgG levels was noted from 0.8 ± 0.28 pretreatment to 0.52 ± 0.25 post-treatment (P < 0.05). On an individual basis, titres decreased in eight patients (44.7 ± 21.3%, Fig. 1). However, in two patients, a mean 23.5% increase was noted (Fig. 1). In contrast, the antibody titres increased in patients belonging to the SAG-unresponsive group (n = 10) from 0.6 ± 0.16 to 0.85 ± 0.11. In the SAG-unresponsive group, 9/10 patients showed an increase, the mean ± s.d. being 30.3 ± 12.4%; one patient showed an 18.0% decrease in post-treatment antibody titre. Analysis of the end-point antibody titres showed a decrease with effective removal of the parasite in the SAG-responsive group, whereas patients who did not respond to conventional SAG therapy showed increased antibody end-point titres (Fig. 2).
Fig. 2.
End point titres of anti-leishmanial antibodies in Indian leishmaniasis and normal human controls. The end point titres were individually determined by ELISA (Patients and Methods). Column 1, sodium antimony gluconate (SAG)-responsive patients before treatment (•); column 2, SAG-responsive patients after treatment (▴); column 3, SAG-unresponsive patients before treatment (○); column 4, SAG-unresponsive patients after treatment (Δ); column 5, non-endemic controls (□). Horizontal bars refer to average titre.
IgG subclass profiles against L. donovani in drug-responsive and unresponsive kala-azar patients
In both SAG-responsive and SAG-resistant groups there was a significant increase in all four IgG subclasses, namely IgG1, IgG2, IgG3 and IgG4, compared with healthy controls (P < 0.05) (Figs 3 and 4). There was no difference in the pretreatment mean OD ± s.d. of parasite-specific antibody levels in SAG-responsive patients versus SAG-non-responsive cases as regards their IgG1 (0.91 ± 0.3 versus 1.1 ± 0.38), IgG2 (0.63 ± 0.3 versus 0.73 ± 0.28), IgG3 (0.36 ± 0.07 versus 0.45 ± 0.2) and IgG4 (0.36 ± 0.11 versus 0.44 ± 0.2) levels (Figs 3 and 4). The rank order of parasite-specific antibody titres in both groups prior to treatment was IgG1 > IgG2 > IgG3 = IgG4.
Fig. 3.
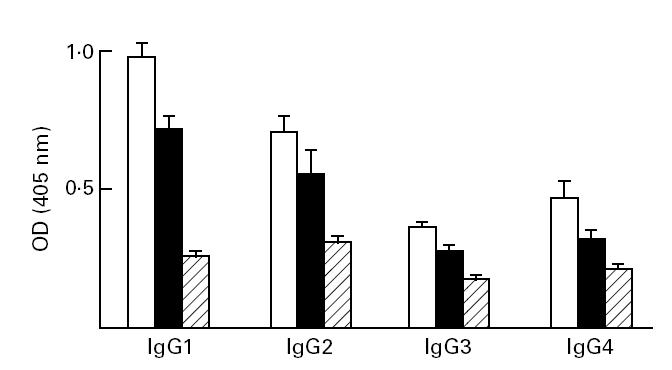
IgG subclass distribution of sodium antimony gluconate (SAG)-responsive kala-azar patients before (□) and after (▪) a single course of SAG treatment compared with healthy controls (??).Sera were diluted 1:50 and assayed by ELISA.
Fig. 4.
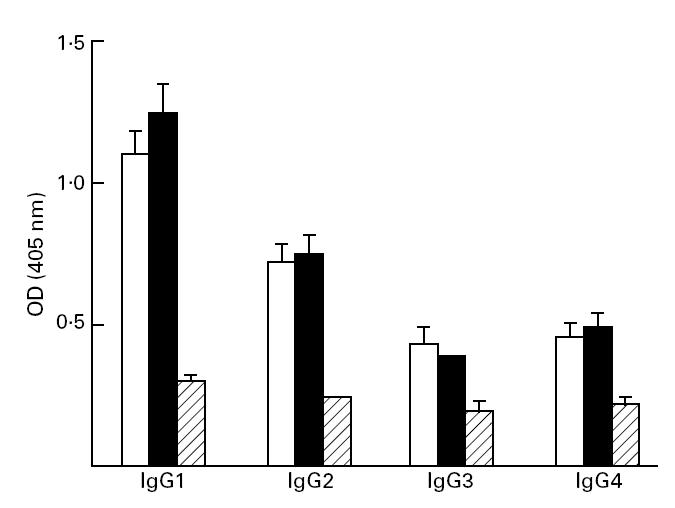
IgG subclass distribution of sodium antimony gluconate (SAG)-responsive kala-azar patients before (□) and after (▪) a single course of SAG treatment compared with healthy controls (??).Sera were diluted 1:50 and assayed by ELISA.
Following a single course of SAG, patients with clinical remission had a concomitant significant decrease in all four IgG1 subclasses (P < 0.05; Fig. 3). However, in patients where clinical features of kala-azar persisted following SAG therapy, there was no difference in the mean ± s.d. of all four subclasses (Fig. 4).
DISCUSSION
Infections with L. donovani can either lead to visceral leishmaniasis or run a subclinical asymptomatic self-healing course [27]. The immunological mechanisms which regulate susceptibility or resistance to disseminated visceral parasitism are still poorly understood. The acute phase of the disease is associated with increased serum levels of IL-4 and IgE [28] and increased IL-10 RNA production in bone marrow and splenic aspirates. In all, this suggests that like other leishmanial species, L. donovani infections trigger a Th2 cell-associated immunosuppressive response. With parasite removal and recovery from disease, the peripheral blood mononuclear cells (PBMC) are seen to produce IL-12 and IFN-γ, suggesting that a switch occurs from Th2 towards a Thl response. However, the Th1–Th2 response is not so clearly demarcated in Indian kala-azar, where measurement of splenic and bone marrow cytokine mRNA levels in the acute phase of the disease showed an increase in both IL-10 and IFN-γ, indicating a mixed Th1–Th2 response; with successful treatment and resolution of infection, levels of both Th1 and Th2 cytokines involute [14]. Similarly, L. donovani-infected BALB/c mice also showed a mixed Th1–Th2 response [29].
The maturation of B lymphocytes into immunoglobulin-secreting cells is regulated by cytokines through its influence on B cell differentiation, proliferation and isotype regulation [30]. Activated CD4+ T cell clones through IL-4 can switch with high frequencies to IgG4 and IgE production [31], while IL-10 causes an increase in IgG1 and IgG3 levels [32]. IFN-γ is also known to be responsible for B cell proliferation [33] and regulates human IgG subclass production. In conjunction with IL-6, IFN-γ increases IgG2 levels, whereas it antagonizes IL-6-mediated IgG1 production [34].
In this study we have utilized the characteristically high Leishmania-specific antibody titres to identify the IgG subclass distribution in longitudinally followed kala-azar patients before and after a single course of chemotherapy (Figs 1,2,3 and 4). Amongst the four subclasses the levels of IgG1 and IgG2 were highest (Figs 3 and 4). The increase in IgG1 levels has also been reported in Somalian VL patients, but no accompanying changes in IgG3 and IgG4 levels occurred and in fact a decrease in parasite-specific IgG2 levels occurred [35]. An increase in IgG1 levels is also a hallmark of Brazilian VL patients [36]. This is in contrast with the isotype profiles of Sudanese VL patients, where IgG3 and IgG4 levels were highest, and with treatment there was a significant decrease in IgG1 and IgG3 levels [21]. These results therefore suggest that the world-wide IgG subclass distribution of VL does show wide variations, possibly due to strain variation as seen in Brazilian VL patients [27]. A need therefore exists for assessing the IgG isotype profiles of Indian kala-azar patients which can indirectly serve as a surrogate measurement of the cell-mediated immune response.
Antimonial unresponsiveness in kala-azar is a major challenge to clinicians, as the cure rate following conventional SAG treatment is approx. 40% [22]. Our study shows that patients who responded to SAG had a decrease in their total IgG levels, as well as individual IgG1, IgG2, IgG3 and IgG4 levels (Figs 1,2,3 and 4). Of the four subclasses, the levels of IgG1 and IgG2 were much higher before treatment and their levels decreased by 26% and 21%, respectively, following reduction of parasite burden (Fig. 3). In contrast, the drug-unresponsive patients had unchanged or slightly increased total IgG (Figs 1 and 2) and IgG1, IgG2, IgG3 and IgG4 levels (Fig. 4).
As Indian kala-azar is a mixed Th1–Th2 response, an increase of IFN-γ (Th1) would account for the increased IgG2 levels. The enhanced IL-4 (Th2) induces an increase in IgG4 levels, whereas the raised levels of IL-10 (Th2) are reflected in increased IgG1 and IgG3 levels. Similarly with parasite disappearance, the levels of IFN-γ, IL-4 and IL-10 decrease [14], accounting for the decreased IgG1, IgG2, IgG3 and IgG4 levels. Conversely, in the drug-resistant group, persistence of the parasite possibly causes increased levels of IFN-γ, IL-4 and IL-10, which may be responsible for the unchanged IgG1, IgG2, IgG3 and IgG4 levels.
As all four IgG subclasses indirectly reflect the presence or absence of parasite, they are suitable serological markers for monitoring disease progression and chemotherapeutic response in kala-azar patients on an individual basis. Longitudinal follow up of individual patients by isotype ELISA offers a non-invasive approach to assessing the disease status and prediction of drug unresponsiveness.
Acknowledgments
This work has received funding from the Department of Biotechnology, Government of India. We thank Ashish Mullick for his technical help and Mr H. N. Dutta for the illustrations.
References
- 1.Alexander J, Russel DG. The interaction of Leishmania species with macrophage. Adv Parasitol. 1992;31:175–254. doi: 10.1016/s0065-308x(08)60022-6. [DOI] [PubMed] [Google Scholar]
- 2.Pearson RD, de Queiroz Sousa A. Clinical spectrum of leishmaniasis. Clin Infect Dis. 1996;22:1–13. doi: 10.1093/clinids/22.1.1. [DOI] [PubMed] [Google Scholar]
- 3.Liew FY, O'Donell CA. Immunology of Leishmaniasis. Adv Parasitol. 1993;32:161–259. doi: 10.1016/s0065-308x(08)60208-0. [DOI] [PubMed] [Google Scholar]
- 4.Mosmann T, Coffman R. Thl and Th2 cells: different patterns of lymphokine activates and secretes proteins. Ann Rev Immunol. 1989;7:145–61. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 5.Reiner SL, Locksley RM. The regulation of immunity to Leishmania major. Ann Rev Immunol. 1995;13:151–7. doi: 10.1146/annurev.iy.13.040195.001055. [DOI] [PubMed] [Google Scholar]
- 6.Heinzel FP, Sadick MD, Mutha SS, Locksley RM. Production of IFN-γ, interleukin 2, interleukin 4 and interleukin 10 by CD4+ lymphocytes in vivo during healing and progressive murine leishmaniasis. Proc Natl Acad Sci USA. 1991;88:7011–5. doi: 10.1073/pnas.88.16.7011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carvalho EM, Teixeira RS, Johnson WD. Cell mediated immunity in American visceral leishmaniasis: reversible immunosuppression during acute infection. Infect Immun. 1981;33:498–502. doi: 10.1128/iai.33.2.498-500.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carvalho EM, Bacellar O. Lymphocyte reactivity to mitogens in American visceral leishmaniasis. Braz J Med Biol Res. 1983;16:35–41. [PubMed] [Google Scholar]
- 9.Haldar JP, Ghose S, Saha KC, Ghose AC. Cell-mediated immune response in Indian kala-azar and post-kala-azar dermal leishmaniasis. Infect Immun. 1983;42:702–7. doi: 10.1128/iai.42.2.702-707.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carvalho EM, Badaro R, Reed SG, Jones TC, Johnson WD. Absence of gamma interferon and interleukin 2 production during active visceral leishmaniasis. J Clin Invest. 1985;76:2066–9. doi: 10.1172/JCI112209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ghalib HW, Piuvezam MR, Skeiky YAW, Siddig M, Hashim FA, el Hassan EM, Russo DM, Reed SG. Interleukin 10 correlates with pathology in human Leishmania donovani infections. J Clin Invest. 1993;92:324–9. doi: 10.1172/JCI116570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carvalho EM, Bacellar O, Brownell C, Regis T, Coffman RL, Reed SG. Restoration of IFN-γ production and lymphocyte proliferation in visceral leishmaniasis. J Immunol. 1994;152:5949–56. [PubMed] [Google Scholar]
- 13.Karp CL, el-Safi SH, Wynn TA, et al. In vivo cytokine profiles in patients with kala-azar. Marked elevation of both interleukin-10 and interferon-gamma. J Clin Invest. 1993;91:1644–8. doi: 10.1172/JCI116372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kenney RT, Sacks DL, Gam AA, Murray HW, Sundar S. Splenic cytokine responses in Indian kala-azar before and after treatment. J Infect Dis. 1998;177:815–8. doi: 10.1086/517817. [DOI] [PubMed] [Google Scholar]
- 15.Rodriguez V, Centeno M, Ulrich M. The IgG isotypes of specific antibodies in patients with American cutaneous leishmaniasis; relationship to the cell mediated immune response. Parasite Immunol. 1996;18:341–5. doi: 10.1046/j.1365-3024.1996.d01-113.x. [DOI] [PubMed] [Google Scholar]
- 16.Yazdanbakhsh M, Paxton WA, Brandenburg A, VanRee R, Lens M, Partons F, Maizels RM, Selkirk ME. Differential antibody isotype reactivity to specific antigens in human lymphatic filariasis: gp 15/400 preferentially induces IgE, Ig4 and IgG2. Infect Immun. 1995;63:3772–9. doi: 10.1128/iai.63.10.3772-3779.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pearce EJ, Caspar P, Grzych JM, Lewis FA, Sher A. Downregulation of Thl cytokine production accompanies induction of Th2 responses by a parasite helminth Schistosoma mansoni. J Exp Med. 1991;173:159–66. doi: 10.1084/jem.173.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cabrera Z, Buttner DW, Parkhouse RME. Unique recognition of a low molecular weight Onchocerca volvulus antigen by IgG3 antibodies in chronic hyper-reactive onchodermatitis. Clin Exp Immunol. 1993;91:500–5. [PMC free article] [PubMed] [Google Scholar]
- 19.Dhandayuthapani S, Izumi S, Anandan D, Bhatia VN. Specificity of IgG subclass antibodies in different clinical manifestations of leprosy. Clin Exp Immunol. 1992;88:253–7. doi: 10.1111/j.1365-2249.1992.tb03069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scott JM, Shreffler WG, Ghalib HW, Elassad A, Siddig M, Badaro R, Reed SG. A rapid and simple diagnostic test for active visceral leishmaniasis. Am J Trop Med Hyg. 1991;44:272–7. doi: 10.4269/ajtmh.1991.44.272. [DOI] [PubMed] [Google Scholar]
- 21.Elassad AMS, Younis SA, Siddig M, Grayson J, Petersen E, Ghalib HW. The significance of blood levels of IgM, IgA, IgG and IgG subclasses in Sudanese visceral leishmaniasis patients. Clin Exp Immunol. 1994;95:294–9. doi: 10.1111/j.1365-2249.1994.tb06526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sundar S, Singh VP, Sharma S, Makharia MK, Murray HW. Response to interferon-gamma plus pentavalent antimony in Indian visceral leishmaniasis. J Infect Dis. 1997;176:1117–9. doi: 10.1086/516526. [DOI] [PubMed] [Google Scholar]
- 23.WHO. The Leishmaniases. Geneva: World Health Organization; 1990. p. 154. Technical Report Series, no. 793. [Google Scholar]
- 24.Chatterjee M, Manna M, Bhaduri AN, Sarkar D. Recent kala-azat cases in India: isozyme profiles of Leishmania parasites. Ind J Med Res. 1995;101:165–72. [PubMed] [Google Scholar]
- 25.Jaffe CL, Bennett E, Grimaldi G Jr, McMahon-Pratt D. Production and characterization of species specific monoclonal antibodies against Leishmania donovani for immunodiagnosis. J Immunol. 1984;133:440–7. [PubMed] [Google Scholar]
- 26.Jaffe CL, McMahon-Pratt D. Serodiagnostic assay for visceral leishmaniasis employing monoclonal antibodies. Trans Roy Soc Trop Med Hyg. 1987;81:587–94. doi: 10.1016/0035-9203(87)90418-4. [DOI] [PubMed] [Google Scholar]
- 27.Badaro R, Jones TC, Carvalho EM, Sampaio D, Reed SG, Teixeira R, Johnson WD. New perspectives on a subclinical form of visceral leishmaniasis. J Infect Dis. 1986;154:1003–11. doi: 10.1093/infdis/154.6.1003. [DOI] [PubMed] [Google Scholar]
- 28.Zwingenberger K, Harms G, Pedrosa Q, Omena S, Sandkamp B, Neifer S. Determinants of the immune response in visceral leishmaniasis: evidence for predominance of endogenous interleukin 4 over interferon-gamma production. Clin Immunol Immunopathol. 1990;57:242–9. doi: 10.1016/0090-1229(90)90038-r. [DOI] [PubMed] [Google Scholar]
- 29.Basak SK, Saha B, Bhattacharyya A, Roy S. Immunobiological studies on experimental visceral leishmaniasis II. Adherent cell mediated down regulation of delayed type hypersensitivity response and up-regulation of B cell activation. Eur J Immunol. 1992;22:2041–5. doi: 10.1002/eji.1830220813. [DOI] [PubMed] [Google Scholar]
- 30.Finkelman FD, Holmes J. Lymphokine control of in vivo immunoglobulin isotype selection. Ann Rev Immunol. 1990;8:303–33. doi: 10.1146/annurev.iy.08.040190.001511. [DOI] [PubMed] [Google Scholar]
- 31.Gascan H, Gauchat JF, Roncarolo MG, Yssel A, Spits H, de Vries JE. Human B cell clones can be induced to proliferate and to switch to IgE and IgG4 synthesis by interleukin 4 and a signal provided by activated CD4+ T cell clones. J Exp Med. 1991;173:747–50. doi: 10.1084/jem.173.3.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Briere F, Servet-Delprat C, Bridon JM, Saint-Remy JM, Banchereau J. Human interleukin 10 induces naive surface immunoglobulin D+ (sIgD+) B cells to secrete IgG1 and IgG3. J Exp Med. 1994;179:757–62. doi: 10.1084/jem.179.2.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakagawa T, Hirano T, Nakagawa N, Yoshizaki K, Kishimoto T. Effect of recombinant IL-2 and IFN-γ on proliferation and differentiation of human B cells. J Immunol. 1985;134:959–66. [PubMed] [Google Scholar]
- 34.Kawano Y, Noma T, Yata J. Regulation of human IgG subclass production by cytokines IFN-γ and IL-6 acts antagonistically in the induction of human IgG1 but additively in the induction of IgG2. J Immunol. 1994;153:4948–58. [PubMed] [Google Scholar]
- 35.Shiddo SA, Huldt G, Nilsson LA, Ouchterlony O, Thorstensson R. Visceral leishmaniasis in Somalia. Significance of IgG subclasses and of IgE response. Immunol Letters. 1996;50:87–93. doi: 10.1016/0165-2478(96)02529-1. [DOI] [PubMed] [Google Scholar]
- 36.Ulrich M, Rodriguez V, Centeno M, Convit J. Differing antibody IgG isotypes in the polar forms of leprosy and cutaneous leishmaniasis characterized by antigen specific T cell anergy. Clin Exp Immunol. 1995;100:54–58.. doi: 10.1111/j.1365-2249.1995.tb03603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]