Abstract
We evaluated possible modes of epithelial cell destruction and restoration in minor salivary gland biopsies from patients with SS. Minor salivary gland biopsies from 10 primary Sjögren's syndrome (pSS) patients and eight control individuals were evaluated by immunohistochemical staining for the expression of apoptosis-related molecules, substances released by activated cytotoxic T cells, as well as proteins involved in epithelial cell repair. The results were analysed by computer screen analysis and they were expressed as average percentages. Apoptosis-promoting molecules, Fas antigen and Fas ligand were observed in ductal and acinar epithelial cells as well as in infiltrating mononuclear cells of minor salivary glands from SS patients in comparison with control biopsies. Bax protein, which acts as a death-promoter message, was expressed in the ductal and acinar epithelial cells and in mononuclear infiltrating cells of SS patients compared with control individuals, while Bcl-2, an inhibitor of apoptosis, was primarily found in the lymphocytic infiltrates. In situ DNA fragmentation assay (TUNEL) revealed that epithelial cells were apoptotic in patients with SS compared with control subjects. Immunohistochemical staining for perforin and granzyme B, released from granules of activated cytotoxic lymphocytes, revealed their presence in lymphocytic infiltrates of patients with SS compared with control biopsies. pS2, a member of the trefoil protein family which functions as promoter of epithelial cell repair and cell proliferation, was expressed in epithelial cells in biopsies from SS patients. These studies suggest that the functional epithelium of minor salivary glands in patients with SS appears to be influenced by both intrinsic and extrinsic mechanisms of destruction, while a defensive mechanism of epithelial restoration seems to be active.
Keywords: Sjögren's syndrome, epithelial cells, apoptosis, regeneration
INTRODUCTION
The major histopathologic lesion of primary Sjögren's syndrome (pSS) is a focal infiltrate around ductal and acinar epithelial cells. The immunocytes that constitute the infiltrates interact with and finally destroy the epithelial cells of minor salivary glands (MSG) of patients with SS. Therefore the autoimmune reaction in SS includes two different participants. Epithelial cells of the exocrine glands in SS are not innocent bystanders but they seem to play a major role in the induction and perpetuation of the autoimmune reaction. Several studies have shown that the epithelial cells in SS present different phenomena, such as spontaneous cytokine production [1], inappropriate HLA-DR [2] and oncogene expression [3] and autoantigen expression on their membrane [4], as well as expression of apoptosis-related proteins [5,6]. T lymphocytes that infiltrate the exocrine glands and surround ductal and acinar epithelial cells constitute the second participant of the histopathologic lesion of SS. Most of these T lymphocytes in SS belong to the CD4 αβ subpopulation and some are CD8 T cells [7].
In this study we examine possible ways by which ductal and acinar epithelial cells are destroyed leading to xerostomia. We examined the pattern of reactivity of apoptosis-related proteins involved in the Fas/Fas ligand (FasL) pathway and perforin/granzyme B of MSG from patients with SS and control subjects. Fas antigen (CD95) and FasL are cell surface molecules belonging to the tumour necrosis factor (TNF)/nerve growth factor receptor family. Fas antigen transduces the apoptotic signal into susceptible target cells [8], while, FasL binds to Fas antigen on cells, inducing Fas-mediated apoptosis [9]. Although FasL expression was initially thought to be restricted to activated T lymphocytes [9], it is also expressed in non-lymphoid cells such as certain epithelial cells [10]. Perforin and granzymes constitute a second extrinsic pathway of apoptosis [11]. Perforin is a cytolytic mediator produced by killer lymphocytes and is stored in and released by cytoplasmic granules [12]. Upon the release of perforin a number of serine proteinases called granzymes, which are stored in the same cytoplasmic granules with perforin, are also released. Granzyme B enters the cytoplasm of the target cell through the perforin pores and induces target cell apoptosis [13]. Apoptosis was confirmed by using in situ terminal deoxytransferase (dTd) catalysed DNA nick end labelling (TUNEL). How do cells respond to these death-inducing messages, and which is the mediator of regeneration in order for these cells to survive? We examine the expression of the trefoil protein pS2, which promotes epithelial repair and cell proliferation, by immunohistochemistry in the same patients and controls. Trefoil proteins (ITF, PSP and pS2) constitute a family of proteins that are characterized by the presence of one to six cysteine-rich P-domains and are found in the cytoplasm of gastric and intestinal epithelial cells. They function as growth factors, protease inhibitors, mucin stabilizers as well as promoters of epithelial repair and cell proliferation [14]. The results of this study indicate that apoptosis may play a crucial role in epithelial cell destruction in SS, while a regenerative mechanism of epithelial cells is probably in operation.
PATIENTS AND METHODS
Patients and MSG biopsies
All patients fulfilled the preliminary classification criteria for pSS [15]. Minor salivary gland biopsies were performed as a routine part of the diagnostic evaluation. A total of 18 labial salivary gland specimens was obtained. A focus score (defined as an aggregate of 50 or more mononuclear cells per 4 mm) was determined for each biopsy specimen [16]. All patients studied (n = 10) had focus scores of > 1. The control group consisted of eight salivary gland specimens derived from individuals who had xerostomia and non-specific sialadenitis.
Immunohistology
Minor salivary glands were obtained, embedded in paraffin and OCT cryoprotectant (Miles Scientific, Napierville, IL) in aluminium foil moulds and snap frozen using pentane and liquid N2 and stored at − 70°C. Sections (4 μm) were cut and mounted on aminoalkylsilane (Sigma Chemical Co., St Louis, MO) glass slides and air-dried for 30 min.
Antigen localization for Fas (goat polyclonal; Santa Cruz, Santa Cruz, CA), FasL (rabbit polyclonal; Santa Cruz) and Bax (rabbit polyclonal; Calbiochem, La Jolla, CA) was demonstrated using the avidin–biotin immunoperoxidase technique. Non-specific antibody binding and endogenous peroxidase activity were blocked by pre-incubating sections in 10% non-immune horse serum (Dako, Glostrup, Denmark) and 3% H2O2/MeOH, respectively. Sections were incubated with primary antibody at 1:25 dilution at room temperature for 60 min in a humidified chamber. This was followed by sequential incubations with appropriate secondary antibody, rabbit anti-goat immunoglobulin biotin-conjugated (Dako) for Fas and goat anti-rabbit immunoglobulin biotin-conjugated (Dako) for FasL and Bax, and avidin–biotin complex (Dako) for 30 min each. Between each antibody application the slides were washed in Tris-buffered saline (TBS) for 15 min. Bound peroxidase was detected using 0.05% diaminobenzidine tetrahydrochloride (DAB) (Sigma)/0.02% H2O2 in TBS. Concurrent negative controls were performed on additional sections, replacing the primary antibody with an irrelevant antibody of the same subclass. Sections were counterstained using Mayer's haematoxylin for 10 s, dehydrated for mounting in DPX (BDH, Poole, UK).
The peroxidase anti-peroxidase (PAP) method was employed for antibodies to Granzyme B (mouse monoclonal; Kamiya Biomedical Co., Seattle, WA), pS2 (mouse monoclonal; Zymed Labs, San Francisco, CA) and Bcl-2 (mouse monoclonal; Zymed). The above procedure was followed by replacing the secondary antibody and complex by anti-mouse immunoglobulins (Dako) and PAP complex (Dako), respectively.
For the detection of perforin, a MoAb (Kamiya) was used and the alkaline-phosphatase anti-alkaline-phosphatase (APAAP) method was employed due to its greater sensitivity. Briefly, sections were incubated with primary antibody diluted in TBS for 60 min. Sequential incubations with rabbit anti-mouse immunoglobulins (Dako) followed by the APAAP complex (Dako) were then applied to the sections. Bound antibody was detected using Fast red substrate (Sigma). The slides were counterstained with haematoxylin, dehydrated and coverslipped with Glycer-Gel (Dako).
TUNEL
Paraffin-embedded tissues (4 μm thick) were dewaxed and rehydrated through xylene and graded alcohol series. Slides were equilibrated in TBS pH 7.6, prior to deproteinization with proteinase K (10 μg/ml) for 10 min at 37°C. DNA fragmentation was detected by using the in situ cell death detection kit, alkaline phosphatase (AP) (Boehringer, Mannheim, Germany) that incorporates FITC into DNA by terminal deoxynucleotidyl transferase (dTd). The reaction was allowed to continue at 37°C for 60 min. Sections were washed in TBS and then incubated with anti-FITC antibody conjugated to alkaline phosphatase for 30 min. Bound alkaline phosphatase was detected using the fast red substrate for 10 min. Sections were counterstained with Mayer's haematoxylin and visualized under a light microscope. Concurrent negative control tissue sections were incubated as described above but omitting the enzyme dTd. Peripheral T lymphocytes pretreated with DNase were used as positive controls. In addition we applied this method to tissue sections from non-Sjögren's individuals.
Quantitative analysis
A semiquantitative assessment of the number of cells expressing apoptosis-related molecules and regenerative molecules in the MSGs was established by examining 10 contiguous fields across the whole section at ×40 magnification. Positive cells in each section were counted by two blinded observers field by field when displayed at ×16 magnification on a television screen connected to a microscope (Image Pro-Plus 2.1 system). The percentage of positive cells of each cell type compared with the total number of cells of that type in the sections as well as the percentage of each cell type compared with the total number of cells in each section were determined. The analysis was performed in age-matched groups of SS patients (average age 50 years) and control individuals (average age 47 years).
Statistical analysis
Results are expressed as percentage mean values ± s.d. Unpaired two-tailed Student's t-tests were used to compare between patients and control subjects. The results were considered significant only if P ≤ 0.05.
RESULTS
Expression of Fas/FasL in MSG biopsies
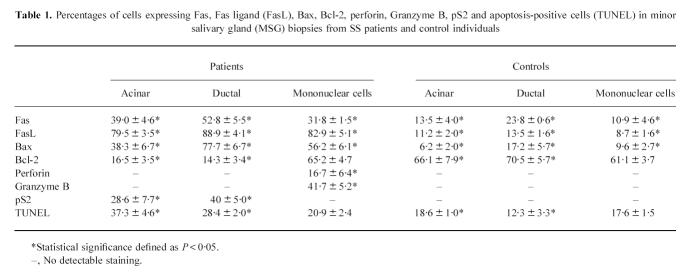
Fas antigen was expressed in ductal (52.8 ± 5.5%) and acinar (39.0 ± 4.6%) epithelial cells as well as in mononuclear cell infiltrates (31.8 ± 1.5%) of MSG biopsies from patients with SS (Table 1). There were less Fas antigen-positive ductal (23.8 ± 0.6%) and acinar (13.5 ± 4.0%) epithelial cells as well as mononuclear cell infiltrates (10.9 ± 4.6%) in control MSG biopsies (Table 1). These differences in Fas antigen expression between SS patients and control individuals were statistically significant (P ≤ 0.05). The expression of FasL was prominent in acinar (79.5 ± 3.5%), ductal (88.9 ± 4.1%) and mononuclear cell infiltrates (82.9 ± 5.1%) of MSGs of patients with SS (Table 1), while in MSG biopsies of control individuals, acinar (11.2 ± 2.0%), ductal (13.5 ± 1.6%), and mononuclear cell (8.7 ± 1.6%) expression of FasL (Table 1) was significantly lower (P ≤ 0.05).
Table 1.
Percentages of cells expressing Fas, Fas ligand (FasL), Bax, Bcl-2, perforin, Granzyme B, pS2 and apoptosis-positive cells (TUNEL) in minor salivary gland (MSG) biopsies from SS patients and control individuals
Expression of Bax and Bcl-2 in MSG biopsies
Bax protein, which acts as a death-promoter message, was prominently expressed in ductal (77.7 ± 6.7%) and acinar (38.3 ± 6.7%) epithelial cells as well as in infiltrating mononuclear cells (56.2 ± 6.1%) of SS MSG biopsies (Table 1 and Fig. 1a), while these were significantly lower in control individuals (Table 1 and Fig. 1b).
Fig. 1.
Immunohistochemical detection of Bax and Bcl-2 in minor salivary gland (MSG) biopsies from patients with SS (a,c) and control individuals (b,d). Positive cells stained brown using immunoperoxidase staining, haematoxylin was used as a counterstain on paraffin-embedded tissue sections. (Mag., a,b,d, ×400; c, ×200.)
Bcl-2, an inhibitor of apoptosis, was expressed by a small percentage of acinar (16.5 ± 3.5%) and ductal (14.3 ± 3.4%) epithelial cells of MSG biopsies from patients with SS (Table 1 and Fig. 1c), while the percentages of acinar (66.1 ± 7.9%) and ductal (70.5 ± 5.7%) epithelial cells positive for Bcl-2 in control individuals (Table 1 and Fig. 1d) were significantly higher (P ≤ 0.05). Most of the mononuclear cell infiltrates in SS patients as well as in control individuals expressed Bcl-2 protein (65.2 ± 4.7%) and (61.1 ± 3.7%), respectively, with no statistical difference (Table 1).
Expression of perforin/Granzyme B in MSG biopsies
Perforin and Granzyme B expression (Table 1 and Fig. 2a,c) was observed by the mononuclear cell infiltrates in MSG biopsies of patients with SS (16.7 ± 6.4% and 41.7 ± 5.2%, respectively), while biopsies from control individuals were negative (Table 1).
Fig. 2.
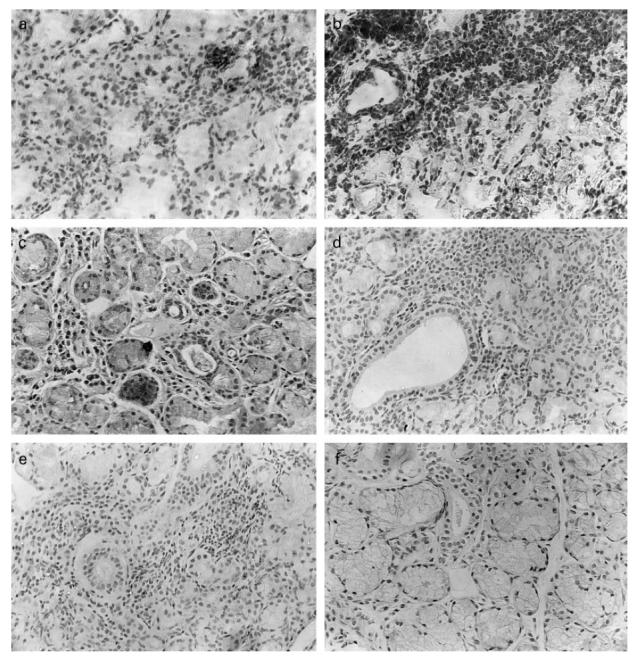
Localization of perforin, Granzyme B and pS2 in minor salivary gland (MSG) biopsies from patients with SS (a,c,e respectively). (a) Mononuclear infiltrating cells stained red with APAAP using anti-human perforin MoAb on a cryostat section with haematoxylin as a counterstain. (c) Granzyme B localization in mononuclear cells on a paraffin-embedded MSG biopsy with immunoperoxidase staining. (e) pS2 localization on acinar and ductal epithelial cells by immunoperoxidase staining. (f) MSG biopsy from a control individual stained with anti-human pS2 MoAb. Control immunostainings on sections from SS patients using irrelevant primary antibodies were also performed (b,d). (Mag. ×200.)
In Situ DNA fragmentation assay (TUNEL)
Apoptotic cell death was confirmed by the TUNEL assay. The percentage of acinar (37.3 ± 4.6%) and ductal (28.4 ± 2.0%) epithelial cells that underwent apoptotic death in MSG biopsies of patients with SS (Table 1) was significantly increased (P < 0.05) compared with that of acinar (18.6 ± 1.0%) and ductal (12.3 ± 3.3%) epithelial cells of control individuals (Table 1). Infiltrating mononuclear cells in MSG biopsies of patients with SS showed a physiological rate of apoptotic death, as the percentage of apoptotic mononuclear cells (20.9 ± 2.4%) was not significantly different from that of apoptotic mononuclear cells (17.6 ± 1.5%) in MSG biopsies of control individuals (Table 1).
Expression of pS2 in MSG biopsies
pS2 was expressed by acinar (28.6 ± 7.7%) and ductal (40 ± 5.0%) epithelial cells of MSG biopsies of patients with SS, while no pS2 expression was found in MSG biopsies of control individuals (P < 0.05) (Table 1 and Fig. 2e,f).
DISCUSSION
In this study we obtained some evidence on the modes of epithelial cell death and repair in SS. The immunohistochemical studies and the in situ DNA fragmentation assay (TUNEL) revealed that apoptosis may be the major mechanism by which epithelial cells die in SS. Apoptosis is an innate cellular mechanism of programmed suicide. It is invoked in disparate biological situations, from embryonic development to suppression of malignancy and regulation of immune cell populations.
Which is the initiating signal for the epithelial cells to enter into an apoptotic programme? Recent studies revealed that FasL and Fas are prominently expressed in acinar and ductal epithelial cells of MSGs of patients with SS [5,6]. In concordance with these studies we observed that the expression of Fas and FasL was significantly up-regulated in acinar and ductal epithelial cells as well as in mononuclear infiltrating cells (results summarized in Table 1). These results may be explained in two different ways. First, epithelial cells may be intrinsically activated and kill themselves through an autocrine interaction of FasL and Fas by membrane folding [17]. Second, the epithelial cells in MSGs of patients with SS may interact with the mononuclear infiltrating cells through the Fas/FasL pathway, thus becoming susceptible to Fas-induced apoptosis.
However, the Fas/FasL pathway is not the only functional apoptotic pathway in the MSGs of patients with SS. Substances released by cytotoxic lymphocytes such as perforin and granzymes constitute the second extrinsic pathway of apoptosis. The expression of perforin and Granzyme B in the infiltrating lymphocytes in SS (Table 1) showed that this pathway of apoptosis may be functional. Previous studies have shown that the majority of the T cells (60–70%) infiltrating the MSGs of patients with SS bear the CD4 phenotype and that most of these CD4 T cells exhibit the memory/inducer marker [7]. In the present study we showed that the cytotoxic T lymphocytes (CTL), either CD4 or CD8 [11,18], which are scattered in the infiltrates are activated, release perforin and Granzyme B, and may play a significant role in the induction of epithelial cell apoptosis. These results are in concordance with previous reports that showed an elevated expression of Granzyme A in SS patients, pointing out the significance of this apoptotic pathway [19].
Although these two apoptotic pathways seem to be responsible for the induction of epithelial cell apoptosis, a distinction has to be made between the decision of a cell to die and the process of death itself. Both extracellular factors, such as cytokines and matrix attachments, and endogenous cellular genes, such as p53, the Bcl-2 family and certain oncogenes, have been shown to modulate the ‘commitment’ of a cell to enter the apoptotic programme [20]. Bcl-2 expression was significantly down-regulated in both acinar and ductal epithelial cells in MSG biopsies of patients with SS (Table 1). In ductal and acinar epithelial cells of MSG biopsies of patients with SS the expression of Bax was significantly up-regulated. Taken together, these results suggest that the over-expression of different apoptosis-inducing messages (Fas and Bax) by the epithelial cells of SS patients and the impaired expression of Bcl-2 by these cells are able to turn the balance towards apoptosis. This suggestion was confirmed by the TUNEL assay, which showed a significant increase in the percentage of apoptotic ductal and acinar epithelial cells in MSG biopsies of patients with SS compared with control individuals.
On the other hand, the expression of Bcl-2 by the mononuclear infiltrating cells of SS MSG biopsies was up-regulated. This up-regulated expression of Bcl-2 seems to account for the inhibition of the apoptotic stimuli given by Bax (the expression of which is also up-regulated in the mononuclear infiltrating cells in SS MSG biopsies) and Fas. The inhibition of apoptosis in these cells is confirmed by the TUNEL assay, where the percentage of apoptotic mononuclear infiltrating cells showed no significant difference compared with that of apoptotic mononuclear cells of control individuals. It is imperative to note that the percentages of apoptotic epithelial and mononuclear infiltrating cells observed in control individuals probably reflect the physiologic turnover of these cells, and not a pathologic condition.
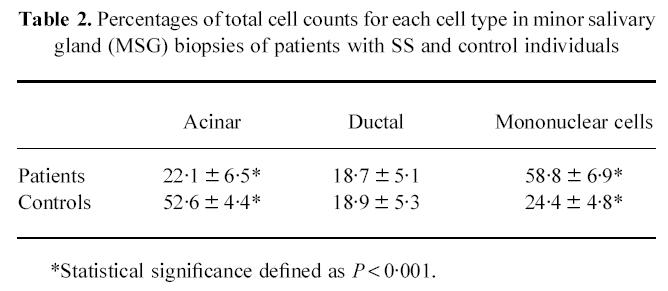
In our study we observed a significant increase in the percentage of apoptotic ductal and acinar epithelial cells in MSG biopsies of patients with SS. Quantitative studies on the percentage of each cell type compared with the total number of cells in each section between age-matched SS patients and control individuals were performed. The results (Table 2) showed a significant reduction in the percentage of acinar cells in SS patients. This phenomenon may be due to apoptotic destruction of the acinar cells and may account for the loss of the secretory function which is observed in SS. On the other hand, the percentage of ductal epithelial cells remained unaltered between SS patients and control individuals. This may be explained by the fact that ductal epithelial cells show a high rate of proliferation [21] and mitotic activity (in contrast to acinar cells, where little or no mitotic activity has been observed [21]), which may overcome the apoptotic degrading messages and retain their population.
Table 2.
Percentages of total cell counts for each cell type in minor salivary gland (MSG) biopsies of patients with SS and control individuals
The pathways of apoptosis that have been described seem to function together in order to lead the epithelial cells to death. We studied the expression of pS2 in MSG biopsies of SS patients to see whether there is a defensive mechanism of the epithelial cells to oppose the action of apoptosis and retain life.
pS2 was expressed by a significant percentage of acinar and ductal epithelial cells in SS patients (Table 1), thus indicating that a regenerative mechanism may be functioning. pS2 is generally expressed by mucus-producing epithelial cells, especially in areas of epithelial cell injury [22], and this could account for the expression of pS2 by epithelial cells in MSG biopsies. Furthermore, pS2, an oestrogen-induced regenerative protein, implicates the involvement of oestrogens which are well known inhibitors of apoptosis [23] in the defensive repertoire of epithelial cells in SS. It is possible that this is a pathway of cell proliferation that is in close contact with the cell cycle, probably acting as a promoter of DNA synthesis, and may antagonize the DNA-degrading messages of the apoptotic stimuli.
The defined phenomenon of apoptotic cell death in the epithelial cells of SS is probably based upon a fine imbalance between proliferative and degrading messages. Our next task is to try to identify this point of imbalance in order to understand the mechanisms of pathogenesis of this syndrome.
Acknowledgments
The authors are grateful to Dr S. Paikos for obtaining the minor salivary gland biopsies. This work was supported by grant 70/3/2826 PENED, the Greek Ministry of Industry, Energy and Technology.
References
- 1.Boumba D, Skopouli FN, Moutsopoulos HM. Cytokine mRNA expression in the labial salivary gland tissues from patients with primary Sjögren's syndrome. Br J Rheumatol. 1995;34:326–33. doi: 10.1093/rheumatology/34.4.326. [DOI] [PubMed] [Google Scholar]
- 2.Moutsopoulos HM, Hooks JJ, Chan CC, et al. HLA-DR expression by labial salivary gland tissues in Sjögren's syndrome. Ann Rheum Dis. 1986;45:677–83. doi: 10.1136/ard.45.8.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Skopouli FN, Kousvelari EE, Mertz P, et al. c-myc m-RNA expression in minor salivary glands of patients with Sjögren's syndrome. J Rheumatol. 1992;19:693–9. [PubMed] [Google Scholar]
- 4.Yannopoulos DI, Roncin S, Lamour A, et al. Conjunctival epithelial cells from patients with Sjögren's syndrome inappropriately express major histocompatibility complex molecules, La (SSB) antigen, and heat-shock proteins. J Clin Immunol. 1992;12:259–65. doi: 10.1007/BF00918149. [DOI] [PubMed] [Google Scholar]
- 5.Kong L, Ogawa N, Nakabayashi T, et al. Fas and Fas ligand expression in the salivary glands of patients with primary Sjögren's syndrome. Arthritis Rheum. 1997;40:87–97. doi: 10.1002/art.1780400113. [DOI] [PubMed] [Google Scholar]
- 6.Matsumura R, Kagami M, Tomioka H, et al. Expression of ductal Fas antigen in sialoadenitis of Sjögren's syndrome. Clin Exp Rheumatol. 1996;14:309–11. [PubMed] [Google Scholar]
- 7.Skopouli FN, Fox PC, Galanopoulou V, et al. T cell subpopulations in the labial minor salivary gland histopathologic lesion of Sjögren's syndrome. J Rheumatol. 1991;18:210–4. [PubMed] [Google Scholar]
- 8.Itoh N, Yonehara S, Ishii A, et al. The polypeptide encoded by the cDNA for human cell surface antigen Fas can mediate apoptosis. Cell. 1991;66:233–43. doi: 10.1016/0092-8674(91)90614-5. [DOI] [PubMed] [Google Scholar]
- 9.Suda T, Takahashi T, Golstein P, et al. Molecular cloning and expression of the Fas ligand, a novel member of the tumor necrosis factor family. Cell. 1993;75:1169–77. doi: 10.1016/0092-8674(93)90326-l. [DOI] [PubMed] [Google Scholar]
- 10.Griffith TS, Brunner T, Fletcher SM, et al. Fas ligand-induced apoptosis as a mechanism of immune privilege. Science. 1995;270:1189–92. doi: 10.1126/science.270.5239.1189. [DOI] [PubMed] [Google Scholar]
- 11.Berke G. The CTL's kiss of death. Cell. 1995;81:9–12. doi: 10.1016/0092-8674(95)90365-8. [DOI] [PubMed] [Google Scholar]
- 12.Liu CC, Walsh CM, Young D. Perforin: structure and function. Immunol Today. 1995;16:194–201. doi: 10.1016/0167-5699(95)80121-9. [DOI] [PubMed] [Google Scholar]
- 13.Smyth M, Trapani JA. Granzymes: exogenous proteinases that induce target cell apoptosis. Immunol Today. 1995;16:202–6. doi: 10.1016/0167-5699(95)80122-7. [DOI] [PubMed] [Google Scholar]
- 14.Lefebvre O, Chenard MP, Masson R, et al. Gastric mucosa abnormalities and tumorigenesis in mice lacking the ps2 trefoil protein. Science. 1996;274:259–62. doi: 10.1126/science.274.5285.259. [DOI] [PubMed] [Google Scholar]
- 15.Vitali C, Bombardieri S, Moutsopoulos HM, et al. Preliminary criteria for the classification of Sjögren's syndrome: results of a prospective concerted action supported by the European Community. Arthritis Rheum. 1993;36:340–7. doi: 10.1002/art.1780360309. [DOI] [PubMed] [Google Scholar]
- 16.Chisholm DM, Mason DK. Labial salivary gland biopsy in Sjögren's disease. J Clin Pathol. 1968;21:656–60. doi: 10.1136/jcp.21.5.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nagata S, Golstein P. The Fas death factor. Science. 1995;267:1449–55. doi: 10.1126/science.7533326. [DOI] [PubMed] [Google Scholar]
- 18.Williams NS, Engelhard VH. Identification of a population of CD4 CTL that utilizes a perforin- rather than a FasL-dependent cytotoxic mechanism. J Immunol. 1996;156:153–9. [PubMed] [Google Scholar]
- 19.Alpert S, Kang HI, Weissman I, Fox RI. Expression of granzyme A in salivary gland biopsies from patients with primary Sjögren's syndrome. Arthritis Rheum. 1994;37:1046–54. doi: 10.1002/art.1780370710. [DOI] [PubMed] [Google Scholar]
- 20.Whyte M. ICE/CED-3 proteases in apoptosis. Trends Cell Biol. 1996;6:245–7. doi: 10.1016/0962-8924(96)20025-x. [DOI] [PubMed] [Google Scholar]
- 21.Durdick I, Byard RW, Carnegie JA. A review of the proliferative capacity of major salivary glands and the relationship to current concepts of neoplasia in salivary glands. Oral Surg Oral Med Oral Pathol. 1990;69:53–67. doi: 10.1016/0030-4220(90)90269-x. [DOI] [PubMed] [Google Scholar]
- 22.Playford RJ, Marchbank T, Goodlad RA, et al. Transgenic mice that overexpress the human trefoil peptide pS2 have an increased resistance to intestinal damage. Proc Natl Acad Sci USA. 1996;93:2137–42. doi: 10.1073/pnas.93.5.2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thompson CB. Apoptosis in the pathogenesis and treatment of disease. Science. 1995;267:1456–62. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]