Abstract
In rheumatoid arthritis peripheral cartilaginous joints are inflamed and eroded. One driving factor may be an immune response towards proteins in the cartilage. Here it is shown that cartilage oligomeric matrix protein (COMP), expressed specifically in cartilage, is arthritogenic in the rat. Both native and denatured rat COMP induced severe arthritis in selected rat strains. The arthritis occurred only in peripheral joints which were attacked by an erosive inflammatory process similar to that seen in the human disease. The disease was self-limited and no permanent destruction of joints was seen macroscopically. Disease development appeared to be dependent on an immune response to autologous (rat) COMP and not on cross-reactivity to other cartilage rat collagens (types II, IX and XI). The disease and the immune response to COMP were genetically controlled by the MHC; the RT1u and RT1l haplotypes were more susceptible than the a, c, d, f and n haplotypes. Both LEW and E3 gene backgrounds were highly permissive for disease induction. These findings suggest that the induction of arthritis with rat COMP represents a unique pathogenesis which is controlled by different genes compared with collagen-induced arthritis or adjuvant-induced arthritis.
Keywords: cartilage, rheumatoid arthritis, autoimmunity, MHC, experimental animal models
INTRODUCTION
Development of rheumatoid arthritis (RA) involves an early erosive inflammatory attack on peripheral cartilaginous joints. The reasons for the tissue specificity of the inflammatory attack are largely unknown, although the characteristic inflammation suggests that it is immune-driven by the presence of activated T cells and abundant class II-expressing synovial cells. Another indication of involvement of the immune system is the highly significant association of certain HLA class II haplotypes with RA. Expression of class II molecules with certain structures in their peptide binding pocket, e.g. the DR4 (DRB1*0401), is three to five times more common in RA-afflicted individuals [1]. Moreover, autoimmune responses to cartilage proteins seem to occur in RA [2–5]. This probably reflects an ongoing autoimmune response.
Taken together, these findings suggest that the immune system in an early phase of disease in fact responds to cartilage-specific proteins and therefore allows a targeted attack on cartilaginous joints. Such a scenario can in fact be reproduced in experimental animal models. It was first demonstrated that immunization with rat type II collagen (CII) induced arthritis (CIA) in rats [6]. The CIA model proved to be highly useful for the elucidation of basic mechanisms leading to arthritis and is now the most commonly used model for RA. Also in CIA, development of arthritis seems to be associated with MHC class II genes. In the rat, the chronic arthritis induced with rat CII is associated with the RT1a haplotype; probably with the expression of the DQa molecule [7].
That lymphocytes are able to recognize cartilage-derived CII as an autoantigen, and that this leads to arthritis, implies that also other cartilage proteins may evoke an arthritogenic immune response. Joint cartilage fulfils unique functions and harbours a number of proteins with tissue-specific locations, constituting potential targets for autoreactive lymphocytes. The major component of fibrils in the cartilage is CII. Another constituent of the fibrils is type XI collagen (CXI), which in fact shares one of its α-chains with CII. The surfaces of the fibrils are decorated with type IX collagen (CIX). There is evidence that both CXI and maybe also CIX can induce arthritis if given as heterologous, i.e. bovine, proteins [8–10]. The major proteoglycan of the joint, aggrecan, has also been shown to induce arthritis in certain mouse strains, if given in a heterologous (human) form [11]. The induction of arthritis with heterologous cartilage proteins may be a way to circumvent the strong tolerance of T cells specific for cartilage proteins [12]. However, it could be anticipated that less abundant cartilage proteins are less efficient in tolerance induction and may therefore be interesting candidates as targets for an autoimmune response leading to arthritis.
One such protein is cartilage oligomeric matrix protein (COMP), and in the present work we investigate its role in arthritis development. COMP is a large protein, with a total molecular weight of 435 kD. It is composed of five identical subunits, disulphide bonded near their N-termini, whereas the C-termini adopt a globular conformation, altogether forming a bouquet-like structure [13–15]. COMP is synthesized by chondrocytes and localized extracellularly. In young tissue and in the growth plate it is found preferentially in the pericellular-territorial matrix of the chondrocytes, while in adult cartilage the protein is found primarily in the interterritorial matrix at distance from the chondrocyte [16]. It has been detected in nasal, tracheal, and meniscal cartilage, and most prominently in articular cartilage [13,17]. It is also present in the vitreous body of the eye [18] and was recently detected in tendon [19,20] and in human synovial tissue [21]. In the growth plate, COMP is primarily observed in the proliferative region. In mature cartilage, COMP is a major non-collagenous matrix protein, primarily located intraterritorially and concentrated in the superficial layers (Lorenzo & Heinegård, unpublished). That COMP may have critical functions in cartilage is underlined by the fact that a mutation in the calcium-binding domain is phenotypically manifested as pseudochondroplasia and multiple epiphyseal dysplasia [22]. An interesting feature of COMP is its release from cartilage during the erosion of the tissue seen in severe arthritis. This makes COMP a potentially useful marker of arthritis in RA [23]. COMP is also released in rats developing chronic arthritis after induction with pristane, a synthetic low molecular weight adjuvant, and the levels in serum correlate strongly with the occurrence of erosive arthritis [24].
To investigate whether COMP is also able to induce arthritis, we have designed a study in which we use COMP of rat origin to ensure we are dealing with an autoimmune process. From our experience with the CIA model we know that rat CII induces a unique arthritis, characterized by the development of chronicity and the involvement of autoreactive T cells [7,25,26]. In the CIA model the disease is induced only with native and not with denatured CII [6,27], indicating that the tertiary triple helical structure in the molecule may be important for antibody recognition. Therefore, not only denatured COMP, but also the native protein was used in this study. Lastly, selected rat strains were used to address both MHC and non-MHC genetic control of the disease. These strains are well characterized with respect to other autoimmune diseases [7,28–31], including various arthritis models, allowing us to compare possible differences in the susceptibility to and phenotype of the developing disease.
MATERIALS AND METHODS
Animal procedures
Inbred rats (originally obtained from Zentralinstitut für Versuchstierzucht, Hannover, Germany) were bred and kept in the Animal Department of Medical Inflammation Research in Lund. This is a pathogen-controlled (virus-free) environment which is climate-controlled with a 12 h light–dark cycle. The rats are housed in polystyrene cages containing wood shavings, and fed with standard rodent chow and water ad libitum. All experiments were performed on age-matched rats (ages between 8 and 15 weeks). Only females were used due to higher disease susceptibility.
COMP purification
The purification of denatured COMP has been described earlier in detail [13]. For purification of native COMP 200 g of rat chondrosarcoma tissue were finely minced and homogenized on ice in 10 volumes of 20 mm Tris–HCl, 0.15 m NaCl, 100 mm e-amino caproic acid, 5 mm benzamidine, 10 mm N-ethylmalemide (NEM), pH 7.4 (TBS) using a Polytron homogenizer operated at full speed. The homogenate was immediately centrifuged for 30 min at 15 300 g (Beckman JA14 rotor; Beckman Instruments, Palo Alto, CA) at 4°C. The tissue pellet was washed once by resuspension in 10 volumes of TBS followed by centrifugation as above. To extract COMP, the tissue was resuspended in 10 volumes of TBS also containing 10 mm EDTA and incubated overnight at 4°C with constant stirring. After sedimentation of the tissue residue, and centrifugation as described above, the EDTA extract was diluted with an equal volume of 10 mm Tris–HCl pH 7.4 and applied to a 100-ml DEAE-Sepharose Fast Flow column (Pharmacia Biotech, Uppsala, Sweden) equilibrated in 20 mm Tris–HCl, 10 mm EDTA, 2 mm NEM pH 7.4. After washing with equilibration buffer, adsorbed material was eluted with a 1000-ml linear 0–0.5 m NaCl gradient in the buffer. COMP-containing fractions, identified by SDS–PAGE, were combined and dialysed against 20 mm Tris–HCl, 10 mm EDTA, 2 mm NEM pH 7.4. The COMP pool was concentrated by adsorption to a 5-ml HiTrap Q-column (Pharmacia Biotech) equilibrated with 20 mm Tris–HCl, 10 mm EDTA, 2 mm NEM pH 7.4 followed by elution with a 10-ml pulse of 0.5 m NaCl in the buffer. The protein was recovered in a volume of 4 ml. Further purification was by gel filtration on a Superdex 200 HR 10/30 column (Pharmacia Biotech) equilibrated and eluted with 20 mm Tris–HCl, 10 mm EDTA, 0.15 m NaCl, 2 mm NEM pH 7.4 at a flow rate of 0.5 ml/min. COMP-containing fractions were identified by SDS–PAGE. As the resulting preparation still contained contaminating thrombospondin-1, the material was passed over a 5-ml HiTrap heparin column (Pharmacia Biotech) equilibrated in 20 mm Tris–HCl, 10 mm EDTA, 2 mm NEM pH 8. After this step, COMP, recovered in the flow through, had been purified to apparent homogeneity as judged by SDS–PAGE and coomassie staining (not shown). The recovery was approx. 15 mg of protein starting with 200 g of chondrosarcoma tissue.
Immunization protocol and measurement of arthritis severity
The native COMP was diluted in PBS and the denatured COMP, originally containing 4 m guanidine chloride, was also diluted in PBS, giving a final concentration of 2 m guanidine chloride. The solubilized COMP preparations were emulsified with an equal volume of Freund's incomplete adjuvant (FIA; Difco, Detroit, MI). Each rat was injected with 150 μg COMP in a volume of 300 μl (denatured COMP) or 400 μl (native COMP) intradermally at the base of the tail. The induction of oil-induced arthritis was achieved as earlier described [32] by injection of 150 μl FIA emulsified with a equal volume of 0.1 m acetic acid, intradermally at the base of the tail. Arthritis development was monitored by a macroscopic scoring system for the four limbs ranging from 0 to 4 (1 = swelling and redness of one joint, 2 = two joints involved, 3 = more than two joints involved, and 4 = severe arthritis in the entire paw).
Quantification of antibody titre in serum
Sera were obtained from the retro-orbital plexus, collected individually and stored at − 80°C until assayed. Antibodies to CII, CIX, CXI, and denatured and native COMP were quantified with a modified ELISA. Briefly, 96-well plates (Dynatech-immunolon 2; Denkendorf, Germany) were coated with 10 μg/ml of respective antigen in PBS overnight at 4°C. Sera were analysed in duplicates and titrated in PBS containing 0.05% Tween 20 and the amounts of captured antibody were detected with affinity-purified donkey anti-rat IgG-specific or goat anti-rat IgM-specific, conjugated with alkaline phosphatase (Jackson ImmunoResearch Labs Inc, West Grove, PA) (diluted 1:16 000 with PBS containing 0.2% Tween 20). Para-nitrophenol was used as a chromogenic substrate and the absorbance determined in a Titertek Multiscan filterphotometer. The titre value was determined from the same absorbance levels in all assays which were run in parallel with both positive (COMP, CII, CXI or CIX immune rat serum) and negative (normal rat serum) controls included on each plate.
Histology
Paws, ankle, sacral, lumbar and cervical vertebra, and mandibular joints, eyes, ears, trachea, and nasal septum were prepared essentially as earlier described [33]. Briefly, bone tissue was decalcified. The tissue samples were fixed in paraformaldehyde or frozen for immunohistochemical analysis. After sectioning, paraffin-embedded tissues were stained with haematoxylin and eosin, and safranin O, and further labelled with antibodies staining macrophage subsets (ED1 and ED2) as markers for inflammation activity. Cryosections were labelled with antibodies OX19 (T cells), OX33 (B cells) and OX6 (RT1 class II). A scoring protocol was used for both active inflammation (A1 to A3) and the healing process (H1 to H3). N, normal; A1, mild synovitis with hyperplastic membrane, small focal cell infiltration, increased number of vessel in and villous formation of synovium. No bone or cartilage erosions; A2, moderate synovitis with pannus formation, bone and cartilage erosions limited to discrete foci. Undisrupted joint architecture; A3, severe pannus formation with extensive erosions of bone and cartilage. Disrupted joint architecture; H1, new formation of cartilage, but not bone formation; H2, cartilage and bone formation; H3, cartilage and bone formation and apparent ankylosis [34].
Statistical analysis
The mean maximum scores were evaluated using the Mann–Whitney U-test and antibody titres using the unpaired Student's t-test.
RESULTS
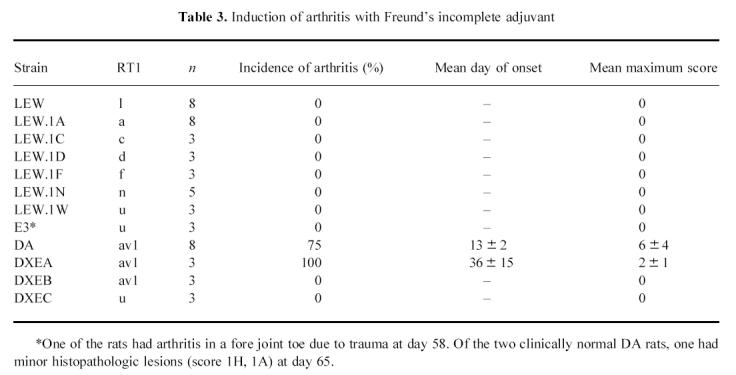
Induction of arthritis
Immunization with COMP in FIA induced arthritis in most strains tested, although with variable frequency and severity (Table 1,Figs 1 and 2). Immunization with FIA alone induced arthritis in the DA and DXEA strains (Table 3). DXEA is a recombinant inbred strain with approx. 50% of its genes originating from DA. Thus, susceptibility to the earlier described oil-induced arthritis [28,32,35] apparently requires DA genes. No other strains showed any sign of oil-induced arthritis during an observation period of 65 days (Table 3), demonstrating that the observed disease, except in DA and DXEA, was specifically dependent on COMP used for immunization.
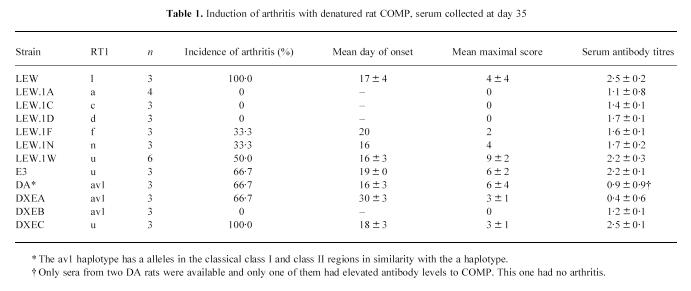
Table 1.
Induction of arthritis with denatured rat COMP, serum collected at day 35
Fig. 1.
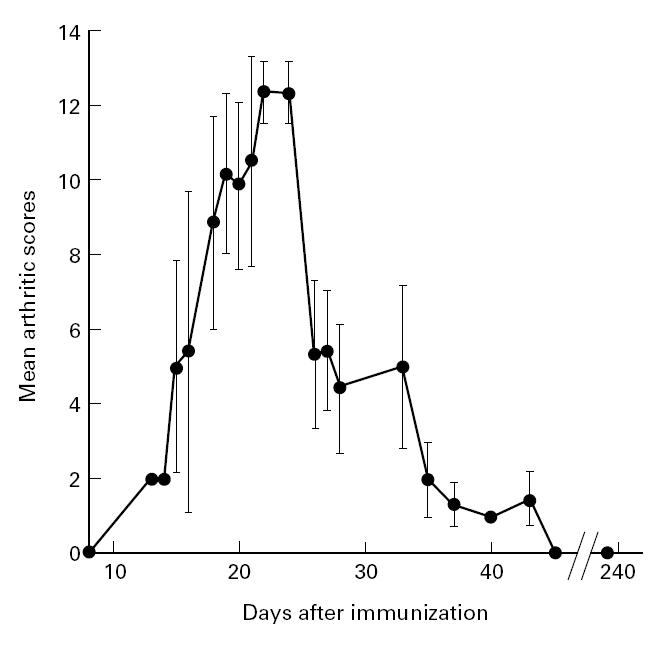
Disease course indicated with mean scores of seven arthritic E3 rats immunized with native COMP. The disease course is acute, with a range of 13–43 days. The rats were further scored twice a week for 240 days, with no reappearance of arthritis.
Fig. 2.
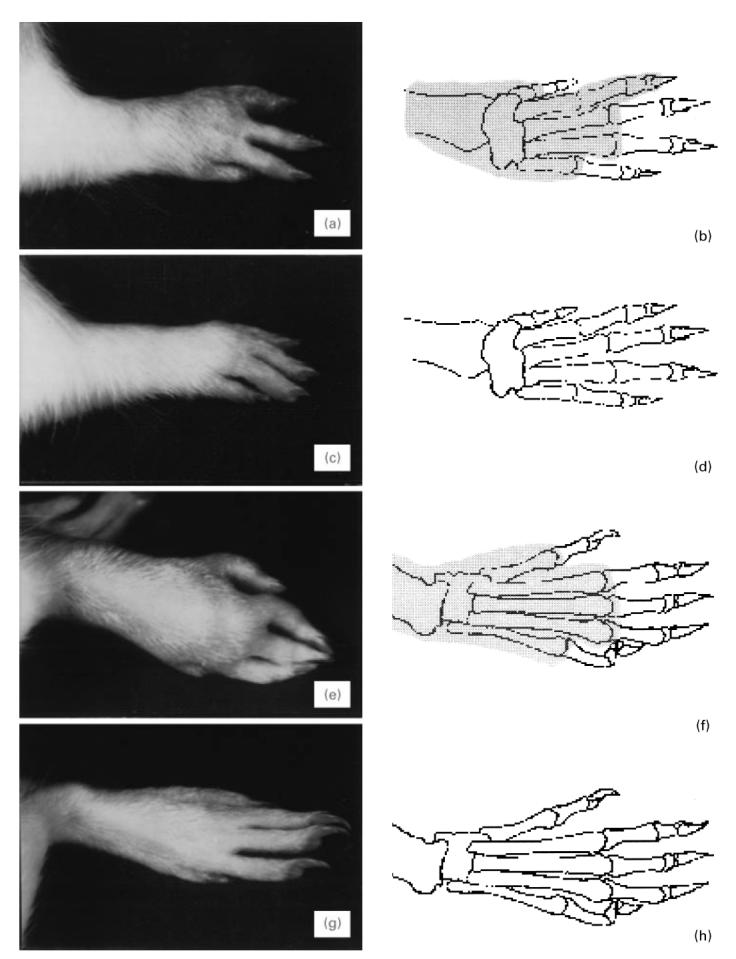
Paws from E3 rats exemplify the appearance of arthritis and the scoring protocol. The hatched areas in the depictions indicate the observed location of swelling and erythema. (a,b) Arthritic hind paw with arthritis given score 3. (c,d) Normal hind paw. (e,f) Arthritic front paw given score 4. (g,h) Normal front paw.
Table 3.
Induction of arthritis with Freund's incomplete adjuvant
The onset of COMP-induced arthritis was characterized by a sudden appearance of erythema and swelling of the hind paws. The arthritis was typically located in the metatarsal region and subsequently spread to the ankle and in severe cases to the knuckles and toes (Fig. 2). In the susceptible E3 strain even front paws were occasionally affected. In most cases the arthritis healed gradually, leaving no signs of inflammation or deformity at 43 days after immunization. No other macroscopic sign of inflammation was observed during a period of 240 days. Immunization with native COMP induced an arthritis which was similar to that induced with denatured COMP, but it tended to be more long-lasting, especially in the E3 strain. No sign of arthritis was observed when immunizing DA and the LEW congenic strains with guanidin chloride and FIA (data not shown).
Some of the LEW.1W (n = 4), DA (n = 3) and E3 (n = 4) rats were analysed histopathologically between days 19 and 240. During the inflammatory period (days 19–61) most rats had scores of A2–A3. A moderate hyperplasia and hypertrophy of the synovium with pannus and vessel formation was seen. Although both bone and cartilage destruction were clearly present, they were limited to the marginal zone while the overall joint architecture was only occasionally remodelled. The most severe inflammation was seen in E3 rats, in which relatively severe erosions were present at day 35 (Fig. 3). The inflammatory infiltrates consisted mainly of activated macrophages, neutrophils and a few lymphocytes. Tendons were mildly affected by inflammatory cells and only in those sections with a high histological score in the active phase. No signs of new bone formation or ankylosis were observed even at late stage. Sacral, lumbar, cervical vertebra, and mandibular joints, eyes, ears, trachea and nasal septum sections contained no detectable inflammation.
Fig. 3.
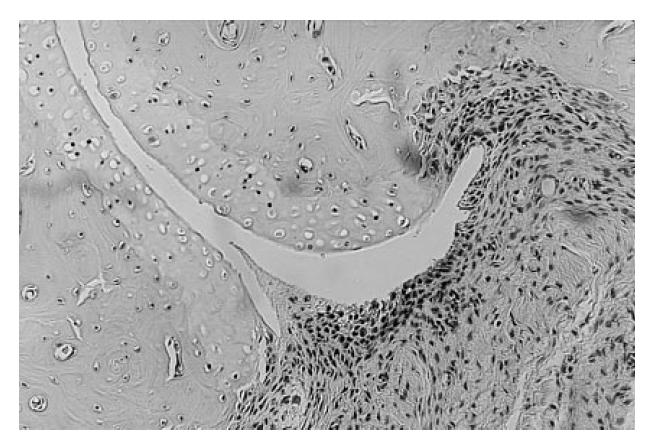
A joint from the mid paw of an E3 rat immunized with native COMP. This section was taken on day 35 after immunization. Both pannus formation and subchondral bone erosion are present, corresponding to score A2. (Haematoxylin and eosin staining, original mag. × 50.)
Histopathological analysis of joints from an E3 rat taken 240 days after immunization with native COMP, well after clinical healing, showed destructive and deforming changes in the joints (scored H1–H2), but no signs of active inflammation.
Antibody response
All rats immunized with denatured or native COMP developed a specific antibody response (Tables 1 and 2,Fig. 4). The response was high only in the arthritis-susceptible E3 and LEW.1W strains. The first appearing response and the subsequent main part were of the IgG type. The IgM fraction appeared a long time after immunization, when most of the active arthritis had disappeared. Rats with arthritis had higher levels of IgG antibodies to COMP in sera in high responder RT1u haplotype rats (P = 0.02 in experiment with native COMP and P = 0.004 in experiment with denatured COMP).
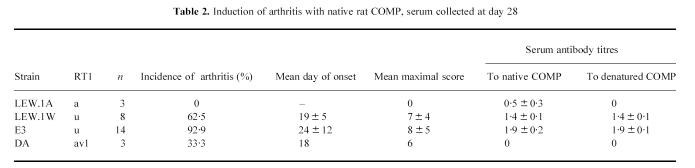
Table 2.
Induction of arthritis with native rat COMP, serum collected at day 28
Fig. 4.
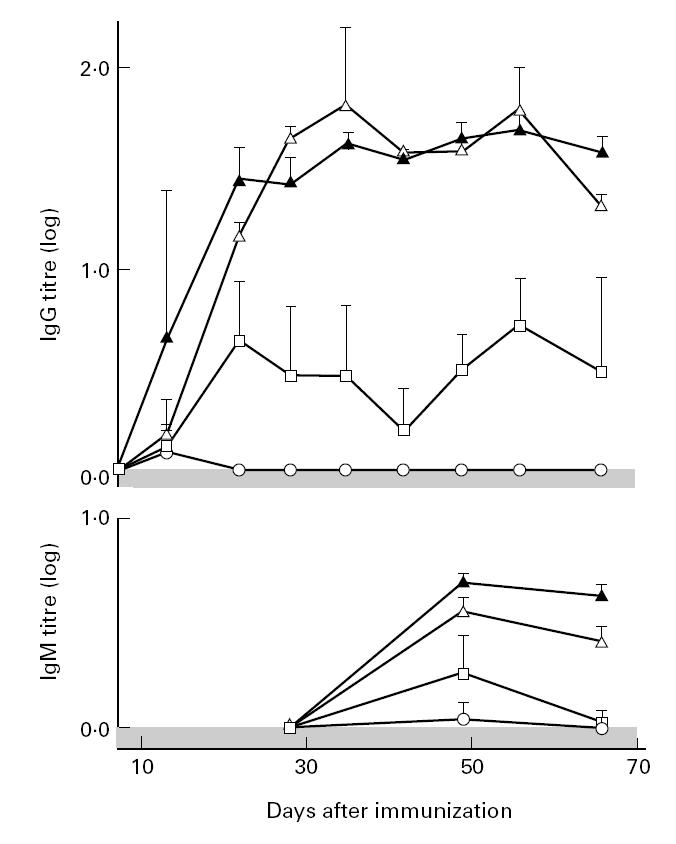
Serum levels of IgG and IgM antibodies to native COMP in DA (○), LEW.1A (□), LEW.1W (▴) and E3 (Δ) rats immunized with native COMP (from day 7 to day 66).
No antibody response to other cartilage proteins, such as rat CIX, CXI or CII, was observed in most strains, although two LEW.1W rats immunized with denatured COMP had low but detectable titres to CII (data not shown). The CII reactivity, however, was not associated with arthritis.
Genetic control
Most strains were susceptible to arthritis after induction with denatured COMP, albeit to different extents (Tables 1 and 2). Strains with the RT1u haplotype made a stronger antibody response towards COMP (P < 0.0001) and these strains had a higher incidence and more severe arthritis (P = 0.0001). This was also observed with the MHC congenic LEW strains; only LEW.1W and LEW rats had a high frequency of arthritis, whereas none of the LEW.1A, LEW.1D and LEW.1C had arthritis. These differences were more pronounced after immunization with native COMP (Table 2). Even in this experiment the expression of RT1u was associated with arthritis susceptibility (compare LEW.1A and LEW.1W). The antibody response after immunization with native COMP was higher in rats with RT1u (P = 0.01).
Comparison of the DA, E3 and the recombinant inbred strains DXEA, DXEB and DXEC (with genes inherited from both DA and E3 in different combinations) strongly suggests that E3 genes promote development of COMP-induced arthritis, whereas DA genes promote oil-induced arthritis and not COMP-induced arthritis. This is at least partly explained by an effect of MHC, since the COMP-induced arthritis-susceptible strains E3 and DXEC both have the RT1u haplotype. However, a contribution from non-MHC genes is also possible, especially since the E3 rats tended to have a higher frequency and a more long-lasting disease than the LEW.1W rats (Table 3).
DISCUSSION
A new model of RA is described in which arthritis is induced after immunization with the non-collagenous cartilage protein COMP. The disease is inducible with autologous rat-derived COMP leading to an autoimmune response and subsequent development of arthritis in peripheral joints of rats. The disease susceptibility is controlled be the MHC region, with the u and l haplotypes being high responders. A surprising finding was that the genetic control differs from other arthritis models, such as CIA [7], avridine-induced arthritis (AvIA) [29], pristane-induced arthritis (PIA) [30] and oil-induced arthritis (OIA) [32,36], suggesting that the pathogenesis of COMP-induced arthritis is unique.
COMP is located in cartilage and in the vitreous humour of the eye, thus having a similar distribution to CII [13,17,18]. The inflammation was directed towards tissues expressing COMP, but as in CIA, only peripheral joints were affected. This possibly reflects the importance of a well developed synovium in which macrophages and fibroblasts are present that could be activated by immune cells. The initiation of arthritis indicates that cartilage-located COMP is recognized by activated lymphocytes, or available for binding to pathogenic antibodies. This also shows that non-collagenous proteins, embedded in the cartilage matrix, can initiate an inflammatory attack on the joints.
Immunization with COMP induced a strong autoantibody response, mainly consisting of IgG rather than IgM antibodies. This, together with the MHC association of the response, suggests an important role for autoreactive T cells in the disease. If COMP, found in all cartilage, can be recognized by lymphocytes the question immediately arises as to why the immune system is not normally tolerized to it. This issue is particularly relevant for COMP, since COMP is apparently released from both normal and diseased joints, and the systemic appearance should effectively tolerize the immune system. Obviously, the induction of an autoimmune response and arthritis after immunization with rat COMP indicate that such a postulated tolerance cannot be complete. Possibly, the circulating COMP molecules and/or fragments might expose other epitopes than provided by the intact COMP chains or by the native bouquet-like molecule when incorporated in the extracellular matrix of cartilage. The predominant location of COMP in cartilage, and the release of COMP fragments, will provide very useful tools for further analyses on how the immune system interacts with cartilage.
The MHC control of the immune response and disease susceptibility may not be surprising, taking into account the disease is induced after immunization with a specific protein. Most likely it reflects that a major immunodominant peptide, derived from COMP, plays an important role in the triggering of autoreactive T cells. This peptide remains to be identified but it is reasonable to postulate that it would bind to RT1u and RT1l class II molecules, but might not form strong enough complexes to induce a complete T cell tolerance [37]. The finding that the COMP-induced disease is associated with RT1u and RT1l rather than RT1a supports the conclusion that CII does not play a role in the disease induction, since CIA induced with rat CII is associated with a rather than u and l [27]. It is also of interest that the failure of the RT1a or RT1av1 haplotype to permit immune response to autologous COMP may be different from the response to bovine COMP, as indicated by the recent observation that bovine COMP induces an immune response and arthritis in RT1av1 haplotype rats [38]. However, it should be emphasized that the linkage to MHC might not necessarily be due to class II genes. It remains to be investigated which genes in the MHC are of importance in controlling COMP-induced arthritis.
One of the most interesting observations is that the E3 rat is highly susceptible. We have found this strain to be highly resistant to the induction of a number of different autoimmune arthritis models such as CIA, PIA, AvIA and, as described here, OIA [7,29,30]. In addition, the E3 rat is resistant to certain other autoimmune diseases such as experimental allergic encephalomyelitis (EAE) [31]. This difference is due not only to the RT1u haplotype but also to the non-MHC genes. This finding indicates that COMP-induced arthritis displays unique features in its genetically controlled pathogenesis, which will be highly useful for further studies elucidating the mechanisms leading to autoimmune arthritis.
The new COMP-induced arthritis model may not only be useful for further work on elucidating the precise mechanisms leading to arthritis in animal models, but might also fuel investigations on the role of various cartilage proteins in RA. So far a response to COMP has not been shown to occur in RA. This could be due to the exposure of this protein during the disease course which might lead to tolerance and thereby absence of detectable immune responses in vitro. Nevertheless, such reactions may play an important role in the maintenance and/or regulation of RA.
Acknowledgments
We thank Lennart Lindström and Yvette Sjöö for taking care of the animals, Margareta Svejme for histopathologic analysis and Andrew Cook for linguistic corrections. The work was supported by grants from the Anna Greta Crafoord Foundation for Rheumatological Research, King Gustaf V:s 80-year Foundation, the Kock and Österlund Foundations, the Swedish Association against Rheumatism and the Swedish Medical Research Council.
References
- 1.Gregersen PK, Silver J, Winchester RJ. The shared epitope hypothesis. An approach to understanding the molecular genetics of susceptibility to rheumatoid arthritis. Arthritis Rheum. 1987;30:1205–13. doi: 10.1002/art.1780301102. [DOI] [PubMed] [Google Scholar]
- 2.Clague RB, Moore LJ. IgG and IgM antibody to native type II collagen in rheumatoid arthritis serum and synovial fluid. Arthritis Rheum. 1984;27:1370–7. doi: 10.1002/art.1780271207. [DOI] [PubMed] [Google Scholar]
- 3.Rowley MJ, Williamson DJ, Mackay IR. Evidence for local synthesis of antibodies to denatured collagen in the synovium in rheumatoid arthritis. Arthritis Rheum. 1987;30:1420–5. doi: 10.1002/art.1780301215. [DOI] [PubMed] [Google Scholar]
- 4.Tarkowski A, Klareskog L, Carlsten H, Herberts P, Koopman WJ. Secretion of antibodies to types I and II collagen by synovial tissue cells in patients with rheumatoid arthritis. Arthritis Rheum. 1989;32:1087–92. doi: 10.1002/anr.1780320906. [DOI] [PubMed] [Google Scholar]
- 5.Cook AD, Rowley MJ, Mackay IR, Gough A, Emery P. Antibodies to type II collagen in early rheumatoid arthritis. Correlation with disease progression. Arthritis Rheum. 1996;39:1720–7. doi: 10.1002/art.1780391015. [DOI] [PubMed] [Google Scholar]
- 6.Trentham DE, Townes AS, Kang AH. Autoimmunity to type II collagen: an experimental model of arthritis. J Exp Med. 1977;146:857–68. doi: 10.1084/jem.146.3.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holmdahl R, Vingsbo C, Hedrich H, Karlsson M, Kvick C, Goldschmidt TJ, Gustafsson K. Homologous collagen-induced arthritis in rats and mice are associated with structurally different major histocompatibility complex DQ-like molecules. Eur J Immunol. 1992;22:419–24. doi: 10.1002/eji.1830220220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morgan K, Evans HB, Firth SA, Smith MN, Ayad S, Weiss JB, Holt PJL. 1a,2a,3a collagen is arthritogenic. Ann Rheum Dis. 1983;42:680–3. doi: 10.1136/ard.42.6.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cremer MA, Griffiths MM, Terato K, Kang AH. Type XI and II collagen-induced arthritis in rats: characterization of inbred strains of rats for arthritis-susceptibility and immune-responsiveness to type XI and II collagen. Autoimmunity. 1995;20:153–61. doi: 10.3109/08916939508993346. [DOI] [PubMed] [Google Scholar]
- 10.Boissier MC, Chiocchia G, Ronziere MC, Herbage D, Fournier C. Arthritogenicity of minor cartilage collagens (types IX and XI) in mice. Arthritis Rheum. 1990;33:1–8. doi: 10.1002/art.1780330101. [DOI] [PubMed] [Google Scholar]
- 11.Glant TT, Mikecz K, Arzoumanian A, Poole AR. Proteoglycan-induced arthritis in Balb/c mice. Arthritis Rheum. 1987;30:201–12. doi: 10.1002/art.1780300211. [DOI] [PubMed] [Google Scholar]
- 12.Holmdahl R, Andersson M, Goldschmidt TJ, Gustafsson K, Jansson L, Mo JA. Type II collagen autoimmunity in animals and provocations leading to arthritis. Immunol Rev. 1990;118:193–232. doi: 10.1111/j.1600-065x.1990.tb00817.x. [DOI] [PubMed] [Google Scholar]
- 13.Hedbom E, Antonsson P, Hjerpe A, et al. Cartilage matrix proteins. An acidic oligomeric protein (COMP) detected only in cartilage. J Biol Chem. 1992;267:6132–6. [PubMed] [Google Scholar]
- 14.Mörgelin M, Heinegård D, Engel J, Paulsson M. Electron microscopy of native cartilage oligomeric matrix protein purified from the Swarm rat chondrosarcoma reveals a five-armed structure. J Biol Chem. 1992;267:6137–41. [PubMed] [Google Scholar]
- 15.Oldberg A, Antonsson P, Lindblom K, Heinegård D. COMP (cartilage oligomeric matrix protein) is structurally related to the thrombospondins. J Biol Chem. 1992;267:22346–50. [PubMed] [Google Scholar]
- 16.Shen Z, Heinegård D, Sommarin Y. Distribution and expression of cartilage oligomeric matrix protein and bone sialoprotein show marked changes during rat femoral head development. Matrix Biol. 1995;14:773–81. doi: 10.1016/s0945-053x(05)80020-4. [DOI] [PubMed] [Google Scholar]
- 17.Fife RS. Identification of cartilage matrix glycoprotein in synovial fluid in human osteoarthritis. Arthritis Rheum. 1988;31:553–6. doi: 10.1002/art.1780310414. [DOI] [PubMed] [Google Scholar]
- 18.Nguyen BQ, Fife RS. Vitreous contains a cartilage-related protein. Exp Eye Res. 1986;43:375–82. doi: 10.1016/s0014-4835(86)80074-4. [DOI] [PubMed] [Google Scholar]
- 19.DiCesare P, Hauser N, Lehman D, Pasumarti S, Paulsson M. Cartilage oligomeric matrix protein (COMP) is an abundant component of tendon. FEBS Letters. 1994;354:237–40. doi: 10.1016/0014-5793(94)01134-6. [DOI] [PubMed] [Google Scholar]
- 20.Smith R, Zunino L, Webbon P, Heinegård D. The distribution of cartilage oligomeric matrix protein (COMP) in tendon and its variation with tendon site, age and load. Matrix Biol. 1997;16:255–71. doi: 10.1016/s0945-053x(97)90014-7. [DOI] [PubMed] [Google Scholar]
- 21.Di Cesare PE, Carlson CS, Stollerman ES, Chen FS, Leslie M, Perris R. Expression of cartilage oligomeric matrix protein by human synovium. FEBS Letters. 1997;412:249–52. doi: 10.1016/s0014-5793(97)00789-8. [DOI] [PubMed] [Google Scholar]
- 22.Briggs MD, Hoffman SM, King LM, et al. Pseudoachondroplasia and multiple epiphyseal dysplasia due to mutations in the cartilage oligomeric matrix protein gene. Nat Genet. 1995;10:330–6. doi: 10.1038/ng0795-330. [DOI] [PubMed] [Google Scholar]
- 23.Saxne T, Heinegård D. Cartilage oligomeric matrix protein: a novel marker of cartilage turnover detectable in synovial fluid and blood. Br J Rheumatol. 1992;31:583–91. doi: 10.1093/rheumatology/31.9.583. [DOI] [PubMed] [Google Scholar]
- 24.Vingsbo-Lundberg C, Saxne T, Olsson H, Holmdahl R. Increased serum levels of cartilage oligomeric matrix protein in chronic erosive arthritis in rats. Arthritis Rheum. 1998;41:544–50. doi: 10.1002/1529-0131(199803)41:3<544::AID-ART21>3.0.CO;2-#. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goldschmidt TJ, Holmdahl R. Anti-T cell receptor antibody treatment of rats with established autologous collagen-induced arthritis. Suppression of arthritis without reduction of anti-type II collagen autoantibody levels. Eur J Immunol. 1991;21:1327–30. doi: 10.1002/eji.1830210536. [DOI] [PubMed] [Google Scholar]
- 26.Vingsbo C, Larsson P, Andersson M, Holmdahl R. Association of pepsin with type II collagen (CII) breaks control of CII autoimmunity and triggers development of arthritis in rats. Scand J Immunol. 1993;37:337–42. doi: 10.1111/j.1365-3083.1993.tb02562.x. [DOI] [PubMed] [Google Scholar]
- 27.Holmdahl ?, Vingsbo C, Malmström V, Jansson L, Holmdahl M. Chronicity of arthritis induced with homologous type II collagen (CII) in rats is dependent on anti-CII B-cell activation. J Autoimmun. 1994;7:739–52. doi: 10.1006/jaut.1994.1058. [DOI] [PubMed] [Google Scholar]
- 28.Holmdahl R, Goldschmidt TJ, Kleinau S, Kvick C, Jonsson R. Arthritis induced in rats with adjuvant oil is a genetically restricted, alpha beta T-cell dependent autoimmune disease. Immunology. 1992;76:197–202. [PMC free article] [PubMed] [Google Scholar]
- 29.Vingsbo C, Jonsson R, Holmdahl R. Avridine-induced arthritis in rats; a T cell-dependent chronic disease influenced both by MHC genes and by non-MHC genes. Clin Exp Immunol. 1995;99:359–63. doi: 10.1111/j.1365-2249.1995.tb05558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vingsbo C, Sahlstrand P, Brun JG, Jonsson R, Saxne T, Holmdahl R. Pristane-induced arthritis in rats: a new model for rheumatoid arthritis with a chronic disease course influenced by both major histocompatibility complex and non-major histocompatibility complex genes. Am J Pathol. 1996;149:1675–83. [PMC free article] [PubMed] [Google Scholar]
- 31.Kjellén P, Issazadeh S, Olsson T, Holmdahl R. Genetic influence on disease course and cytokine response in relapsing experimental allergic encephalomyelitis. Int Immunol. 1998;10:333–40. doi: 10.1093/intimm/10.3.333. [DOI] [PubMed] [Google Scholar]
- 32.Holmdahl R, Kvick C. Vaccination and genetic experiments demonstrate that adjuvant oil induced arthritis and homologous type II collagen induced arthritis in the same rat strain are different diseases. Clin Exp Immunol. 1992;88:96–100. doi: 10.1111/j.1365-2249.1992.tb03045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Holmdahl R, Jonsson R, Larsson P, Klareskog L. Early appearance of activated CD4 positive T lymphocytes and Ia-expressing cells in joints of DBA/1 mice immunized with type II collagen. Lab Invest. 1988;58:53–60. [PubMed] [Google Scholar]
- 34.Holmdahl R, Carlsén S, Mikulowska A, et al. Genetic analysis of murine models for rheumatoid arthritis. In: Adolpho K, editor. Human genome methods. Boca Raton/New York: CRC Press; 1998. pp. 215–38. [Google Scholar]
- 35.Kleinau S, Erlandsson H, Holmdahl R, Klareskog L. Adjuvant oils induce arthritis in the DA rat. I. Characterization of the disease and evidence for an immunological involvement. J Autoimmun. 1991;4:871–80. doi: 10.1016/0896-8411(91)90050-M. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lorentzen JC, Klareskog L. Susceptibility of DA rats to arthritis induced with adjuvant oil or rat collagen is determined by genes both within and outside the major histocompatibility complex. Scand J Immunol. 1996;44:592–8. doi: 10.1046/j.1365-3083.1996.d01-354.x. [DOI] [PubMed] [Google Scholar]
- 37.Liu GY, Wraith DC. Affinity for class II MHC determines the extent to which soluble peptides tolerize autoreactive T cells in naive and primed adult mice—implications for autoimmunity. Int Immunol. 1995;7:1255–63. doi: 10.1093/intimm/7.8.1255. [DOI] [PubMed] [Google Scholar]
- 38.Erlandsson-Harris H. Stockholm: Karoliska Institute, Dept of Medicine; 1997. Studies of the immunopathogensis in three arthritis models in rats. [Google Scholar]