Abstract
In the frog embryo, a sub-population of trunk neural crest (NC) cells undergoes a dorsal route of migration to contribute to the mesenchyme in the core of the dorsal fin. Here we show that a second population of cells, originally located in the dorsomedial region of the somite, also contributes to the fin mesenchyme. We find that the frog orthologue of Wnt11 (Wnt11-R) is expressed in both the NC and somite cell populations that migrate into the fin matrix. Wnt11-R is expressed prior to migration and persists in the mesenchymal cells after they have distributed throughout the fin. Loss of function studies demonstrate that Wnt11-R activity is required for an epithelial to mesenchymal transformation (EMT) event that precedes migration of cells into the fin matrix. In Wnt11-R depleted embryos, the absence of fin core cells leads to defective dorsal fin development and to collapse of the fin structure. Experiments using small molecule inhibitors indicate that dorsal migration of fin core cells depends on calcium signaling through calcium/calmodulin-dependent kinase II (CaMKII). In Wnt11-R depleted embryos, normal migration of NC cells and dorsal somite cells into the fin and normal fin development can be rescued by stimulation of calcium release. These studies are consistent with a model in which Wnt11-R signaling, via a downstream calcium pathway, regulates fin cell migration and, more generally, indicates a role for non-canonical Wnt signaling in regulation of EMT.
Keywords: Wnt11, Wnt11-R, Xenopus, trunk neural crest, fine core cells, CaMKII, non-canonical Wnt signaling
INTRODUCTION
The dorsal fin is a prominent feature of the Amphibian embryo that extends along the dorsal surface of the animal, from behind the head to the tip of the tail. The mature dorsal fin is a simple keel-shaped out-pocketing of the dorsal epidermis that is supported by mesenchymal cells and extracellular matrix (Tucker, 1986). Development of the fin begins during the early tail bud stage and the structure remains present until metamorphosis when both the fin and tail regress. The dorsal fin develops as a result of inductive signals from the neural crest (NC) (DuShane, 1935; Bodenstein, 1952; Tucker and Slack, 2004). At first, epidermis of the nascent fin undergoes cell proliferation and extends dorsally, accompanied by an accumulation of extracellular matrix, but the interior of the fin is largely acellular. During tailbud stages, non-pigmented NC cells migrate into the fin matrix (Twitty and Bodenstein, 1941; Tucker and Erickson, 1986; Collazo et al., 1993), followed by a later migration of pigmented melanocytes occurring during the early tadpole stages. In contrast, the ventral fin of Xenopus is induced by mesoderm, not neural crest, and is populated primarily by mesoderm-derived cells (Tucker and Slack, 2004), although at least some neural crest cells are also present (Collazo et al., 1993). Recent studies of dorsal fin development in the axolotl indicate that, in addition to the well-characterized NC population, cells originally located in the somite also contribute to the fin mesenchyme (Sobkow et al., 2006).
Expression studies in Xenopus have shown that a Wnt11 related sequence, Wnt11-R, is expressed in the heart, the neural tube, the dorsal somite and mesenchymal cells within the dorsal fin (Garriock et al., 2005). Xenopus contains two Wnt11 genes and Wnt11-R is the orthologue of avian and mammalian Wnt11 (Garriock et al., 2005 and data not shown). Wnt11 proteins are members of the non-canonical family of Wnt-ligands which bind to cysteine-rich frizzled and ROR receptors and LRP5/6 coreceptors (Bhanot et al., 1996; Wang et al., 1996; Wehrli et al., 2000; Hikasa et al., 2002). After ligand binding, Wnt11 and related non-canonical Wnt-proteins signal through the Wnt/calcium pathway and the planar cell polarity (PCP) pathway, both of which involve the intracellular protein Dishevelled (Dsh) (Sokol, 1996; Sheldahl et al., 2003; reviewed in Fanto and McNeill, 2004; Kohn and Moon, 2005). Through the Wnt/calcium pathway, Wnt11 can elicit intracellular calcium fluctuations resulting in the activation of PKC, calmodulin and CaMKII (Sheldahl et al., 1999; Kuhl et al., 2000). The PCP pathway signals through RhoA and can result in the activation of JNK (Li et al., 1999; Yamanaka et al., 2002;Kim and Han, 2005). Non-canonical Wnt-signaling through the PCP and Wnt/calcium pathways modulates a variety of cell behaviors including convergent extension movements during gastrulation (Heisenberg et al., 2000; Tada and Smith, 2000; Choi and Han, 2002; Yamanaka et al., 2002, Wallingford et al., 2000; Wallingford and Harland, 2001), neural tube closure (Wallingford and Harland, 2002), dendritic outgrowth (Rosso et al., 2005), heart tube morphogenesis (Garriock et al., 2005) and cranial neural crest migration (De Calisto et al., 2005).
In mouse, chicken, frog and zebrafish embryos, Wnt11/Wnt11-R expression marks populations of cells that undergo morphogenetic movements and cell shape change (Ku and Melton, 1993; Kispert et al., 1996; Heisenberg et al., 2000; Olivera-Martinez et al., 2002; De Calisto et al., 2005; Garriock et al., 2005). In this report we show that Wnt11-R expression marks a subset of somite cells and of trunk NC cells that will migrate dorsally to occupy the core of the Xenopus dorsal fin. The function of Wnt11-R is initially required for an EMT event that precedes migration of cells into the fin and ultimately for maintenance of fin structure. These Wnt11-R activities are mediated through a calcium sensitive pathway involving CamKII.
MATERIALS AND METHODS
Embryology and microinjection
Xenopus laevis embryos were staged according to Nieuwkoop and Faber (1994) and cultured in 0.2×MMR. Microinjections occurred in 4% Ficoll in 0.4xMMR and embryos were maintained in this medium for the first 12 hours. Embryos were then cultured in 0.2×MMR until harvested. A morpholino oligo (MO) complementary to sequences in the 5′ UTR and shared by both pseudo-tetraploid copies of the Wnt11-R transcript has previously been shown to inhibit translation in vivo (Garriock et al., 2005). The sequence of the Wnt11-R MO1 is (5′-AATCATCTTCAAACCCAATAACAA-3′) and control mismatched MO is (5′-CTTGTACTTCTATAGCCTATAAGAA-3′). MOs were stored in 50 mM HEPES pH 8.0, diluted in water and heated to 65 °C for 10 minutes prior to injection. For neural crest targeting and somite targeting, 15-30 ng of MO was injected at the 8 and 16 cell stage targeted to the D1.2 and V1.2 blastomeres (Dale and Slack, 1987; Moody, 1987). KN-93, CaMKII inhibitor, was prepared as 10 mM stocks in DMSO and used immediately at 10 μM concentrations diluted in 0.2×MMR media (KN-93 Cat# S-2022, A.G. Scientific Inc). The media containing inhibitor was exchanged every three hours. Calcium signaling was activated with 100 nM thapsigargin (Sigma) or 10 nM A23187 ionophore (Sigma) for 1 hour.
Whole-mount in situ hybridization
Whole-mount in situ hybridization was carried out using a modification of the protocol by Harland, (1991), with antisense digoxigenin-labeled probes for Wnt11-R (Garriock et al., 2005). Plasmids were linearized with Not1 and transcribed with T7 RNA polymerase using the MEGAscript kit (Ambion). For serial sections, embryos were post-fixed in 4% paraformaldehyde, embedded in Paraplast and 10 μm transverse sections were prepared. DAPI was used to stain nuclei for cell counts.
Cell lineage studies and neural tube transplants
Cell lineage studies utilized 2.5 mg/ml DiI (1,1′-dioctadecyl - 3,3,3′,3′-tetramethylindocarbocyanine perchlorate, Molecular probes, Cat# D282). Lineage tracer prepared and stored using standard methods (Collazo et al., 1993) were microinjected in a volume of 2.6 pl into the somite or the neural tube at St 19 and location of labeled cells was then followed directly by fluorescence. Neural tubes from St 19–20 embryos (i.e. prior to NC migration) were excised from donor embryos injected with 750 pg of synthetic GFP mRNA alone or in combination with 15 ng of Wnt11-R morpholino or 15 ng control morpholino. Tissue was implanted into the region of the dorsal somite, beneath the epidermis, of a similarly staged uninjected host embryo in 0.2×MMR. Embryos were cultured until St 37 and the dorsal fins of those embryos were examined for the presence of GFP-expressing cells within the fin. For neural tube ablations, a segment of the neural tube was carefully excised from St 19 embryos, which were then allowed to develop until St 27 when they were assayed.
RESULTS
Wnt11-R marks neural crest cells, dorsal somite cells and fin mesenchyme
We have carried out in situ hybridization analysis of Wnt11-R expression in the trunk region of the embryo, encompassing the time that mesenchymal cells migrate and populate the dorsal fin (Fig. 1). At late neurula (St 22) Wnt11-R-expressing cells were observed along the length of the neural tube (Fig. 1A). In transverse sections (Fig. 1B), Wnt11-R expression was located at the dorsal region of the neural tube corresponding to the location of NC progenitor cells (Davidson and Keller, 1999; Linker et al., 2000). At St 24, Wnt11-R expression was observed in the neural tube and at the dorsal margin of the somite (Fig. 1C) and this latter domain of expression became more prominent by St 26 (Fig. 1D, G), by which time it extends along almost the entire length of the embryo (Fig. 1E,F). At St 26 the presumptive dorsal fin is only a small raised keel of epidermis and does not contain any mesenchymal cells. Transverse sections showed that Wnt11-R was expressed in a group of cells located at the dorsal and lateral margin of the somite bundle and, at lower levels, in the dorsal neural tube (Fig. 1G). Approximately 2 hours later (St 27), the fin has become larger as the epidermis protrudes dorsally creating an ECM-filled space above the neural tube (Tucker, 1986) and, for the first time, a few detached Wnt11-R-expressing cells became visible within the fin (Fig. 1H,I). Transverse sections (Fig. 1J) show Wnt11-R expression in dorsal regions of the somite and neural tube and at higher levels in a subset of cells that have separated from surrounding tissues. At these early stages we sometimes observe cells within the fin that are not expressing Wnt11-R, but these are not detected at later stages. We have not pursued the identity of these cells, though it is possible that they represent precursors of melanocytes. At St 34 (Fig. 1K-L), Wnt11-R expression extends significantly to more lateral regions of the somite and numerous isolated cells occupy positions within the fin (Fig. 1M).
Figure 1.
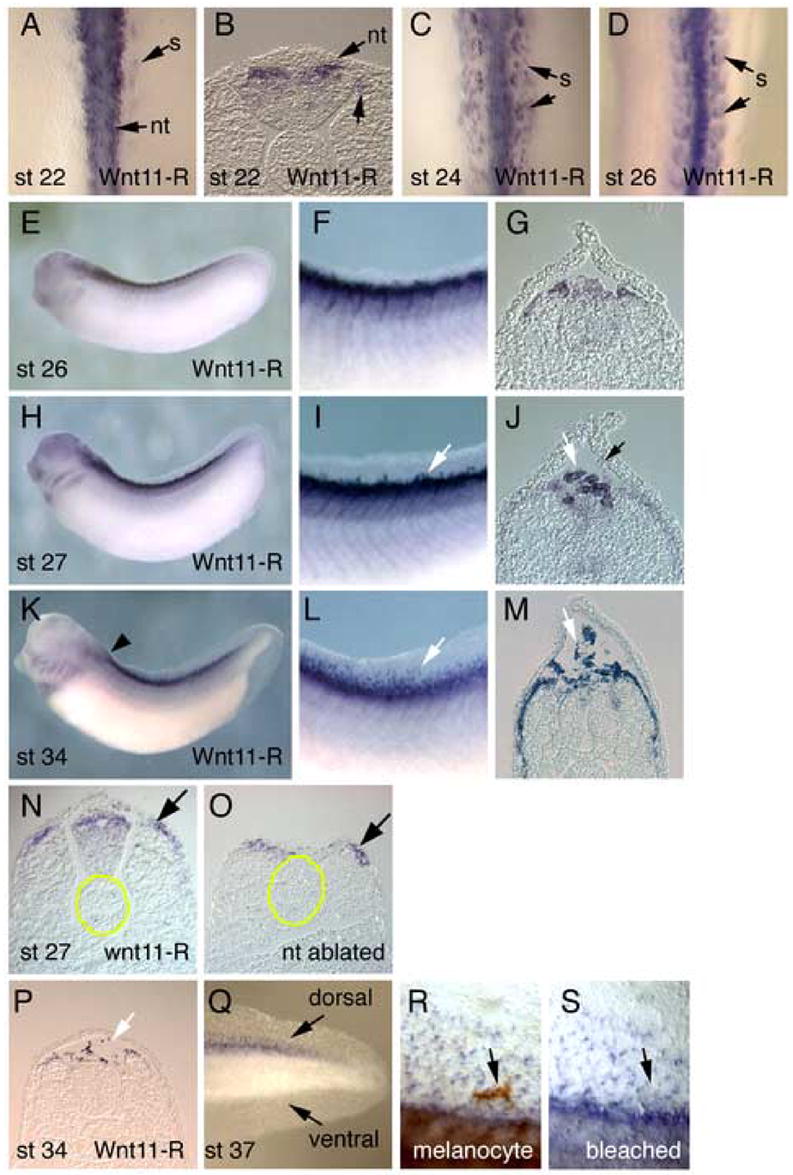
In situ hybridization analysis of Wnt11-R expression during Xenopus fin development. (A) Dorsal view of St 22 embryo showing Wnt11-R transcripts in the neural tube and in cells at the medial border of the somite. (B) Transverse section through the embryo in (A) showing Wnt11-R expression in the dorsal neural tube. (C) Dorsal view of St 24 embryo showing expression of Wnt11-R in the neural tube and in the somite. (D) Dorsal view of St 26 embryo with Wnt11-R expression in the neural tube and more extensively in the somite. (E) Lateral view of St 26 embryo showing expression of Wnt11-R in the pharyngeal arches and in dorsal tissues of the embryo. (F) Enlarged view of (E) showing expression of Wnt11-R in the dorsal somite. (G) Transverse section showing Wnt11-R transcripts in dorsal neural tube and the dorsal region of the somites. (H) Lateral view of St 27 embryo showing expression of Wnt11-R in dorsal tissues and in cranial NC migrating into the pharyngeal arch region. (I) Enlarged view of (H) showing expression of Wnt11-R in the somite and in individual cells at the base of the dorsal fin (arrow). (J) Transverse section through St 27 embryo showing expression of Wnt11-R in detached cells at the base of the dorsal fin (white arrow). We sometimes observe a small number of cells within the fin that at not expressing Wnt11-R (black arrow). (K) Lateral view of St 34 embryo showing expression of Wnt11-R in the heart, cranial neural crest cells and dorsal tissues. (L) Enlarged view of (K) showing numerous separate stained cells within the fin. (M) Transverse section through St 34 embryo showing Wnt11-R expressing cells dispersed within the dorsal fin core and along the dorsal and lateral surface of the somite. (N, O) Transverse sections through the trunk of a St 25 embryo from which a region of the neural tube has been ablated. For reference, the notochord is outlined in yellow. The section in (N) is located anterior to the region where the neural tube has been removed. Note Wnt11-R expression in the dorsal neural tube and the dorsomedial region of the somite. The section in (O) shows the region where the neural tube is missing. Wnt11-R expression in the dorsal somite (arrow) is equivalent to that in the control section. (P) Transverse section through St 34 embryo at the level indicated by the arrowhead in (K) showing stained cells dorsal to the neural tube. (Q) Lateral view of the tail of St 37 embryo showing Wnt11-R expressing cells within the dorsal fin but not the ventral fin. (R) Magnified view of the dorsal fin of an unbleached St 37 embryo showing Wnt11-R expressing cells, plus a large pigmented melanocyte (arrow). (S) Identical region of the fin shown in (R) after bleaching. Note that the melanocyte does not express detectable levels of Wnt11-R. Abbreviations: nt, neural tube; s, somite.
In the chick and mouse embryo, Wnt11 is expressed in the dorsomedial region of the somite, but not in neural crest cells (Kispert et al., 1996; Olivera-Martinez et al., 2002). In the Xenopus embryo, lineage-tracing studies have demonstrated that a minor population of trunk NC cells migrates from the neural tube and laterally across the dorsal surface of the somites (Collazo et al., 1993). This migration route raises the possibility that the domain of Wnt11-R expression in the Xenopus somite (Fig. 1D, G) represents NC cells in the process of lateral migration. To specifically address this question we have carried out ablation experiments to excise premigratory trunk NC. At St 19, well before any migration of trunk NC, the dorsal region of the neural tube was dissected from both sides of the embryo. The manipulated embryos were then allowed to develop until St 25/26, when they were assayed for Wnt11-R expression. As shown in Fig. 1O, even though these embryos completely lacked detectable neural tube in the region of ablation, the domain of Wnt11-R expression was still present in the dorsal somite at levels comparable to those observed in control regions of the embryo (Fig. 1N). We conclude that the Wnt11-R expressing cells in the dorsal somite are not of NC origin and that this domain of expression probably corresponds to the equivalent somitic region in the chick embryo (Olivera-Martinez et al., 2002).
We also detected Wnt11-R expressing cells at anterior locations of the embryo where no dorsal fin is present (Fig. 1P) and once again, the Wnt11-R expressing cells occupied a position dorsal to the neural tube. Furthermore, Wnt11-R marked fin core cells within the dorsal fin but not the ventral fin (Fig. 1Q), which contains cells primarily of mesodermal origin (Tucker and Slack, 2004). Finally, Wnt11-R expression only marked the mesenchymal population of fin cells and not the much larger pigmented melanocyte NC cells (Fig. 1R,S).
Cell lineage studies of dorsal migrating neural crest
Numerous embryological studies have demonstrated that NC cells can migrate from the dorsal neural tube, into the matrix of the fin (Raven, 1931; DuShane, 1935; Krotoski et al., 1988; Collazo et al., 1993; Tucker and Slack, 2004). We examined serial sections to determine the location of Wnt11-R expressing cells when dorsal migration of fin mesenchyme first occurs (St 27) and observed cells detaching from the somite rather than the neural tube (Fig. 2A–C). This observation suggested that at least some fin mesenchymal cells were migrating from the dorsal somite rather than the neural tube. This would be consistent with observations of fin development in the axolotl embryo, where a significant proportion of fin mesenchyme cells originate from the somites (Sobkow et al., 2006). To specifically address this issue for the Xenopus embryo, we carried out lineage tracing experiments. At St 19, prior to any migration of trunk NC cells (Collazo et al., 1993), single somite bundles were labeled by injection of DiI into the dorsal surface (Fig. 2D). Embryos were allowed to develop for about 36 hours (until St 38) when the location of labeled cells was recorded. In 86% of embryos examined (n=22), DiI labeled cells were observed to migrate into the fin (Fig. 2E,F). We could demonstrate that the DiI injections were accurate because sectioning showed no DiI labeling within the neural tube (Fig. 3G,H). Based on these experiments, we conclude that cells of the dorsal somite contribute to the fin core cell population. When the neural tube was labeled at St 19 (Fig. 2I), subsequent tracing of fluorescent cells also showed contribution to the fin mesenchyme (Fig. 2J,K – 63% of embryos examined, n=11) and sectioning confirmed that the labeling was accurately directed to the neural tube (Fig. 2L,M). This experiment confirms previous results that dorsal migrating neural crest cells contribute to the fin mesenchyme (Raven, 1931; DuShane, 1935; Krotoski et al., 1988; Collazo et al., 1993; Tucker and Slack, 2004). Due to the limitations of lineage tracing methods however, these studies do not permit an estimate of the relative contributions of the somite and the neural crest to the final population fin core cells.
Figure 2.
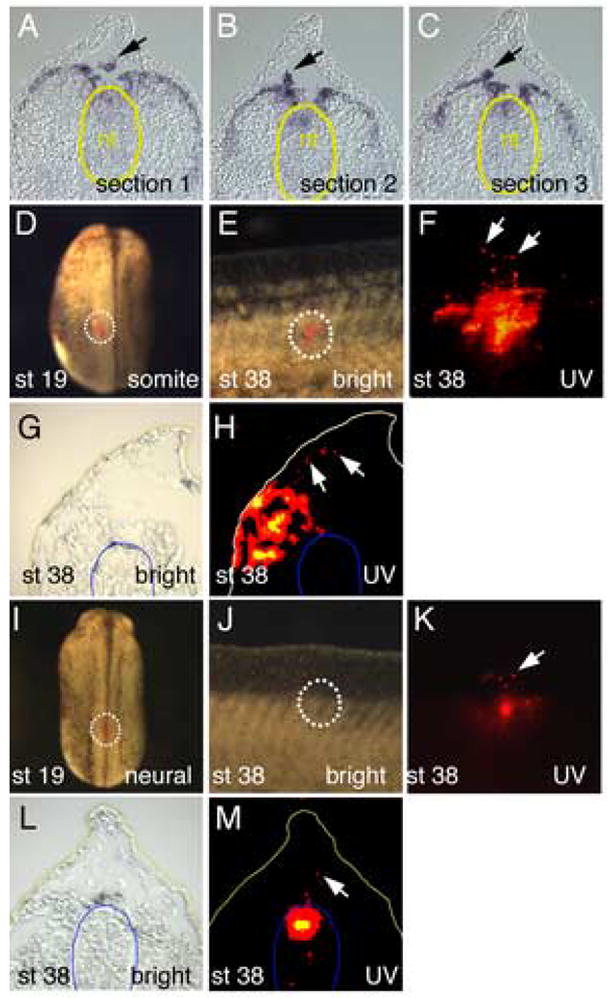
Lineage tracing of cells migrating into the dorsal fin matrix. (A–C) Serial sections through the trunk region of St 27 embryo stained for Wnt11-R transcripts. Cells moving into the fin (arrows) appear to be detaching from the dorsal somites rather than the neural tube (yellow outline) (D) Embryo at St 19 showing location of DiI label on the dorsal surface of the somite (outline). (E) Merged UV and white light image of the dorsal fin of a DiI labeled embryo at St 38, showing the presence of labeled cells within the fin matrix. (F) UV image of same region in (E) showing presence of DiI labeled cells inside the fin (white arrows). (G, H) Bright field and UV image respectively of frozen section through a DiI labeled embryo at St 38. Cells originating in the labeled somite have migrated into the matrix of the dorsal fin (arrows). The position of the neural tube is outlined in blue for reference. (I). Embryo at St 19 showing location of DiI label on the dorsal surface of the forming neural tube (outline). (J). Merged UV and white light image of the dorsal fin of a DiI labeled embryo at St 38, showing the presence of labeled cells within the fin matrix. (K). UV image of same region in (E) showing presence of DiI labeled cells inside the fin (white arrow). Labeling of the neural tube is faint because it is viewed through the body of the embryo. (L, M). Bright field and UV image respectively of frozen section through a DiI labeled embryo at St 38. Cells originating in the labeled neural tube have migrated into the matrix of the dorsal fin (arrow). The position of the neural tube is outlined in blue.
Figure 3.
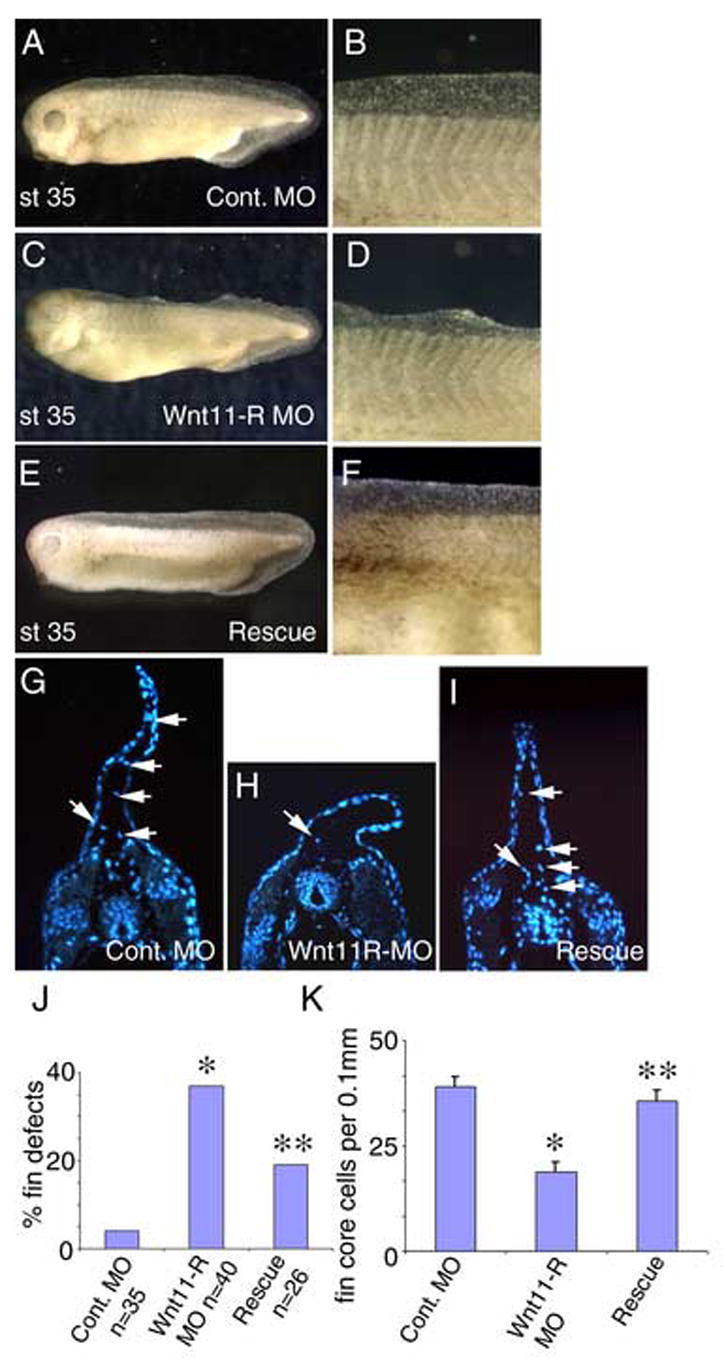
Inhibition of Wnt11-R expression disrupts normal fin development. (A) St 35 embryo injected with 15 ng of control MO showing normal fin morphology. (B) Magnified view of the dorsal fin of the embryo pictured in (A). (C) St 35 embryo injected with Wnt11-R MO showing collapsed appearance of the dorsal fin. (D) Magnified view of the dorsal fin of the embryo pictured in (C). (E) Rescue experiment showing normal dorsal fin development where the embryo was injected with 15 ng of Wnt11-R MO plus 100 pg of Wnt11-R mRNA. (F) Magnified view of the dorsal fin of the embryo pictured in (E). (G–I) UV images of transverse sections through the trunk region of control and MO-treated embryos. Cells within the fin matrix (arrows) can be identified using DAPI nuclear stain. (G) Control. (H) Wnt11-R MO treated embryo showing very few mesenchymal cells within the fin (arrow). Note also the altered morphology of the dorsal fin epidermis. (I) Rescued embryo. The number of cells within the fin is comparable to the control embryo and the dorsal fin morphology is normal. (J) Frequency of embryos showing fin morphological defects in Wnt11-R MO experiments. Statistical difference from control is indicated (*). Coinjection of 100 pg of Wnt11-R mRNA resulted in a reduction in frequency of observed fin defects relative to Wnt11-R MO alone. The rescue was statistically significant (**) using the Chi squared test, p < 0.02. (K) Quantitation of fin core cells per 0.1 mm of dorsal fin in Wnt11-R MO experiments. The decrease in fin core cells in Wnt11-R MO treated embryos was significant, p = 0.00001, by T-Test (Two-Sample Assuming Unequal Variances). The restoration of the number of fin core cells in rescue experiments was also significant (p=0.00009).
Wnt11-R function is required for normal fin development
Wnt11-R is expressed in both the neural crest and the dorsal somite cell populations that contribute to the fin mesenchyme. In the subsequent studies, we will treat the dorsal migrating Wnt11-R expressing cells as a single population, even though we acknowledge the distinct germ layer origins of the migrating cells. To determine whether Wnt11-R function is required for fin core cell migration and fin morphogenesis we used a morpholino oligomer (MO) that has previously been shown to inhibit translation of Wnt11-R (Garriock et al., 2005). Targeting of the MO to dorsal trunk tissues produced embryos that were phenotypically normal at early stages, but which showed defects in fin morphology that were first visible at about St 33 and became pronounced by St 35. When assayed at St 34/35 the most common defects were collapsed or deficient fin tissue, especially in more anterior regions of the embryo (Fig. 3C, D). Severe disruption of fin morphology was observed in 37% of Wnt11-R MO treated embryos but only rarely (4%) in control MO injected embryos (Fig. 3J). Co-injection with 100 pg of Wnt11-R mRNA that does not contain the MO target sequences results in partial rescue of fin morphological defects (defects reduced to 19%) (Fig. 3E, F, I, J). This reduction is statistically significant, p < 0.02. Examination of transverse sections through Wnt11-R MO-treated embryos showed a greatly reduced number of mesenchymal cells within the fin matrix, with the remaining cells located proximal to the neural tube and somites (Fig. 3H). This contrasts with control fins, and mRNA rescued fins, where mesenchymal cells were distributed throughout the extent of the fin (Fig. 3G, I). Quantitation showed that inhibition of Wnt11-R expression reduced the number of isolated mesenchyme cells in the fin matrix to 48% of control levels (Fig. 3K). The reduction in fin core cell number in MO-treated embryos was restored to near wild type numbers by the co-injection of Wnt11-R mRNA (Fig. 3K). As can be seen in Fig. 3H, the overall fin structure of Wnt11-R knockdown embryos also appeared shorter than controls (Fig. 3G). Counting of cells in the epidermal layer of the fin in knockdown embryos showed a significant reduction relative to controls (29.8 +/− 1.3 for MO-treated compared to 44.3 +/− 1.2 for controls). Since Wnt11-R is not expressed in fin epidermis, this observation suggests the possibility of interactions between fin core cells and the nearby epidermal tissue.
There are two likely explanations for the absence of fin mesenchymal cells following MO treatment. First, that cells failed to migrate into the fin or second, that cells migrated normally but failed to survive in the absence of Wnt11-R activity. To distinguish between these options, we examined MO-treated embryos at St 32, soon after initial migration of cells into the fin matrix. Examination of MO treated embryos (Fig. 4D–F), revealed a severe inhibition of separation of Wnt11-R expressing cells from the somite and from the dorsal neural tube, compared to control MO-treated embryos (Fig. 4A–C). Although not ideal, it was necessary to use Wnt11-R as an in situ marker for these experiments because we are not aware of any other sequence that marks the migratory population and that maintains expression for the duration of the delamination and migration process. As presented in graphical form in Fig. 4G, approximately 62% of MO-treated embryos showed reduced migration of cells into the fins. Since both the neural tube and the somites are epithelialized structures after early neurula stages (Keller, 2000), we propose that inhibition of Wnt11-R function is blocking the EMT event that allows separation of premigratory cells from the somite and dorsal neural tube.
Figure 4.
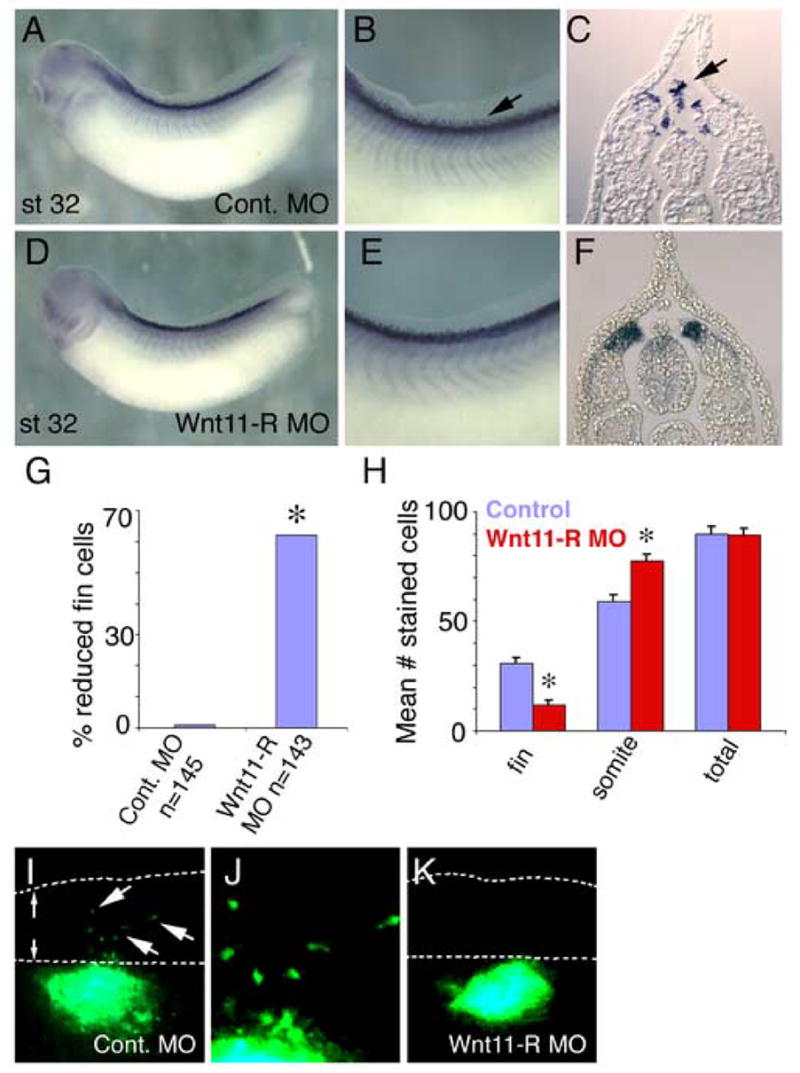
Inhibition of Wnt11-R expression or non-canonical Wnt signaling results in fin cell migration defects. (A–F) Migratory and premigratory cells were detected by in situ hybridization using Wnt11-R probe. (A) Lateral view of St 32 embryo injected with control MO showing expression of Wnt11-R. (B) Magnified view of the dorsal region of the embryo pictured in (A) showing numerous mesenchymal cells within the dorsal fin (arrow). (C) Transverse section showing Wnt11-R expressing mesenchymal cells detached from the neural tube or somite and entering the dorsal fin. (D) Lateral view of Wnt11-R MO-treated embryo (bilateral injection) showing normal overall morphology and expression of Wnt11-R. (E) Magnified view of the dorsal region of the embryo pictured in (D) showing an absence of separated mesenchymal cells within the fin. (F) Transverse section showing Wnt11-R expressing cells in contact with the neural tube and dorsal somite. (G) Chart showing the percent of embryos with a reduced number of Wnt11-R expressing cells within the fin in MO knockdown experiments. Statistically significant differences from controls are indicated (*). (H) Chart showing quantitation of Wnt11-R expressing cells in 0.1 mm of trunk fin of control and Wnt11-R MO-treated embryos. The number of DAPI stained/Wnt11-R expressing cells within the matrix or attached to the neural tube or the somite is presented, together with total cell number. The decrease in fin core cell number was significant (*) for Wnt11-R MO treatment, (T-Test Two-Sample Assuming Unequal Variances). (I–K) UV images of GFP expressing neural tube implants. (I) GFP labeled cells from control neural tube have migrated from the position of the implant into the dorsal fin. (J) Magnified view of (I) showing isolated mesenchymal cells within the fin. (K) Wnt11-R MO-treated neural tube implant showing absence of detectable mesenchymal cells in the dorsal fin.
To determine whether cell death might contribute to the reduction in number of fin core cells, we counted the number of Wnt11-R expressing cells located within the fin or remaining adjacent to the somite (Fig. 4H). While MO treatment decreased the number of cells within the fin matrix, we observed a corresponding increase in the number of Wnt11-R expressing cells adjacent to, or in, the somite. The total number of Wnt11-R-expressing cells was unchanged in MO knockdown embryos relative to controls (Fig. 4H) and therefore, cell death was not a primary cause of the reduction in fin core cells. We conclude that inhibition of Wnt11-R activity did not lead to reduction in cell number, but instead inhibited cell separation and subsequent cell migration into the fin.
To directly test whether Wnt11-R function is required within the migrating cell population we carried out transplant experiments using tissue isolated from control and Wnt11-R MO-treated embryos. In these studies, neural tube tissue from donor embryos was implanted into the equivalent location of recipient wild type embryos. Implanted tissue was distinguished from host cells by the presence of GFP tracer. In control experiments, implants of un-manipulated tissue showed migration of labeled cells in 91% of embryos (n=11) (Fig. 4I, J). On the other hand, inhibition of Wnt11-R function in transplanted neural tubes reduced observed migration to 50% of embryos (n=10) (Fig. 4K). These results suggest that Wnt11-R activity is required within the migrating cells, rather than in adjacent tissues.
Calcium signaling via CaMKII is required for the EMT prior to migration of fin core cells into the fin
Previous studies have shown that Wnt11 is capable of signaling through the Wnt/calcium pathway, which results in the activation of CaMKII (Kuhl et al., 2000). We utilized a specific inhibitor for CaMKII (KN-93) to determine if calcium signaling via CaMKII was required for migration of cells into the fin. Previous studies have demonstrated that KN-93 effectively inhibits CaMKII activation in Xenopus embryos (Wu and Cline, 1998). Embryos were cultured with KN-93 from St 26 to St 34/35 during which time migration of cells into the fin is taking place (Fig. 1). As previously observed for Wnt11-R knockdown embryos (Fig. 3C, D, Fig. 5C), KN-93 treated embryos exhibited dorsal fin defects (62%, n=60) (Fig. 5D, E). Examination of transverse sections from KN-93 treated embryos showed a reduced number of mesenchymal cells within the fin matrix (Fig. 5H), again similar to the situation in Wnt11-R MO-treated embryos (Fig. 5G). Counts of cells in serial sections (Fig. 5I) showed that inhibition of CaMKII-signaling reduced the number of mesenchyme cells within the fin to 36% of controls, slightly more efficient than the reduction observed with Wnt11-R MO treatment (48%).
Figure 5.
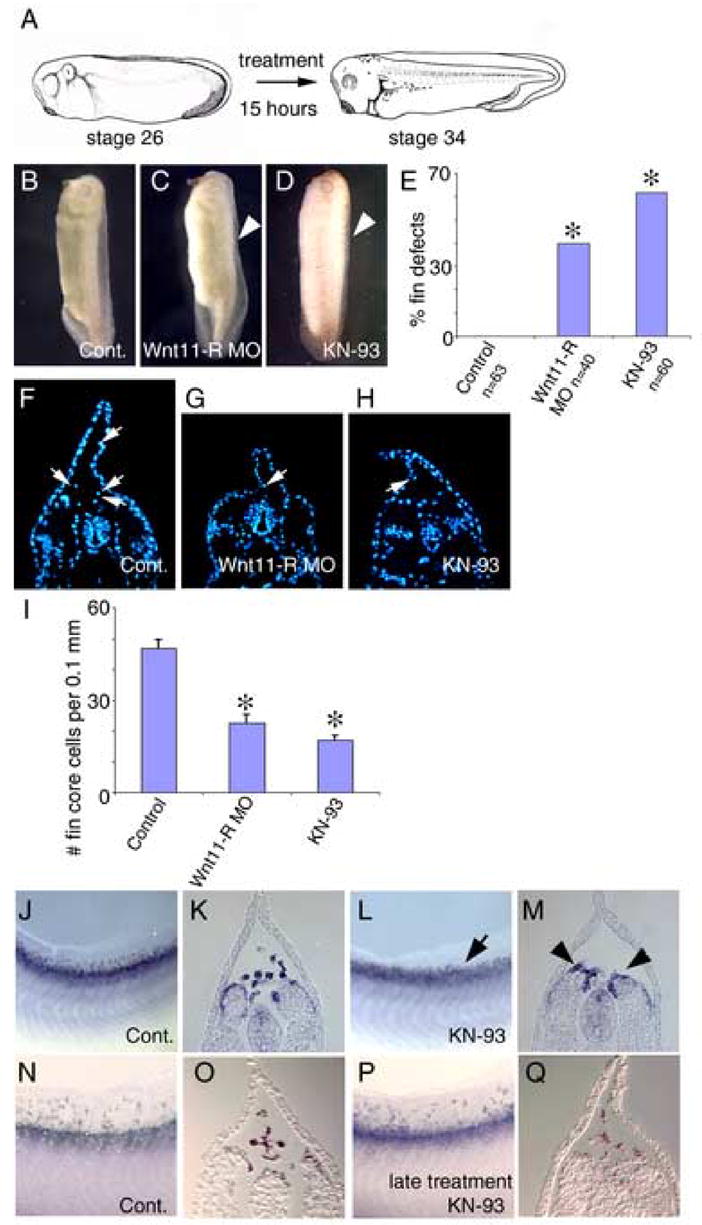
Inhibition of CaMKII activity results in defective migration of cells into the fin. (A) Diagram illustrating the administration of the inhibitor. Embryos were treated with inhibitor from St 26, prior to migration of cells into the fin, until St 34. (B) Control embryo incubated in DMSO carrier medium showing normal dorsal fin development. (C) Wnt11-R MO injected embryo showing disrupted dorsal fin development with a collapsed dorsal fin (arrowhead). (D) CaMKII inhibitor treated (KN-93) embryo showing disrupted dorsal fin development (arrowhead). Note that that dorsal fin is collapsed along the entire length of the embryo. (E) Chart showing the frequency of embryos with fin morphological defects in KN-93 experiments compared to Wnt11-R MO treatments. Statistically significant differences from controls are indicated (*). (F–H) UV images of a transverse sections through the trunk region of control, Wnt11-R MO treated and KN-93 treated embryos. (F) Control embryo showing DAPI-stained fin core cells (arrows). (G) Wnt11-R knockdown embryo showing very few mesenchymal cells within the fin (arrow). (H) KN-93 treated embryo showing very few mesenchymal cells within the fin. Note also the altered morphology of the dorsal fin epidermis compared to control. (I) Quantitation of dorsal fin core cells per 0.1 mm of dorsal fin in Wnt11-R MO and KN-93 experiments. Statistically significant differences from control are indicated (*). (T-Test -Two-Sample Assuming Unequal Variances). (J–Q) Fin mesenchyme cells were detected by in situ hybridization using Wnt11-R probe. (J) Lateral view of the fin region of St 34 control embryo showing numerous mesenchymal cells within the dorsal fin. (K) Transverse section of the embryo in (J) showing Wnt11-R expressing mesenchymal cells detached from the neural tube or somite and entering the dorsal fin. (L) Lateral view of the fin region of KN-93 treated embryo at St 34 showing very few mesenchymal cells within the dorsal fin (arrow). (M) Transverse section of the embryo in (L) showing and absence of fin core cells and Wnt11-R expressing cells attached to the somite (arrowheads). (N) Lateral view of the fin region of St 36 control embryo showing numerous mesenchymal cells within the dorsal fin. (O) Transverse section of the embryo in (N) showing mesenchymal cells within the fin matrix. (P) Lateral view and (Q) transverse section of embryo treated with KN-93 from St 28–36 and assayed at St 36 showing numerous Wnt11-R expressing mesenchymal cells distributed throughout the dorsal fin.
We used in situ hybridization to examine the distribution of presumptive fin core cells between the fin matrix and adjacent neural tube and somite tissues. As in previous experiments, Wnt11-R was used as a marker for fin mesenchyme and KN-93 treatments produced no detectable effect on expression levels of Wnt11-R transcript (compare Fig. 5J to Fig. 5L). Sectioning showed that inhibition of CaMKII prevented Wnt11-R expressing cells from undergoing EMT from the somite, resulting in an accumulation of cells in the somite and neural tube (compare Fig. 5K and M). This appears to be very similar to the effect observed in Wnt11-R MO treated embryos (Fig. 4F). To determine whether CaMKII activity is required for subsequent dorsal migration of cells into the fin matrix we treated embryos with CaMKII inhibitor commencing at St 28, after cells have delaminated from the dorsal somite (Fig. 1H–J). Late stage inhibition of CaMKII produced no detectable effect on migration and mesenchymal cells were distributed throughout the fin (Fig. 5P, Q), indistinguishable from carrier treated controls (Fig. 5N, O). This series of experiments implies that CaMKII activity is required for EMT of Wnt11-R cells from the somite, but not for subsequent migration within the fin matrix.
If activation of CaMKII is one of the primary steps in the pathway downstream of Wnt11-R during regulation of EMT and cell migration, then increasing calcium signaling may be able to rescue the fin defects observed in Wnt11-R knockdown embryos. To directly test this possibility, we cultured Wnt11-R MO treated embryos in thapsigargin or A23187 to increase intracellular calcium signaling. Thapsigargin and A23187 are well characterized calcium activators, previously shown to be effective in Xenopus oocytes and embryos (Osborn et al., 1997; Lupu-Meiri et al., 1993; Matifat et al., 1997). Brief treatment with either thapsigargin or A23187 at St 26–27 decreased the incidence of dorsal fin defects in Wnt11-R MO treated embryos (Fig. 6A–D). Thapsigargin rescued fin defects by 57% while A23187 rescued fin defects by 19% (Fig. 6E). The modest rescue by A23187 was probably due to toxicity because A23187 alone caused fin malformations in 25% of treated embryos (n=24 – data not shown). Examination of transverse sections through Wnt11-R MO knockdown embryos treated with thapsigargin or A23187 MO showed a rescue in the number of mesenchymal cells within the fin when compared to Wnt11-R MO alone (Fig. 6F–I). Quantitation of cells in serial sections showed that the reduction in fin core cell number in Wnt11-R knockdown embryos was completely rescued by thapsigargin or A23187 (Fig. 6J). Although treatment with thapsigargin alone resulted in a 20% increase in fin core cells, this number was not statistically different from controls (p = 0.08).
Figure 6.
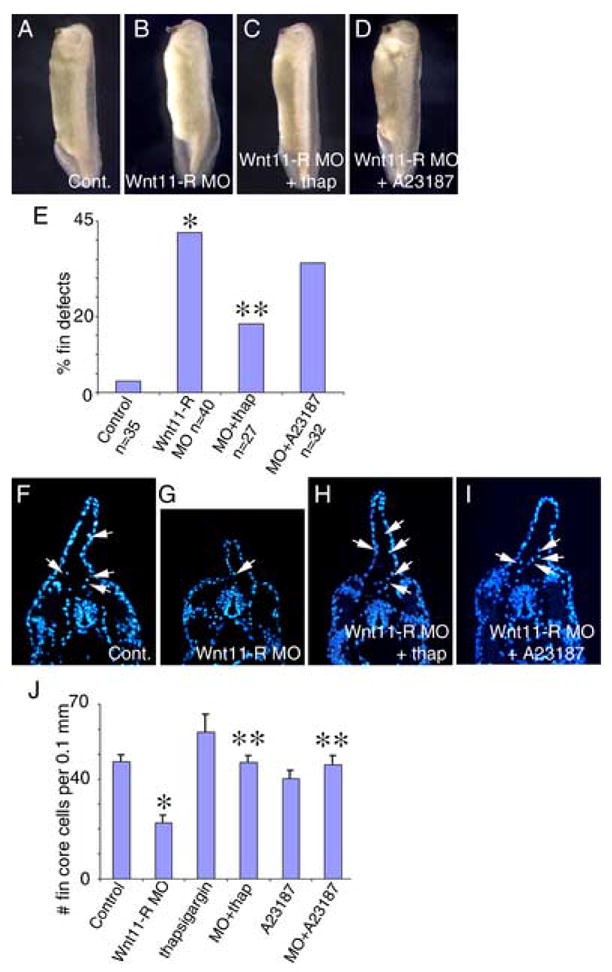
Activation of calcium signaling rescues dorsal fin development in Wnt11-R knockdown embryos. Calcium signaling was activated using thapsigargin or A23187 for 1 hour at St 26–27 in control MO or Wnt11-R MO-treated embryos. Embryos were subsequently cultured for 20 hours in fresh medium until St 35. (A) Control MO treated embryo showing normal fin development. (B) Wnt11-R MO treated embryo showing defective dorsal fin development. (C) Wnt11-R MO embryo treated with thapsigargin showing normal fin development. (D) Wnt11-R MO embryo treated with A23187 showing normal fin development. (E) Quantitation of dorsal fin morphological defects in Wnt11-R MO embryos (*) and rescue of Wnt11-R MO embryos treated with thapsigargin (**) and A23187. (F–I) UV images of transverse sections through the trunk region of DAPI stained embryos. (F) Control embryo showing numerous DAPI-stained cells within the fin matrix (arrows). (G) Wnt11-R knockdown embryo showing few DAPI-stained cells within the fin matrix. (H) Thapsigargin treated Wnt11-R knockdown embryo showing numerous stained cells within the dorsal fin core. (I) A23187 treated Wnt11-R knockdown embryo showing numerous stained cells within the fin core. (J) Quantitation of fin core cells in 0.1 mm trunk region of experimental embryos. Note the statistically significant decrease in fin cell number in Wnt11-R MO treated embryos (*) and the rescue of fin cell number in Wnt11-R MO embryos treated with thapsigargin or A23187 (**).
DISCUSSION
The fin mesenchymal cells in Xenopus
Wnt11-R is a marker for a unique population of cells that populate the dorsal fin matrix. The Wnt11-R expressing cells originate in two distinct locations, the dorsal region of the neural tube that will contribute trunk neural crest and the dorsomedial region of the somites. The Wnt11-R expressing cells populating the fin matrix are quite distinct from, and much more numerous than, the better characterized melanocyte NC population (Fig. 1R, S). Loss of function studies show that Wnt11-R activity is required for normal formation and maintenance of fin structure in the embryo. MO inhibition of Wnt11-R expression results in a severe block of dorsal migration of presumptive fin core cells into the fin and, presumably as a result of this failure, the fin fails to undergo correct morphogenesis. First, the fin lacks rigidity and is often seen to be collapsed to one side of the embryonic midline (Fig. 3C–D). Second, the epidermal layer of the fin in Wnt11-R deficient embryos contains less total cells than control embryos (Fig. 3G,H). It is possible that both morphological defects are due to lack of adequate matrix deposition within the fin. If the Wnt11-R expressing population is a source of matrix components, then failure to distribute throughout the fin, may lead to a lack of space filling structures, especially in more distal regions.
Signaling pathways downstream of Wnt11-R
Non-canonical Wnt-signaling is mediated through the PCP, Wnt/calcium and Wnt/JNK pathways (reviewed in Pandur et al, 2002; Kohn and Moon, 2005). PCP signaling involves several proteins, including Flamingo, Strabismus and Prickle that are required for convergent extension movements during gastrulation (reviewed in Ueno and Greene, 2003; Fanto and McNeill, 2004; Takeuchi et al., 2003). However, none of these factors appear to be expressed in the NC cells or dorsal somite cells that express Wnt11-R (Darken et al., 2002;Wallingford et al., 2002; Morgan et al., 2003). This strongly suggests that PCP signaling, as it occurs during gastrulation, is not required for dorsal migration of fin core cells. Using small molecule inhibitors, we found that blocking calcium signaling through CaMKII prevented EMT of cells from the dorsal somite (Fig. 5H). The effects of CaMKII inhibition were apparently identical to those obtained by blocking Wnt11-R signaling by MO knockdown. Considering that CaMKII is a target of Wnt11-signaling (Kuhl et al, 2000), we conclude that CaMKII activity is required downstream of Wnt11-R and is essential for the delamination event when cells leave the neural tube and dorsal somite. In contrast, CaMKII activity is not required for subsequent migration of isolated mesenchymal cells into the fin (Fig. 5P, Q). In support of a calcium dependent pathway, stimulation of calcium signaling using thapsigargin or A23187 releases cells from the somite when Wnt11-R function is blocked (Fig. 6). It is important to note that, although these studies are consistent with a mechanism in which EMT of Wnt11-R expressing cells is regulated by the Wnt/calcium pathway, our experiments cannot distinguish whether the calcium signaling event is a direct or an indirect response to Wnt11-signaling.
Taken together, these studies suggest that Wnt11-R regulates delamination of cells from the neural tube and the dorsal somite through a calcium dependent pathway. This would represent a novel role for non-canonical Wnt signaling, involving CaMKII, in the separation of cells during EMT. Similar Wnt-mediated, calcium dependent EMT events might be involved in regulation of other developmental processes. For example, cells of the endothelial layer of the atrio-ventricular (AV) canal undergo a similar calcium-sensitive EMT to populate the matrix layer of the cardiac cushion (Runyan et al., 1990). While there is currently no evidence that non-canonical Wnt activity is required for EMT of the AV canal, several Wnt ligands capable of activating non-canonical Wnt-signaling are expressed in the relevant heart tissues at the appropriate time (Zakin et al., 1998; Yamaguchi et al., 1999; Le Floch et al., 2005).
Is Wnt11-R function cell autonomous?
Studies exploring the role of Wnt11 and related genes during gastrulation and heart morphogenesis have suggested that the primary effects of Wnt signaling may be cell- autonomous (Heisenberg et al., 2000; Tada and Smith, 2000; Garriock et al., 2005). In these examples, the cells expressing Wnt11 are also the cells that exhibit altered behavior when Wnt function is reduced. Similarly, our transplant studies showed that neural crest cells in which Wnt11-R function had been inhibited, failed to undergo migration into the fin (Fig. 4K) even though the transplanted tissue was immediately adjacent to normal somite tissue expressing Wnt11-R. On the other hand, studies of migrating cranial neural crest cells implied that signaling by Wnt11 is required cell non-autonomously (De Calisto et al., 2005). This is based on the observation that Wnt11 is initially expressed only at the leading edge of the migrating NC cells with the trailing cells expressing the Wnt receptor, Frizzled-7. The suggestion is that cranial NC cells may be migrating along a chemotactic gradient towards the source of Wnt11 (De Calisto et al., 2005). Our results appear to indicate that Wnt11-R function is required cell-autonomously during dorsal migration into the fin because the cells actively undergoing migration are the only cells in the vicinity that are expressing Wnt11-R (Fig. 1 and 4). These somewhat differing viewpoints can be reconciled of if we propose that Wnt11/Wnt11-R signaling is required to alter the properties of the local environment around the cell. This is consistent with the established role of non-canonical Wnt signaling to cell-autonomously facilitate cell movements through regulation of polarity and adhesion (Tada and Smith, 2000; Wallingford et al., 2000). It is also consistent with models in which non-canonical Wnt signaling regulates the polarity of fibronectin fibril deposition (Goto et al., 2005). Overall, failure to modulate the environment surrounding the cells, including the structure of the fibronectin network, may explain the inhibition of EMT and migration following inhibition of Wnt11-R expression. It seems likely that additional, currently uncharacterized factors, direct migration of fin core cells towards distal regions of the fin, although it is possible that the distribution of cells within the matrix is stochastic.
Evolutionary conservation of a dorsal migrating cell populations
Recent studies of the axolotl have shown that fin core cells are derived from both neural crest and from the somites (Sobkow et al., 2006). Our studies indicate that the same cell populations contribute to the mesenchyme of the dorsal fin in Xenopus and that these cells are marked by expression of Wnt11-R. Although birds do not possess a structure equivalent to a dorsal fin it is intriguing that a dorsal migrating cell population, originating in the dorsomedial somites (but with no neural crest component) also expresses Wnt11, the chick orthologue of frog Wnt11-R (Olivera-Martinez et al., 2002). During avian embryogenesis, cells from the medial dermatome undergo an EMT and then migrate dorsally from the somites to populate the dorsal feather field and it has been proposed that this process might be regulated by Wnt11 (Olivera-Martinez et al., 2004). Our studies provide strong experimental evidence that Wnt11 signaling is indeed mechanistically involved in delamination of somite cells prior to migration. The mouse embryo also expresses Wnt11 in the dorsomedial somite (Kispert et al., 1996) but it has not been determined whether this expression serves a developmental function. The fact that neural crest cells contribute to the mesenchyme of the Amphibian dorsal fin has been known for some time (Twitty and Bodenstein, 1941; Tucker and Erickson, 1986; Krotoski et al., 1986; Collazo et al., 1993; Tucker and Slack, 2004). Furthermore, it has been observed that neural crest cells of the lamprey and neural crest-like cells of the ascidian, a urochordate, also migrate dorsally into the fin structures of the embryo (Newth, 1956; McCauley and Bronner-Fraser, 2003; Jeffery et al, 2004). It seems likely therefore, that the NC component of the fin core cells in Xenopus has preserved an extremely ancient function of the NC lineage, and it will be interesting to determine whether dorsal migration of lamprey and ascidian NC cells is also regulated by Wnt11.
Acknowledgments
Special thanks to Florence Broders and Roberto Mayor for sharing results prior to publication. P.A.K. is the Allan C. Hudson and Helen Lovaas Endowed Professor of the Sarver Heart Center at the University of Arizona College of Medicine and is supported by the Sarver Heart Center and by the NHLBI of the NIH, grants #HL63926 and HL74184.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bhanot P, Brink M, Samos CH, Hsieh JC, Wang Y, Macke JP, Andrew D, Nathans J, Nusse R. A new member of the frizzled family from Drosophila functions as a Wingless receptor. Nature. 1996;382:225–230. doi: 10.1038/382225a0. [DOI] [PubMed] [Google Scholar]
- Bodenstein D. Studies on the development of the dorsal fin in amphibians. J Exp Zool. 1952;120:213–243. [Google Scholar]
- Choi SC, Han JK. Xenopus Cdc42 regulates convergent extension movements during gastrulation through Wnt/Ca2+ signaling pathway. Dev Biol. 2002;244:342–357. doi: 10.1006/dbio.2002.0602. [DOI] [PubMed] [Google Scholar]
- Collazo A, Bronner-Fraser M, Fraser SE. Vital dye labelling of Xenopus laevis trunk neural crest reveals multipotency and novel pathways of migration. Development. 1993;118:363–376. doi: 10.1242/dev.118.2.363. [DOI] [PubMed] [Google Scholar]
- Darken RS, Scola AM, Rakeman AS, Das G, Mlodzik M, Wilson PA. The planar polarity gene strabismus regulates convergent extension movements in Xenopus. EMBO J. 2002;21:976–985. doi: 10.1093/emboj/21.5.976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale L, Slack JM. Fate map for the 32-cell stage of Xenopus laevis. Development. 1987;99:527–551. doi: 10.1242/dev.99.4.527. [DOI] [PubMed] [Google Scholar]
- Davidson LA, Keller RE. Neural tube closure in Xenopus laevis involves medial migration, directed protrusive activity, cell intercalation and convergent extension. Development. 1999;126:4547–4556. doi: 10.1242/dev.126.20.4547. [DOI] [PubMed] [Google Scholar]
- De Calisto J, Araya C, Marchant L, Riaz CF, Mayor R. Essential role of non-canonical Wnt signalling in neural crest migration. Development. 2005;132:2587–2597. doi: 10.1242/dev.01857. [DOI] [PubMed] [Google Scholar]
- DuShane GP. An experimental study of the origin of pigment cells in Amphibia. J Exp Zool. 1935;72:1–31. [Google Scholar]
- Fanto M, McNeill H. Planar polarity from flies to vertebrates. J Cell Sci. 2004;117:527–533. doi: 10.1242/jcs.00973. [DOI] [PubMed] [Google Scholar]
- Garriock RJ, D’Agostino SL, Pilcher KC, Krieg PA. Wnt11-R, a protein closely related to mammalian Wnt11, is required for heart morphogenesis in Xenopus. Dev Biol. 2005;279:179–192. doi: 10.1016/j.ydbio.2004.12.013. [DOI] [PubMed] [Google Scholar]
- Goto T, Davidson L, Asashima M, Keller R. Planar cell polarity genes regulate polarized extracellular matrix deposition during frog gastrulation. Curr Biol. 2005;15:787–793. doi: 10.1016/j.cub.2005.03.040. [DOI] [PubMed] [Google Scholar]
- Harland RM. In situ hybridization: an improved whole-mount method for Xenopus embryos. Methods Cell Biol. 1991;36:685–695. doi: 10.1016/s0091-679x(08)60307-6. [DOI] [PubMed] [Google Scholar]
- Heisenberg CP, Tada M, Rauch GJ, Saude L, Concha ML, Geisler R, Stemple DL, Smith JC. Silberblick/Wnt11 mediates convergent extension movements during zebrafish gastrulation. Nature. 2000;405:76–81. doi: 10.1038/35011068. [DOI] [PubMed] [Google Scholar]
- Hikasa H, Shibata M, Hiratani I, Taira M. The Xenopus receptor tyrosine kinase Xror2 modulates morphogenetic movements of the axial mesoderm and neuroectoderm via Wnt signaling. Development. 2002;129:5227–5239. doi: 10.1242/dev.129.22.5227. [DOI] [PubMed] [Google Scholar]
- Jeffery WR, Strickler AG, Yamamoto Y. Migratory neural crest-like cells form body pigmentation in a urochordate embryo. Nature. 2004;431:696–699. doi: 10.1038/nature02975. [DOI] [PubMed] [Google Scholar]
- Keller R. The origin and morphogenesis of Amphibian somites. Curr Top Dev Biol. 2000;47:183–246. doi: 10.1016/s0070-2153(08)60726-7. [DOI] [PubMed] [Google Scholar]
- Kim GH, Han JK. JNK and ROKalpha function in the noncanonical Wnt/RhoA signaling pathway to regulate Xenopus convergent extension movements. Dev Dyn. 2005;232:958–968. doi: 10.1002/dvdy.20262. [DOI] [PubMed] [Google Scholar]
- Kispert A, Vainio S, Shen L, Rowitch DH, McMahon AP. Proteoglycans are required for maintenance of Wnt-11 expression in the ureter tips. Development. 1996;122:3627–3637. doi: 10.1242/dev.122.11.3627. [DOI] [PubMed] [Google Scholar]
- Kohn AD, Moon RT. Wnt and calcium signaling: beta-Catenin-independent pathways. Cell Calcium. 2005;38:439–446. doi: 10.1016/j.ceca.2005.06.022. [DOI] [PubMed] [Google Scholar]
- Krotoski DM, Fraser SE, Bronner-Fraser M. Mapping of neural crest pathways in Xenopus laevis using inter- and intra-specific cell markers. Dev Biol. 1988;127:119–132. doi: 10.1016/0012-1606(88)90194-7. [DOI] [PubMed] [Google Scholar]
- Ku M, Melton DA. XWnt-11: a maternally expressed Xenopus Wnt gene. Development. 1993;119:1116–1173. doi: 10.1242/dev.119.4.1161. [DOI] [PubMed] [Google Scholar]
- Kuhl M, Sheldahl LC, Malbon CC, Moon RT. Ca(2+)/calmodulin-dependent protein kinase II is stimulated by Wnt and Frizzled homologs and promotes ventral cell fates in Xenopus. J Biol Chem. 2000;275:12701–12711. doi: 10.1074/jbc.275.17.12701. [DOI] [PubMed] [Google Scholar]
- Le Floch N, Rivat C, De Wever O, Bruyneel E, Mareel M, Dale T, Gespach C. The proinvasive activity of Wnt-2 is mediated through a noncanonical Wnt pathway coupled to GSK-3beta and c-Jun/AP-1 signaling. FASEB J. 2005;19:144–146. doi: 10.1096/fj.04-2373fje. [DOI] [PubMed] [Google Scholar]
- Li L, Yuan H, Xie W, Mao J, Caruso AM, McMahon A, Sussman DJ, Wu D. Dishevelled proteins lead to two signaling pathways. Regulation of LEF-1 and c-Jun N-terminal kinase in mammalian cells. J Biol Chem. 1999;274:129–134. doi: 10.1074/jbc.274.1.129. [DOI] [PubMed] [Google Scholar]
- Linker C, Bronner-Fraser M, Mayor R. Relationship between gene expression domains of Xsnail, Xslug, and Xtwist and cell movement within the prospective neural crest of Xenopus. Dev Biol. 2000;224:215–225. doi: 10.1006/dbio.2000.9723. [DOI] [PubMed] [Google Scholar]
- Lupu-Meiri M, Beit-Or A, Christensen SB, Oron Y. Calcium entry in Xenopus oocytes: effects of inositol trisphosphate, thapsigargin and DMSO. Cell Calcium. 1993;14:101–10. doi: 10.1016/0143-4160(93)90080-p. [DOI] [PubMed] [Google Scholar]
- Matifat F, Fournier F, Lorca T, Capony JP, Brule G, Collin T. Involvement of the Ca2+/calmodulin-dependent protein kinase II pathway in the Ca2+-mediated regulation of the capacitative Ca2+ entry in Xenopus oocytes. Biochem J. 1997;322:267–272. doi: 10.1042/bj3220267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCauley DW, Bronner-Fraser M. Neural crest contributions to the lamprey head. Development. 2003;130:2317–2327. doi: 10.1242/dev.00451. [DOI] [PubMed] [Google Scholar]
- Moody SA. Fates of the blastomeres of the 32-cell-stage Xenopus embryo. Dev Biol. 1987;122:300–319. doi: 10.1016/0012-1606(87)90296-x. [DOI] [PubMed] [Google Scholar]
- Morgan R, El-Kadi AM, Theokli C. Flamingo, a cadherin-type receptor involved in the Drosophila planar polarity pathway, can block signaling via the canonical Wnt pathway in Xenopus laevis. Int J Dev Biol. 2003;47:245–252. [PubMed] [Google Scholar]
- Newth DR. On the neural crest of the lamprey. J Exp Morph. 1956;4:358–375. [Google Scholar]
- Nieuwkoop PD, Faber J. Normal Table of Xenopus laevis (Daudin) Garland; New York: 1994. [Google Scholar]
- Olivera-Martinez I, Missier S, Fraboulet S, Thelu J, Dhouailly D. Differential regulation of the chick dorsal thoracic dermal progenitors from the medial dermomyotome. Development. 2002;129:4763–4772. doi: 10.1242/dev.129.20.4763. [DOI] [PubMed] [Google Scholar]
- Olivera-Martinez I, Thelu J, Dhouailly D. Molecular mechanisms controlling dorsal dermis generation from the somitic dermomyotome. Int J Dev Biol. 2004;48:93–101. doi: 10.1387/ijdb.15272374. [DOI] [PubMed] [Google Scholar]
- Osborn JC, Duncan CJ, Smith JL. Role of calcium ions in the control of embryogenesis of Xenopus. Changes in the subcellular distribution of calcium in early cleavage embryos after treatment with the ionophore A23187. J Cell Biol. 1997;80:589–604. doi: 10.1083/jcb.80.3.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandur P, Maurus D, Kuhl M. Increasingly complex: new players enter the Wnt signaling network. Bioessays. 2002;24:881–884. doi: 10.1002/bies.10164. [DOI] [PubMed] [Google Scholar]
- Raven CP. Zur entwicklung der Ganglienleiste. 1 Die kinematik der Ganglienleistenentwicklung bei den Urodelen. Arch F Entw Mechan. 1931;125:210–292. doi: 10.1007/BF00576356. [DOI] [PubMed] [Google Scholar]
- Rosso SB, Sussman D, Wynshaw-Boris A, Salinas PC. Wnt signaling through Dishevelled, Rac and JNK regulates dendritic development. Nat Neurosci. 2005;8:34–42. doi: 10.1038/nn1374. [DOI] [PubMed] [Google Scholar]
- Runyan RB, Potts JD, Sharma RV, Loeber CP, Chiang JJ, Bhalla RC. Signal transduction of a tissue interaction during embryonic heart development. Cell Regul. 1990;1:301–313. doi: 10.1091/mbc.1.3.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldahl LC, Park M, Malbon CC, Moon RT. Protein kinase C is differentially stimulated by Wnt and Frizzled homologs in a G-protein-dependent manner. Curr Biol. 1999;9:695–698. doi: 10.1016/s0960-9822(99)80310-8. [DOI] [PubMed] [Google Scholar]
- Sheldahl LC, Slusarski DC, Pandur P, Miller JR, Kuhl M, Moon RT. Dishevelled activates Ca2+ flux, PKC, and CaMKII in vertebrate embryos. J Cell Biol. 2003;161:769–777. doi: 10.1083/jcb.200211094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobkow L, Epperlein HH, Herklotz S, Sttrabue WL, Tanaka EM. A germline GFP transgenic axolotl and its use to track cell fate: Dual origin of the fin mesenchyme during development and the fate of blood cells during regeneration. Dev Biol. 2006;290:386–397. doi: 10.1016/j.ydbio.2005.11.037. [DOI] [PubMed] [Google Scholar]
- Sokol SY. Analysis of Dishevelled signalling pathways during Xenopus development. Curr Biol. 1996;6:1456–1467. doi: 10.1016/s0960-9822(96)00750-6. [DOI] [PubMed] [Google Scholar]
- Tada M, Smith JC. XWnt11 is a target of Xenopus Brachyury: regulation of gastrulation movements via Dishevelled, but not through the canonical Wnt pathway. Development. 2000;127:2227–2238. doi: 10.1242/dev.127.10.2227. [DOI] [PubMed] [Google Scholar]
- Takeuchi M, Nakabayashi J, Sakaguchi T, Yamamoto TS, Takahashi H, Takeda H, Ueno N. The prickle-related gene in vertebrates is essential for gastrulation cell movements. Curr Biol. 2003;13:674–679. doi: 10.1016/s0960-9822(03)00245-8. [DOI] [PubMed] [Google Scholar]
- Tucker RP. The role of glycosaminoglycans in anuran pigment cell migration. J Embryol Exp Morphol. 1986;92:145–164. [PubMed] [Google Scholar]
- Tucker RP, Erickson CA. Pigment cell pattern formation in Taricha torsora: the role of the extracellular matrix in controlling pigment cell migration and differentiation. Dev Biol. 1986;118:268–285. doi: 10.1016/0012-1606(86)90094-1. [DOI] [PubMed] [Google Scholar]
- Tucker AS, Slack JM. Independent induction and formation of the dorsal and ventral fins in Xenopus laevis. Dev Dyn. 2004;230:461–467. doi: 10.1002/dvdy.20071. [DOI] [PubMed] [Google Scholar]
- Twitty VC, Bodenstein D. Experiments on the determination problem. I The roles of ectoderm and neural crest in development of the dorsal fin in Amphibia. J Exp Zool. 1941;86:343–380. [Google Scholar]
- Ueno N, Greene ND. Planar cell polarity genes and neural tube closure. Birth Defects Res C Embryo Today. 2003;69:318–324. doi: 10.1002/bdrc.10029. [DOI] [PubMed] [Google Scholar]
- Wallingford JB, Rowning BA, Vogeli KM, Rothbacher U, Fraser SE, Harland RM. Dishevelled controls cell polarity during Xenopus gastrulation. Nature. 2000;405:81–85. doi: 10.1038/35011077. [DOI] [PubMed] [Google Scholar]
- Wallingford JB, Harland RM. Xenopus Dishevelled signaling regulates both neural and mesodermal convergent extension: parallel forces elongating the body axis. Development. 2001;128:2581–2592. doi: 10.1242/dev.128.13.2581. [DOI] [PubMed] [Google Scholar]
- Wallingford JB, Harland RM. Neural tube closure requires Dishevelled-dependent convergent extension of the midline. Development. 2002;129:5815–5825. doi: 10.1242/dev.00123. [DOI] [PubMed] [Google Scholar]
- Wallingford JB, Goto T, Keller R, Harland RM. Cloning and expression of Xenopus Prickle, an orthologue of a Drosophila planar cell polarity gene. Mech Dev. 2002;116:183–186. doi: 10.1016/s0925-4773(02)00133-8. [DOI] [PubMed] [Google Scholar]
- Wang Y, Macke JP, Abella BS, Andreasson K, Worley P, Gilbert DJ, Copeland NG, Jenkins NA, Nathans J. A large family of putative transmembrane receptors homologous to the product of the Drosophila tissue polarity gene frizzled. J Biol Chem. 1996;271:4468–4476. doi: 10.1074/jbc.271.8.4468. [DOI] [PubMed] [Google Scholar]
- Wehrli M, Dougan ST, Caldwell K, O’Keefe L, Schwartz S, Vaizel-Ohayon D, Schejter E, Tomlinson A, DiNardo S. Arrow encodes an LDL-receptor-related protein essential for Wingless signaling. Nature. 2000;407:527–530. doi: 10.1038/35035110. [DOI] [PubMed] [Google Scholar]
- Wu GY, Cline HT. Stabilization of dendritic arbor structure in vivo by CaMKII. Science. 1998;279:222–226. doi: 10.1126/science.279.5348.222. [DOI] [PubMed] [Google Scholar]
- Yamaguchi TP, Bradley A, McMahon AP, Jones SA. Wnt5a pathway underlies outgrowth of multiple structures in the vertebrate embryo. Development. 1999;126:1211–1223. doi: 10.1242/dev.126.6.1211. [DOI] [PubMed] [Google Scholar]
- Yamanaka H, Moriguchi T, Masuyama N, Kusakabe M, Hanafusa H, Takada R, Takada S, Nishida E. JNK functions in the non-canonical Wnt pathway to regulate convergent extension movements in vertebrates. EMBO Rep. 2002;3:69–75. doi: 10.1093/embo-reports/kvf008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakin LD, Mazan S, Maury M, Martin N, Guenet JL, Brulet P. Structure and expression of Wnt13, a novel mouse Wnt2 related gene. Mech Dev. 1998;73:107–116. doi: 10.1016/s0925-4773(98)00040-9. [DOI] [PubMed] [Google Scholar]


