Abstract
CD8+ cell-secreted CC-chemokines, MIP-1α, and MIP-β have recently been identified as factors which suppress HIV. In this study we co-inoculated MIP-1α expression plasmid with a DNA vaccine constructed from HIV-1 pCMV160IIIB and pcREV, and evaluated the effect of the adjuvant on HIV-specific immune responses following intramuscular and intranasal immunization. The levels of both cytotoxic T lymphocyte (CTL) activity and DTH showed that HIV-specific cell-mediated immunity (CMI) was significantly enhanced by co-inoculation of the MIP-1α expression plasmid with the DNA vaccine compared with inoculation of the DNA vaccine alone. The HIV-specific serum IgG1/IgG2a ratio was significantly lowered when the plasmid was co-inoculated in both intramuscular and intranasal routes, suggesting a strong elicitation of the T helper (Th) 1-type response. When the MIP-1α expression plasmid was inoculated intramuscularly with the DNA vaccine, an infiltration of mononuclear cells was observed at the injection site. After intranasal administration, the level of mucosal secretory IgA antibody was markedly enhanced. These findings demonstrate that MIP-1α expression plasmid inoculated together with DNA vaccine acts as a strong adjuvant for eliciting Th1-derived immunity.
Keywords: DNA vaccine, HIV-1, MIP-1α, CTL
INTRODUCTION
DNA vaccines against HIV-1 have been proven to be an effective means of generating immune responses and protection in a wide variety of preclinical models [1–3]. DNA vaccines provide a means to generate antibodies and cytotoxic T lymphocytes (CTL); they can be a tool for studying mechanisms of antigen presentation, the role of cytokines, and the effects of bacterial DNA in the generation of immune responses; and they also provide a technology for the discovery of novel vaccine antigens. In a previous study, an HIV DNA vaccine consisting of a mixture of cytomegalovirus (CMV) promoter DNA linked to the HIV env gene and CMV promoter DNA linked to the rev gene (IIIB/REV) induced a certain level of HIV-1-specific humoral and cellular immune responses [4]. However, the immunogenicity of the DNA vaccine was not as strong as expected. The use of expression plasmids as adjuvants for DNA vaccination against AIDS has also been explored to optimize the preparations employed in immunization [5,6]. DNA co-inoculation can lead to the expression of proteins which may help in inducing a stronger and longer lasting immunity [7–10]. To achieve protective immunity against HIV-1 infection, virus-specific CTL have been shown to play an important role in the clearance of persistent virus infections in both human and animal models [11,12].
To enhance the HIV-specific cell-mediated immunity (CMI), we tested co-inoculation of the DNA vaccine with MIP-1α expression plasmid. MIP-1α, a member of the β-chemokine family, acts as a chemoattractant for inflammatory cells and modulates functions of monocytes and B and T lymphocytes [13–16], and it also affects haematopoietic stem/progenitor cell growth [17,18]. Several studies have shown that MIP-1α stimulation enhances interferon-gamma (IFN-γ) production [19], which is essential for the induction of Th1-derived CMI. These observations suggest that MIP-1α would be useful as an effective adjuvant in DNA vaccination by activating macrophages and Th1-type cells. Since DNA is amenable to genetic manipulation, we designed a MIP-1α expression plasmid which we co-inoculated with an immunogenic HIV DNA vaccine [4] to determine whether this plasmid enhances HIV-1-specific immunity.
MATERIALS AND METHODS
Animals
We used only 6–10-week-old BALB/c female mice purchased from Japan SLC, Inc. (Shizuoka, Japan).
Plasmids
pCMV160IIIB encoding gp160 of HIV-1IIIB and pcREV encoding rev were described previously [4]. Murine MIP-1α cDNA [20] was kindly donated by Dr T. Yoshimura (Department of Immunopathology Section and Laboratory of Immunology, NCI-FCRDC, Frederick). The pCAGGS expression vector [21] was donated by Dr J. Miyazaki (Department of Nutrition and Physiological Chemistry, Osaka Medical University, Osaka, Japan). Murine MIP-1α cDNA was inserted into the Xho I site of the pCAGGS expression vector to obtain the pCAGGSMIP-1α plasmid (Fig. 1).
Fig. 1.
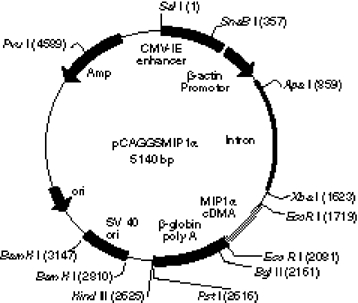
Construction of expression plasmid pCAGGSMIP-1α. pCAGGS vector was digested with Xho I restriction enzyme, blunted, and ligated with blunted MIP-1α cDNA.
DNA inoculation
Mice were inoculated by injection or intranasally. A total of 100 μl of DNA mixture containing 2 μg each of pCMV160IIIB and pcREV (hereafter referred to as pCMV160IIIB/REV) and a 5–50 μg dose of pCAGGSMIP-1α diluted in sterile PBS was injected into the right biceps femoral muscle of mice [4]. For the intranasal route, mice were anaesthetized with diethyl ether. After about 20 s, 30 μl of the DNA vaccine preparation containing 2 μg each of pCMV160IIIB/REV and a 1, 10, or 50-μg dose of pCAGGSMIP-1α diluted in PBS were dropped into the nostrils little by little, so as to avoid suffocation [22].
DTH response
Two weeks after DNA inoculation, a total of 25 μl PBS containing 4 μg of the HIV-1IIIB V3 peptide RIQRGPRAFVTIGK was injected into the rear footpads of each mouse. After 24 h, the extent of footpad swelling was measured with a microdial meter (Ozaki Seisakusho, Tokyo, Japan) in units of 10−2 mm. Control mice were injected with the same dose of the sperm whale myoglobin peptide ALVEADVA [4,22].
HIV-1-specific cytotoxic test
As described previously [4], 3 weeks after DNA injection, splenic mononuclear cells were collected and 1 × 106 lymphoid cells were restimulated in vitro in the presence of the same amount of irradiated (30 Gy) syngeneic spleen cells with 3 μg/ml of the HIV-1 V3 peptide RGPGRAFVTI, a known CTL epitope of HIV-1IIIB. After being cultured for 5 days, the cytotoxic activity of these spleen cells was measured by a 6-h 51Cr-release assay using V3 peptide-pulsed target cells. The target cells were prepared using the same HIV-1 V3 peptide-pulsed P815 cells (H-2d). The bulk splenocytes used as effector cells were co-cultivated with the target cells at effector-to-target cell (E:T) ratios that ranged from 5:1 to 80:1. Target cell lysis was measured by gamma-ray counting of 100 μl of cell-free supernatant to determine the amount of 51Cr released. The percentage of specific 51Cr released was calculated as 100 × (experimental release — spontaneous release)/(maximun release — spontaneous release). Target cells incubated in medium alone and with medium plus 5% Triton X-100 were used to determine spontaneous and maximum chromium release, respectively.
ELISA
ELISA was used to determine antibody responses against HIV-1IIIB. Serum and faecal specimens were collected on day 28 after immunization. Sample blood was collected by retro-orbital puncture under anaesthesia with diethyl ether and the serum obtained was stored at 4°C until the antibody assay as described elsewhere [22]. Faecal extract samples were prepared by suspending 100 mg of faecal pellets in 1 ml of PBS. After centrifugation at 12 000 rev/min, the supernatant was collected and stored at −20°C until use. ELISA for HIV-1IIIB was done according to the protocol already described [4,22]. Ninety-six-well microplates (Falcon, NJ) were coated with HIV-1IIIB V3 peptide-multiple antigenic peptide (MAP) at a concentration of 5 μg/ml. Serial dilutions of sera or faecal samples from immunized mice were added to the wells after blocking with 1% bovine serum albumin (BSA) in PBS and washing with PBS–T (0.05% Tween 20). Wells were then treated with peroxidase-labelled anti-mouse IgG, IgG1 or IgG2a (Organon Teknika, West Chester, PA) as the secondary antibody. The plates were coloured with o-phenylenediamine dihydrochloride (Wako Chemical, Osaka, Japan) and read at 490 nm on a plate reader. For estimation of secretory IgA (sIgA), antibody against the V3 peptide was also used. Antibody titres were expressed as the reciprocal of the final detectable dilution which gave an optical density (OD) at 490 nm of > 0.2 OD units above the pretreated control. The IgG1/IgG2a ratio was calculated from the reciprocal log2 titres.
Histopathological analysis
Two micrograms of pCMV160IIIB/REV formulated with 10 μg of MIP-1α plasmid dissolved in PBS were injected into the biceps femoral muscles of BALB/c mice. At 1, 3, 5, 7 and 14 days after injection, muscles were resected, fixed with 10% buffered formalin, and embedded in paraffin. Thin sections were then prepared and stained with haematoxylin and eosin for light microscopic observation. Negative control mice were injected with the same amount of DNA vaccine alone.
Statistical analysis
Statistical analyses for comparison of two groups were conducted using an unpaired two-tailed t-test or one-way factorial analysis of variance (anova) for distribution parameters. Significance was defined as P < 0.05 in both analyses.
RESULTS
HIV-1-specific antibody responses
To examine dose-related immune responses to MIP-1α-mediated DNA vaccination, we administered the DNA vaccine with 1, 10 and 50 μg of MIP-1α expression plasmid by intramuscular and intranasal immunization. The HIV-1-specific serum IgG and faecal IgA antibodies were analysed by ELISA on day 28 (Table 1). Antibody responses were significantly enhanced when 10 μg of MIP-1α expression plasmid were administered together with the immunogen (P < 0.05). Therefore, this dose was used in subsequent assays to evaluate HIV-1-specific immunity, including analyses of immunoglobulin subclasses and histological examinations. Intramuscularly the DNA vaccine alone was able to induce a serum IgG antibody titre of 26, and similar titres were observed when the adjuvants were included. Intranasal administration with the adjuvant similarly enhanced the IgG responses of the DNA vaccine. Intranasal administration was also effective in generating high levels of faecal IgA antibody. The antibody titres obtained with the DNA vaccine alone and with vaccine plus pCAGGS-empty were the same with either route, suggesting that the adjuvant effect was not caused by the pCAGGS expression vector. Mice serving as negative controls showed no detectable antibody response.
Table 1.
Titres of HIV-1-specific antibody induced by intramuscular (i.m.) and intranasal (i.n.) administration of the DNA vaccine with or without MIP-1α expression plasmid
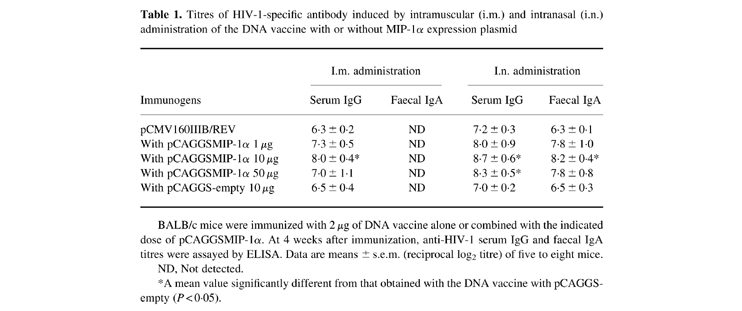
BALB/c mice were immunized with 2 μg of DNA vaccine alone or combined with the indicated dose of pCAGGSMIP-1α. At 4 weeks after immunization, anti-HIV-1 serum IgG and faecal IgA titres were assayed by ELISA. Data are means ± s.e.m. (reciprocal log2 titre) of five to eight mice.
ND, Not detected.
*A mean value significantly different from that obtained with the DNA vaccine with pCAGGS-empty (P < 0.05).
HIV-1-specific immunoglobulin subclass analysis
The vaccine-induced antibodies were then examined for their immunoglobulin subclass and titre using serum samples from the intramuscular and intranasal groups. As shown in Fig. 2, IgG2a titres increased and IgG1 titres decreased, causing a sizeable drop in the IgG1/IgG2a ratio, which was significantly lower than that obtained with inoculation of DNA vaccine alone in both routes, suggesting that a Th1-type response was induced by co-inoculation with the DNA vaccine and MIP-1α expression plasmid.
Fig. 2.
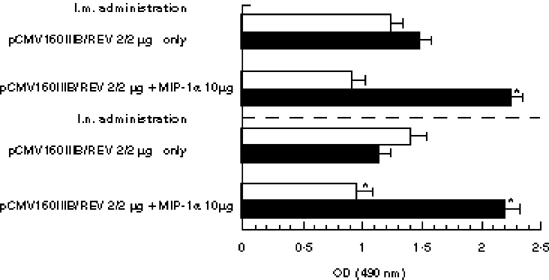
Immunoglobulin subclasses of HIV-specific antibody. BALB/c mice were given 2 μg of DNA vaccine alone or vaccine combined with 10 μg of pCAGGSMIP-1α by intramuscular (i.m.) and intranasal (i.n.) immunization. After 4 weeks, anti-HIV-IgG1 (□) and IgG2a titres (▪) were assayed by ELISA. Optical density (OD) data are expressed as means OD ± s.e.m. *A significant difference from that obtained using the DNA vaccine alone (P < 0.05).
HIV-1-specific CMI responses
Dose-related DTH responses and CTL activity were also evaluated in both intramuscularly and intranasally immunized mice. HIV-1-specific DTH was analysed using the footpad swelling response 2 weeks after immunization (Table 2), and this response was found to be similar in both groups of mice. When the DNA vaccine was inoculated with 10 μg of the MIP-1α plasmid, the footpad swelling response was strongly enhanced compared with that of mice injected with the DNA vaccine alone. There was no significant difference between mice immunized with the DNA vaccine alone and with the vaccine plus pCAGGS-empty. Mice serving as negative controls showed no significant increase in the footpad swelling response.
Table 2.
Footpad swelling responses of mice co-inoculated with DNA vaccine and MIP-1α expression plasmid
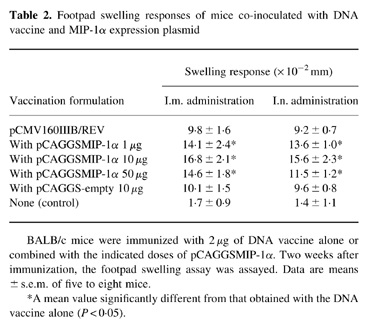
BALB/c mice were immunized with 2 μg of DNA vaccine alone or combined with the indicated doses of pCAGGSMIP-1α. Two weeks after immunization, the footpad swelling assay was assayed. Data are means ± s.e.m. of five to eight mice.
*A mean value significantly different from that obtained with the DNA vaccine alone (P < 0.05).
Another experiment was conducted to determine whether co-inoculation of the DNA vaccine with the MIP-1α expression plasmid via the two routes could induce stronger CTL responses than inoculation of the immunogen alone. As shown in Fig. 3, strongly enhanced CTL activity was noted not only at an E:T ratio of 80 but also at a ratio of 20, when the vaccine was co-inoculated with 10 μg of the MIP-1α plasmid. There were no substantial differences between the two routes with respect to either HIV-1-specific DTH or CTL activity. The data of three other CTL experiments gave the same results, suggesting that the DNA vaccine with MIP-1α expression plasmid induced a higher level of HIV-1-specific CMI than did the vaccine alone.
Fig. 3.
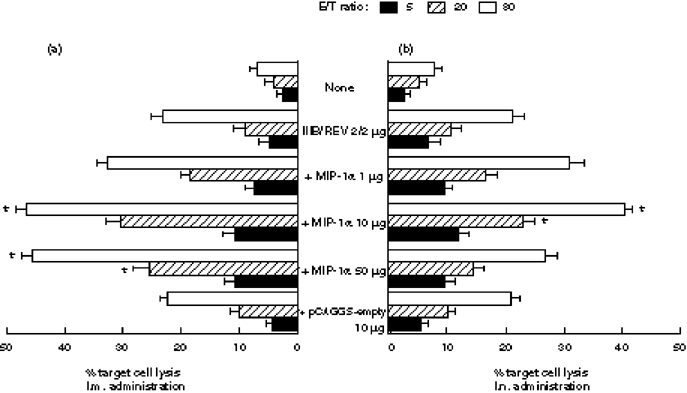
Cytotoxic T lymphocyte (CTL) activity of MIP-1α expression plasmid. (a) By intramuscular administration. (b) By intranasal administration. Two separate groups of mice were immunized with 2 μg of DNA vaccine formulated with the indicated dose of MIP-1α expression plasmid. Splenocytes were isolated after 4 weeks and cultured for 5 days with the V3 peptide. The CTL activity was titrated at E:T ratios of 5, 20 and 80. Data are means ± s.e.m. of three to four mice. *A significant difference from that obtained using the expression vector alone (P < 0.01).
Inflammatory cell accumulation in the MIP-1α-injected muscles
On histopathological examination, a substantial level of inflammatory cell infiltration composed of histocytes and lymphocytes was observed lasting from 1 to 7 days after injection. The maximal accumulation of lymphocytes in the injected muscles was observed 3 days after plasmid injection (Fig. 4b,c), indicating that strong inflammation was caused by MIP-1α injection. This was not observed in muscles injected with the DNA vaccine alone (Fig. 4a) and injected after 14 days. These data demonstrate that MIP-1α is a cytokine which displays chemoattractive activity for inflammatory cells.
Fig. 4.
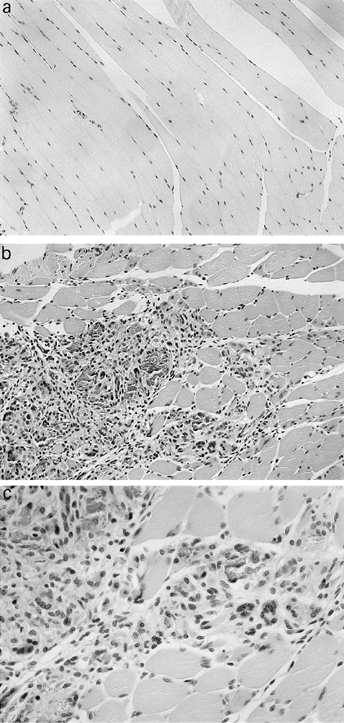
Histological examination of injected mice. (a) Injected with 2 μg of DNA vaccine alone. (b) Injected with 2 μg of DNA vaccine and 10 μg of MIP-1α expression plasmid. (Mag. × 100.) (c) Injected with 2 μg of DNA vaccine and 10 μg of MIP-1α expression plasmid. (Mag. × 200.) At 1, 3, 5, 7 and 14 days after injection, muscles were resected, fixed with 10% buffered formalin, and embedded in paraffin. Thin sections were then prepared and stained with haematoxylin and eosin for light microscopic observation.
DISCUSSION
We previously reported that the use of expression plasmids for certain cytokines or costimulatory factors enhanced the immune responses induced with a DNA vaccine [7–10]. There have been several studies suggesting that DNA plasmids encoding a functional gene can elicit protective immunity in mice against certain pathogenic viruses [4,23,24]. Techniques employing immunological adjuvants together with plasmid DNA are not only simple to perform but also offer other several advantages including low cost, easy production, facilitated quality control, a continuous production of protein, and no risk of inadvertent infection. Our preliminary experiments revealed that the half-life of IL-2 and IL-12 protein in vivo is short compared with their expression plasmid forms (unpublished data). Therefore, in an effort to develop more effective adjuvants, we considered MIP-1α co-inoculation with DNA vaccine to promote various forms of the immune response.
In this study, we examined the immune modifications elicited by MIP-1α expression plasmid. Although DNA vaccines without adjuvant are able to generate antigen-specific CMI, the faecal IgA antibodies were significantly activated and DTH response and CTL activity were consistently enhanced when the DNA vaccine was co-inoculated with MIP-1α either intramuscularly or intranasally (Table 2 and Fig. 3). Therefore, co-inoculating immunogenic DNA with MIP-1α augments the potency of DNA vaccinations. Moreover, the IgG1/IgG2a ratio was significantly lower than that obtained using the DNA vaccine alone, suggesting activation of Th1-type cells (Fig. 2). Activation of a Th1-type immune response is vital for HIV-1 protection or therapeutic efficacy. In murine models, cytokines such as IL-2 and IFN-γ produced by Th1 cells support the development of cellular immunity, including the CTL response and production of the IgG2a immunoglobulin isotype. Cytokines such as IL-4 and IL-5 produced by Th2 cells promote B cell activation and immunoglobulin class switching processes, which are typified by a predominance of the IgG1 immunoglobulin isotype [25,26]. The relative predominance of IgG1 over IgG2a or vice versa can be influenced by the method of DNA inoculation (gene gun versus saline injection) as well as by the form of expressed antigen (membrane-bound or secreted) [27,28]. We used MIP-1α because of its known activation of macrophages and its ability to induce IFN-γ production [29]. Activation of Th1-derived immunity elicited a DTH response, CTL activity, and IgG2a antibody production, and our results accommodate this. Recently, three murine CC-chemokine (MIP-1α, MIP-1β, RANTES) receptors which display different forms or chemokine binding have been described [30,31]. The chemokine ligands for these receptors provide signalling in T cells and deliver different intracellular signals that activate a Th1 response and enhance IFN-γ production. In the present study, we did not obtain direct data about in vivo efficacy of MIP-1α expression plasmids because of the limitations of HIV-1 infection in mice.
We also demonstrated that injection with MIP-1α caused massive inflammatory infiltration in the injected site (Fig. 4). Histopathological examination demonstrated that MIP-1α has chemoattractive activity, which is consistent with previous studies [7]. In addition to adjuvant activity, MIP-1α suppresses the growth of HIV, possibly by interfering with HIV binding to CC-CKR3 and CC-CKR5 [30,31]. Molecular identification of these fusion cofactors is critical for understanding the pathogenesis of HIV-1 infection and would be useful for designing therapeutic strategies. MIP-1α also plays an important role in vivo in the development of both acute and relapsing experimental autoimmune encephalomyelitis (EAE) [32]. In support of a vital role for MIP-1α in Th1-mediated inflammatory disease, Cook and others have shown recently that MIP-1α gene-deficient mice fail to develop virus-induced autoimmune heart disease [33]. These reports along with the data of the present study emphasize the importance of CC chemokine selectivity during the inflammatory immune response in terms of their roles as mediators of chemotaxis to the Th1 subtype.
Including the CpG motif in DNA vaccines as an adjuvant may increase humoral and cellular immunity to a weakly antigenic protein β-galactosidase encoded by it or a co-injected plasmid [34]. There are reports that CpG-based DNA sequences in the plasmid backbone of DNA vaccines promote antigen-specific immunity [35,36]. To eliminate the promoter region function as a confusing variable, we inoculated MIP-1α-free pCAGGS with the DNA vaccine using both routes of immunization (Tables 1 and 2). The antibody titres and the DTH response were not significantly different from those obtained using the DNA vaccine alone, suggesting the adjuvant activity of MIP-1α was not due to the CpG motif.
DNA vaccine therapy is thought to be an effective method for treating patients with AIDS. Intramuscular injection has been favoured for eliciting immune response over the past years because of its many advantages [7]. Recent reports have shown that intranasal administration of DNA vaccine induces strong antibody production, particularly production of mucosal sIgA antibody, and a high level of HIV-specific CTL activity [37–39]. We found in the present study that the DTH response and CTL activity were significantly enhanced when the DNA vaccine was inoculated intranasally with MIP-1α expression plasmid (Table 2; Fig. 3b). A high level of HIV-specific mucosal sIgA antibody was also observed when we used this route of administration. Compared with the intramuscular route, intranasal administration of DNA vaccine is safe, easily carried out, and has fewer side-effects. However, further detailed analysis is necessary to evaluate this method fully. Taking all data together, we consider that the present approach of formulating adjuvants for use with plasmid DNA and administration via the intranasal route is the simplest and most economical method for providing immunity against this disease.
In conclusion, our present findings clearly show that DNA vaccine co-inoculated with MIP-1α expression plasmid induces a substantial level of HIV-specific CMI and that similar vaccine–plasmid combinations may be useful for designing therapeutic strategies to combat HIV infection.
Acknowledgments
We would like to thank Ms T. Honsho for her technical assistance and Ms T. Kaneko and Ms T. Ito for their secretarial assistance. This work was supported by a Grant-in-Aid from the Ministry of Education, Science, Sports and Culture of Japan, and by a Grant-in-Aid from the Kihara Memorial Yokohama Foundation for the Advancement of Life Sciences.
References
- 1.Davis HL, Michel ML, Whalen RG. Use of plasmid DNA for direct gene transfer and immunization. Ann NY Acad Sci. 1995;772:21–29. doi: 10.1111/j.1749-6632.1995.tb44728.x. [DOI] [PubMed] [Google Scholar]
- 2.Hassett DE, Whitton JL. DNA immunization. Trends Microbiol. 1996;4:307–12. doi: 10.1016/0966-842x(96)10048-2. [DOI] [PubMed] [Google Scholar]
- 3.Kumar V, Szercar E. Genetic vaccination: the advantages of going naked. Nature Med. 1996;2:857–9. doi: 10.1038/nm0896-857. [DOI] [PubMed] [Google Scholar]
- 4.Okuda K, Bukawa H, Hamajima K, et al. Induction of potent humoral and cell-mediated immune responses following direct injection of DNA encoding the HIV-1 env and rev gene products. AIDS Res Hum Retrovir. 1995;11:933–43. doi: 10.1089/aid.1995.11.933. [DOI] [PubMed] [Google Scholar]
- 5.Wang B, Boyer J, Srikantan V, et al. DNA inoculation induces neutralizing immune responses against human immunodeficiency virus type-1 in mice and nonhuman primates. DNA Cell Biol. 1993;12:799–05. doi: 10.1089/dna.1993.12.799. [DOI] [PubMed] [Google Scholar]
- 6.Wang B, Ugen KE, Srikantan V, et al. Gene inoculation generates immunoresponses against human immunodeficiency virus type 1. Proc Natl Acad Sci USA. 1993;90:4156–60. doi: 10.1073/pnas.90.9.4156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsuji T, Fukushima J, Hamajima K, et al. HIV-1-specific cell-mediated immunity is enhanced by coinoculation of TCA-3 expression plasmid with DNA vaccine. Immunol. 1997;90:1–6. doi: 10.1046/j.1365-2567.1997.00117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ishii N, Fukushima J, Kaneko T, et al. Cationic liposomes are a strong adjuvant for a DNA vaccine of human immunodeficiency virus type 1. AIDS Res Hum Retrovir. 1997;13:1421–8. doi: 10.1089/aid.1997.13.1421. [DOI] [PubMed] [Google Scholar]
- 9.Tsuji T, Kawamoto S, Sasaki S, et al. Immunomodulatory effects of plasmid expressing B7-2 on human immunodeficiency virus-1-specific cell-mediated immunity induced by a plasmid encoding the viral antigen. Eur J Immunol. 1997;27:782–7. doi: 10.1002/eji.1830270329. [DOI] [PubMed] [Google Scholar]
- 10.Sasaki S, Tsuji T, Hamajima K, et al. Monophosphoryl Lip A enhances both humoral and cell-mediated immune responses to DNA vaccination against human immunodeficiency virus type 1. Infect Immun. 1997;65:3520–8. doi: 10.1128/iai.65.9.3520-3528.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Douglas FN, Alain RMT, John GE, Charles RR, John G, Andrew JM. HIV-1 gag-specific cytotoxic T lymphocytes defined with recombinant vaccinia virus and synthetic peptides. Nature. 1988;336:484–7. doi: 10.1038/336484a0. [DOI] [PubMed] [Google Scholar]
- 12.Carmichael A, Jin X, Sissons P, Borysiewicz L. Quantitative analysis of the human immunodeficiency virus type 1 (HIV-1)-specific cytotoxic T lymphocyte (CTL) responses at different stages of HIV-1 infection: differential CTL responses to HIV-1 and Epstein–Barr virus in late disease. J Exp Med. 1993;177:249–56. doi: 10.1084/jem.177.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baggiolini M, Dahinden CA. CC chemokines in allergic inflammation. Immunol Today. 1994;15:127–33. doi: 10.1016/0167-5699(94)90156-2. [DOI] [PubMed] [Google Scholar]
- 14.Schall TJ, Bacon K, Camp R, Kaspari JW, Goeddel DV. Human macrophage inflammatory protein 1α (MIP-1α) and MIP-1β chemokines attract distinct populations of lymphocytes. J Exp Med. 1993;177:1821–6. doi: 10.1084/jem.177.6.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taub DD, Conlon K, Lloyd AR, Oppenhein JJ, Kelvin DJ. Preferential migration of activated CD4+ and CD8+ T cells in response to MIP-1α and MIP-1β. Science. 1993;260:355–8. doi: 10.1126/science.7682337. [DOI] [PubMed] [Google Scholar]
- 16.Fahey TJ, Tracey KJ, Tekamp-Olson P, Cousens LS, Jones WG, Shires GT, Gerami A, Sherry B. Macrophage inflammatory protein 1α modulates macrophage function. J Immunol. 1992;148:2764–9. [PubMed] [Google Scholar]
- 17.Graham GJ, Wright EG, Hewick R, Wolpe SD, Wilkie NM, Donaldson D, Lorimore S, Pragnell IB. Identification and characterization of an inhibitor of haemopoietic stem cell proliferation. Nature. 1990;344:442–7. doi: 10.1038/344442a0. [DOI] [PubMed] [Google Scholar]
- 18.Verfaillie CM, Catanzarro P, Li W. Macrophage inflammatory protein 1α, interleukin-3 and diffusible bone marrow stromal factors maintain human hematopoietic stem cells for at least eight weeks in vitro. J Exp Med. 1994;179:643–9. doi: 10.1084/jem.179.2.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.William JK, Nicholas WL, Kevin JK, Nicholas WL, Kevin JK, Wendy SS, Stephen DH, Terrence AB. Differential CC chemokine-induced enhancement of T helper cell cytokine production. J Immunol. 1997;158:4129–36. [PubMed] [Google Scholar]
- 20.Davatelis G, Tekamp-Olson P, Wolpe SD, et al. Cloning and characterization of a cDNA for murine macrophage inflammatory protein (MIP), a novel monokine with inflammatory and chemokinetic properties. J Exp Med. 1988;167:1939–44. doi: 10.1084/jem.167.6.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Niwa K, Yamamura K, Miyazaki J. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene. 1991;108:193–6. doi: 10.1016/0378-1119(91)90434-d. [DOI] [PubMed] [Google Scholar]
- 22.Bukawa H, Sekigawa K, Hamajima K, Fukushima J, Yamada Y, Kiyono H, Okuda K. Neutralization of HIV-1 by secretory IgA induced by oral immunization with a new macromolecular multicomponent peptide vaccine candidate. Nature Med. 1995;1:681–5. doi: 10.1038/nm0795-681. [DOI] [PubMed] [Google Scholar]
- 23.Wolff JA, Malone RW, Williams P, Chong W, Acsadi G, Jani A, Felgner PL. Direct gene transfer into mouse muscle in vivo. Science. 1990;247:1465–8. doi: 10.1126/science.1690918. [DOI] [PubMed] [Google Scholar]
- 24.Eisenbaun MD, Fuller DH, Haynes JR. Examination of parameters affecting the elicitation of humoral immune responses by particle bombardment-mediated genetic immunization. DNA Cell Biol. 1993;12:791–7. doi: 10.1089/dna.1993.12.791. [DOI] [PubMed] [Google Scholar]
- 25.Seder RA, Paul WE. Acquisition of lymphokine-producing phenotype by CD4+ T cells. Annu Rev Immunol. 1994;12:635–73. doi: 10.1146/annurev.iy.12.040194.003223. [DOI] [PubMed] [Google Scholar]
- 26.Pertmer TM, Roberts TR, Haynes JR. Influenza virus nucleoprotein-specific immunoglobulin G subclass and cytokine responses elicited by DNA vaccination are dependent on the route of vector DNA delivery. J Virol. 1996;70:6119–25. doi: 10.1128/jvi.70.9.6119-6125.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Feltquate DM, Heaney S, Webster RG, Robinson HL. Different T helper cell types and antibody isotypes generated by saline and gene gun DNA immunization. J Immunol. 1997;158:2278–84. [PubMed] [Google Scholar]
- 28.Babiuk LA, Lewis PJ, Cox G, et al. DNA immunization with bovine herpesvirus-1 genes. Ann NY Acad Sci. 1995;772:47a. doi: 10.1111/j.1749-6632.1995.tb44731.x. [DOI] [PubMed] [Google Scholar]
- 29.Schrum S, Probst P, Fleischer B, Zipel PF. Synthesis of the CC-chemokines MIP-1α, MIP-1β, and RANTES is associated with a type 1 immune response. J Immunol. 1996;157:3598–604. [PubMed] [Google Scholar]
- 30.Doranz BJ, Rucker J, Yi Y, et al. A dual-tropic primary HIV-1 isolate that uses fusion and the β-chemokine receptors CKR-5, CKR-3, and CKR-2b as fusion cofactors. Cell. 1996;85:1149–58. doi: 10.1016/s0092-8674(00)81314-8. [DOI] [PubMed] [Google Scholar]
- 31.Choe H, Farzan M, Sun Y, et al. The β-chemokine receptors CCR3 and CCR5 facilitate infection by primary HIV-1 isolates. Cell. 1996;85:1135–48. doi: 10.1016/s0092-8674(00)81313-6. [DOI] [PubMed] [Google Scholar]
- 32.Karpus WJ, Lukacs NW, McRae BL, Strieter RM, Kunkel SL, Miller SD. An important role for the chemokine macrophage inflammatory protein-1α in the pathogenesis of the T cell-mediated autoimmune disease experimental autoimmune encephalomyelitis. J Immunol. 1995;155:5003–10. [PubMed] [Google Scholar]
- 33.Cook DN, Beck MA, Coffman TM, Kirby SL, Sheridan JF, Pragnell IB, Smithies O. Requirement of MIP-1α for an inflammatory response to viral infection. Science. 1995;269:1583–5. doi: 10.1126/science.7667639. [DOI] [PubMed] [Google Scholar]
- 34.Krieg AM, Yi A-K, Matson S, Waldschmldt TJ, Bishop GA, Teasdale R, Koretzky GA, Kllnman DM. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature. 1995;374:546–9. doi: 10.1038/374546a0. [DOI] [PubMed] [Google Scholar]
- 35.Klinman DM, Yamshchikov G, Ishigatsubo Y. Contribution of CpG motifs to the immunogenicity of DNA vaccines. J Immunol. 1997;158:3635–9. [PubMed] [Google Scholar]
- 36.Roman M, Martin-Orozco E, Goodman JS, et al. Immunostimulatory DNA sequences function as T help-1-promoting adjuvants. Nature Med. 1997;3:849–54. doi: 10.1038/nm0897-849. [DOI] [PubMed] [Google Scholar]
- 37.Tamura S, Samegai Y, Kurata H, et al. Protection against influenza virus infection by vaccine inoculated intranasally with cholera toxin B subunit. Vaccine. 1988;6:409–13. doi: 10.1016/0264-410x(88)90140-5. [DOI] [PubMed] [Google Scholar]
- 38.Abraham E. Intranasal immunization with bacterial polysaccharide containing liposomes enhances antigen-specific pulmonary secretory antibody response. Vaccine. 1992;10:461–8. doi: 10.1016/0264-410x(92)90395-z. [DOI] [PubMed] [Google Scholar]
- 39.Okada E, Sasaki S, Ishii N, et al. Intranasal immunization of a DNA vaccine with IL-12 and granulocyte-macrophage colony-stimulating factor (GM-CSF)-expressing plasmids in liposomes induces strong mucosal and cell-mediated immune responses against HIV-1 antigens. J Immunol. 1997;159:3638–47. [PubMed] [Google Scholar]


