Abstract
Hantaviruses cause an important human illness, HFRS. Blood samples from 22 HFRS-positive, six seronegative patients and 15 healthy controls were examined in 1995, during the largest HFRS epidemic in Croatia. Results of double- and triple-colour immunofluorescence analysis showed an increased percentage of cytotoxic T cells (CD3+CD8+) in seropositive patients compared with seronegatives and healthy controls. The majority of seropositive HFRS patients expressed activation and memory antigens on T and B lymphocytes. The percentage of CD23+ and CD21+ B lymphocytes was lower in seropositive patients. HFRS patients had elevated levels of sCD23 and five had elevated total IgE. The increased expression of both early and late T cell activation antigens, e.g. CD25, CD71 and HLA-DR, memory cells and sCD23 positively correlated with biochemical parameters (AST, ALT, urea, α2-globulin) during the acute phase of HFRS. The phenotypic changes observed, especially early and late T cell activation markers, as well as memory cells, could be useful parameters in the evaluation of HFRS course, and prognostic factors of HFRS severity. Additional attention should be paid to liver involvement in the pathogenesis of HFRS.
Keywords: haemorrhagic fever with renal syndrome, flow cytometry, T and B lymphocytes, sCD23, liver transaminases
INTRODUCTION
Hantaviruses (HTV), family Bunyaviridae, include a number of pathogens that cause HFRS in humans. The phylogenetic analyses of virus isolates identified 14 related groups of hantaviruses and eight probable species [1]. Some of them also cause hantavirus pulmonary syndrome [2].
A broad spectrum of clinical conditions has been recognized in HFRS, ranging from inapparent or mild illness to a fulminant haemorrhagic process with severe renal failure and death. Xu & Wang suggest that antigenic variations could explain variations in the HFRS severity and different clinical symptoms as well as epidemiological characteristics [3]. In spite of numerous genetic and serologic analyses of different hantaviral isolates worldwide, especially since the hantaviral pulmonary syndrome (HPS) has been recognized in the USA, little is known about the pathogenesis of HFRS.
There is some evidence that immune complexes have a role in the HFRS pathogenesis [4]. Also, activation of both classic and alternative complement pathways in HFRS has been documented [5]. In HFRS patients, a specific antibody can be detected at or very close to the onset of symptoms. It is generally accepted that specific IgM antibodies are measurable as early as the first 3 days of the disease [6,7]. Specific IgG rise more slowly and do not reach maximum until about the second week of the disease [6]. In contrast to some other haemorrhagic fevers, HFRS patients may die with high antibody titres and no detectable viraemia [8]. Additionally, specific IgE as well as soluble CD23 (sCD23) have been detected in HFRS in the febrile phase [9]. T cell activation occurs very early in HFRS and is associated with an increase in the absolute number of neutrophils, monocytes, and B and T cells. There is no change in CD4+ T cell population [4,10]. In mice, T cells seem to play a crucial role in the resistance to hantaviral infection through the cytotoxic T cell response, and to help in antibody production, production of cytokines including interferon (IFN) and induction of DTH reactions [11]. Recent investigations of Linderholm et al. [12] demonstrated elevated plasma levels of tumour necrosis factor-alpha (TNF-α), soluble TNF receptors, IL-6 and IL-10 in patients with HFRS.
Activation of resting, antigen-specific lymphocytes with viral antigens, and non-specific activation of lymphocytes with soluble factors (e.g. cytokines) results in changes of the cell surface phenotype. Among the earliest activation changes are transient expression of the early activation markers, CD25 (IL-2 receptor) and CD71 (transferrin receptor), with later up-regulation of MHC II class (HLA-DR) antigen [13].
At the beginning of the greatest HFRS epidemic in Croatia [14,15], we wanted to evaluate immunophenotypic changes occurring in HFRS patients, considering the main lymphocyte populations, especially in regard to activation markers. For that purpose, we analysed the immunophenotypic changes in the main lymphocyte populations and lymphocyte activation markers in HFRS patients, seronegative and healthy controls, by use of whole blood two- and three-colour flow cytometry. Total IgE and soluble CD23 were also determined in some patients.
PATIENTS AND METHODS
Patients
In this study, 22 soldiers with serologically confirmed HFRS (Dobrava and Puumala infection), males (aged 21–37 years) hospitalized at the University Hospital for Infectious Diseases in Zagreb and General Hospital in Varaždin in April–May 1995, were tested. Blood samples were taken at admission to the hospital. The patients were hospitalized in the acute phase of illness, between 2 and 29 days (mean 12 days) after the onset of HFRS. Seventeen of them were in febrile, hypotensive or oliguric phase, and five entered polyuric phase. Six HFRS suspected but seronegative controls and 15 healthy controls were also included in the study. Three of the seronegative patients had pneumonia atypica, two had catarrhus febrilis respiratoris, and one had gastroenterocolitis acuta as the final diagnosis. Clinical and biochemical findings in the HFRS patients were also analysed.
Flow cytometry analysis
Two- and three-colour immunofluorescence cytometry were performed with MoAbs identifying the following surface antigens for: T cells and T cell subpopulations (CD3, TCR, CD4, CD8, CD45RA, CD45RO); B cells (CD20, CD21); natural killer (NK) cells (CD16, CD56); and activation markers on T cells (CD25, CD71, HLA-DR), and on B cells (CD23). All MoAbs except CD21 (Immunotech, Marseille, France) were purchased from Becton Dickinson (Heidelberg, Germany). MoAbs were directly conjugated to fluorochromes (FITC, PE and PerCP). In each experiment, FITC-, PE- and PerCP-conjugated isotypic controls were used for determination of non-specific binding. Simultaneous staining with different MoAbs was performed on whole blood samples as described previously [16]. Briefly, 50 μl of heparinized blood were incubated in the dark at 4°C with 10 μl of fluorochrome-conjugated antibodies for 30 min. Erythrocytes were lysed by adding 2 ml of 10% FACS lysing solution (Becton Dickinson, San Jose, CA) for 10 min at room temperature in the dark. After extensive washing, the cells were resuspended in 0.5 ml of the fixative (1% formaldehyde) solution. Control suspensions were prepared by the same procedure.
Cell fluorescence of the control and patient samples was analysed on a FACScan flow cytometer (Becton Dickinson, Mountain View, CA) and 5000 and 20 000 cells within the lymphocyte gate were collected for two- and three-colour analysis, respectively. Data were analysed using CELLQuest software (Becton Dickinson).
Measurement of total IgE and sCD23
Total IgE was determined in 36 sera of 21 patients, using PRIST (Institute of Immunology, Zagreb, Croatia), as previously described [16]. Total IgE values > 120 kU/l were considered elevated.
Soluble CD23 was measured in sera of 13 patients, by use of ELISA kit (The Binding Site Limited, Birmingham, UK). According to the manufacturer's protocol, the range for sCD23 for normal sera was 1–6 μg/l.
Statistical analysis
Multiple comparison of the three groups tested was performed by computing Conover's inequality if H0 was rejected by Kruskal–Wallis test corrected for ties [17]. Pearson product — moment correlation was used for describing the relationship between the clinical and immunological data.
RESULTS
Percentage of major lymphocytic subpopulations in hantavirus+ and hantavirus− patients
Analysis of the major lymphocyte subpopulations (T, B and NK cells) in the groups of seropositive, seronegative and healthy controls revealed no changes between the groups in the percentage of total T lymphocytes (CD3+ or TCR+), B lymphocytes (CD20+ or CD21+) and NK cells (CD3−CD16,CD56+) (data not shown). The percentage of cytotoxic T lymphocytes (CD3+CD8+) was significantly elevated in the group of seropositive patients compared with seronegative and healthy controls (Fig. 1a). Although there was no difference in the percentage of either CD20+ or CD21+ B lymphocytes between the tested groups, seropositive patients showed a significant decrease in CD20+CD21+ double-positive subpopulations as a percentage of total B lymphocytes (CD20+) when compared with healthy controls (Fig. 1b).
Fig. 1.
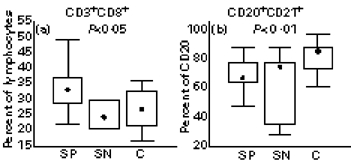
Percentage of CD3+CD8+, cytotoxic T lymphocytes (a), and CD20+CD21+ subpopulation of B lymphocytes (b) in seropositive patients (SP; n = 22), seronegative patients (SN; n = 6) and healthy controls (C; n = 11). Box indicates 25th and 75th percentile, central point median, and whiskers indicate minimum and maximum data values. Probabilities (P) of rejecting null hypothesis for computed Conover's inequalities are indicated by dotted lines where found.
Expression of activation and memory antigens on T and B lymphocytes
Within T lymphocytes, we found an increased percentage of total activated T cells (TCR+HLA-DR+) in seropositive patients compared with both seronegatives and controls (Fig. 2a). Further analysis of early activation markers on T cell subpopulations revealed an increased percentage of activated cytotoxic (CD8+CD71+) and helper (CD4+CD25+) lymphocytes in seropositive patients (Fig. 2b,c). In contrast, the percentage of activated B lymphocytes (CD20+CD23+) within total B cells was decreased in the seropositive group compared with healthy controls (Fig. 2d).
Fig. 2.
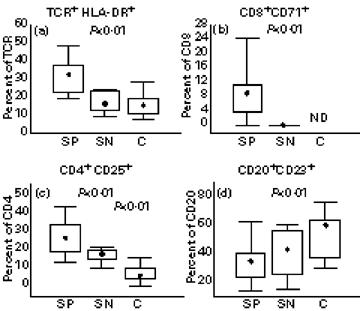
Percentage of TCR+HLA-DR+ (a), CD8+CD71+ (b), CD4+CD25+ (c) T lymphocytes, and CD20+CD23+ (d) B lymphocytes in seropositive patients (SP; n = 22), seronegative patients (SN; n = 6) and healthy controls (C; n = 15 (a), 14 (b), and 13 (d)). Box indicates 25th and 75th percentile, central point median, and whiskers indicate min-max data values. Probabilities (P) of rejecting null hypothesis for computed Conover's inequalities are indicated by dotted lines where found. ND, Not done.
The expression of naive (CD45RA) and memory (CD45RO) markers was analysed on CD4+ and CD8+ T lymphocytes. There was a decrease in the percentage of total CD45RA lymphocytes (Fig. 3a) and an increase in the percentage of CD45RO+ lymphocytes (Fig. 3b) in seropositive patients in comparison with healthy controls. We also found an increased percentage of CD4+ and CD8+ lymphocytes simultaneously expressing both CD45RA and CD45RO markers in seropositive patients when compared with seronegatives and healthy controls (Fig. 3c,d).
Fig. 3.
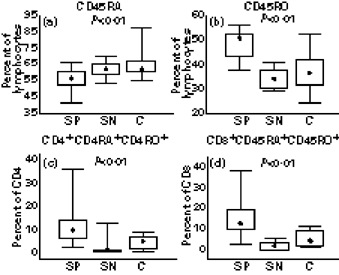
Percentage of lymphocytes with CD45RA+ (a) and CD45RO+ markers (b), and percentage of CD4+CD45RA+CD45RO+ (c) and CD8+CD45RA+CD45RO+ (d) phenotype, in seropositive (SP; n = 22), seronegative patients (SN; n = 6) and healthy controls (C; n = 13). Box indicates 25th and 75th percentile, central point median, and whiskers indicate minimum and maximum data values. Probabilities (P) of rejecting null hypothesis for computed Conover's inequalities are indicated by dotted lines where found.
Clinical and biochemical findings
All except three (see below) of the tested HFRS patients developed a mild form of HFRS. All patients had fever and most of them suffered headache, backache, myalgia and arthralgia, but they also developed oliguria and polyuria, visual disturbance, conjunctival injection and face and neck flush. We also observed pulmonary and liver disorders in our patients. Among the three severe HFRS cases, one patient needed dialysis, and two patients developed epileptic seizures. In one of these patients, cerebral haemorrhage was found on computed tomography (CT). Biochemical data are shown in Table 1.
Table 1.
Distribution of increased and decreased laboratory parameters in patients with HFRS
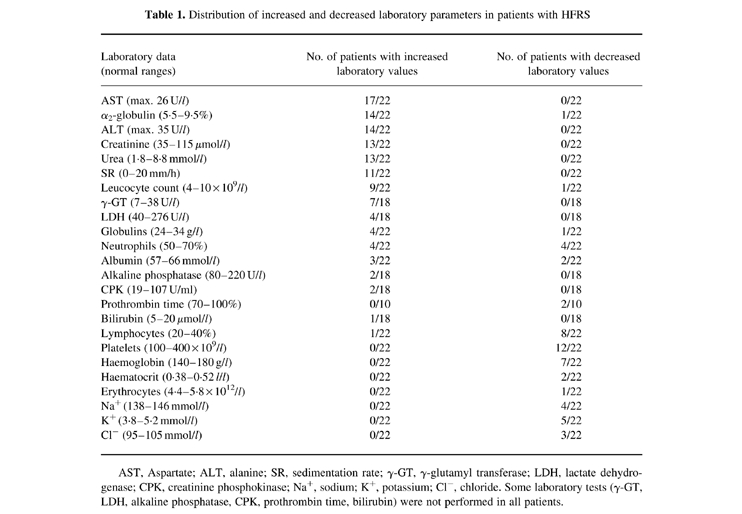
AST, Aspartate; ALT, alanine; SR, sedimentation rate; γ-GT, γ-glutamyl transferase; LDH, lactate dehydrogenase; CPK, creatinine phosphokinase; Na+, sodium; K+, potassium; Cl−, chloride. Some laboratory tests (γ-GT, LDH, alkaline phosphatase, CPK, prothrombin time, bilirubin) were not performed in all patients.
Total IgE and sCD23
In five out of 21 HFRS patients we found elevated total IgE values (mean 412 kU/l; range 147–770 kU/l). All patients with increased total IgE values showed a slight decrease of IgE in paired sera (data not shown).
All tested HFRS patients (n = 13) had elevated levels of sCD23 (mean 17 μg/l; range 6.5–53.7 μg/l).
Relationship between the expression of lymphocyte surface antigens and biochemical data
Several correlations between the expressed lymphocyte surface antigens and biochemical data are shown in Table 2. In short, liver transaminases (AST, ALT) were in positive correlation with activation markers on T cells and sCD23, while platelets showed negative correlation with CD4+CD25+. AST, ALT, urea and α2-globulin were in positive correlation with CD4+ and CD8+ lymphocytes which simultaneously expressed both CD45RA and CD45RO markers. Biochemical parameters of renal and liver function were in positive interrelationships, while platelets showed negative correlation with a majority of them.
Table 2.
Relationship between the expression of lymphocyte surface antigens and the biochemical data
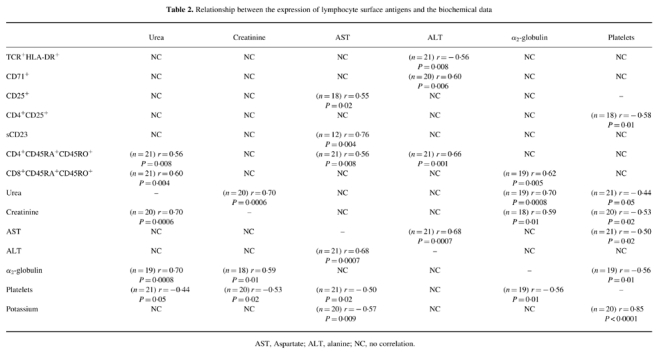
AST, Aspartate; ALT, alanine; NC, no correlation.
DISCUSSION
Immune mechanisms involved in the pathogenesis of HFRS have been the subject of research in the last 20 years. However, this is the first study in Europe to analyse in detail the immunophenotypic changes in peripheral blood lymphocytes, and their relationship with the clinical parameters and pathogenesis of HFRS. In our study, we performed whole blood two- and three-colour immunofluorescence analysis, a powerful tool in the phenotypic determination of different lymphocyte subsets [18,19].
We found an increased percentage of cytotoxic T lymphocytes (CD3+CD8+) in seropositive patients. In mice, it was previously postulated that hantaviral infections could induce changes in the cell-mediated response with generation of an effective CD8+ cell-mediated immune response. These findings were found to be closely linked to the resolution of acute hantaviral illness [11,20–23]. The ability of cytotoxic cells to attack and destroy virus-infected cells early in the replicative cycle of a virus is modulated via cytokine production [24]. IL-12 promotes the development of Th1 lymphocytes which then produce cytokines (e.g. IFN-γ) important for their cytotoxic function [25]. IFN-γ was shown to decrease the expression of CD23 antigen on B cells [26]. Therefore, the decreased percentage of CD23+ B lymphocytes in seropositive HFRS patients in comparison with seronegatives and healthy controls could be the result of IFN-γ produced by HFRS-specific CD4+ T lymphocytes.
Soluble CD23 has been found to be implicated in the regulation of many immunological functions of T and B lymphocytes, macrophages and myeloid cells in humans [9,19]. We found elevated levels of sCD23 which highly correlated with AST. Although we found no correlation between the levels of sCD23 and IL-2 receptor (CD25), both of them were increased in HFRS patients and could have a regulatory interaction [27]. Both could be involved in the activation of HFRS. Elevated total IgE could also be one of the parameters of HFRS activation in some patients. IgE immune complexes may induce increased expression of TNF-α, resulting in increased vascular permeability. TNF-α has recently been found to be one of the major parameters in the pathogenesis of HFRS [5]. In our patients, total IgE slightly decreased in the later phase of HFRS.
We found that in seropositive patients, the percentage of T cells expressing early activation markers (CD71, CD25) was also increased. An increased expression of the IL-2 receptor (CD25) antigen was also found in the study of Huang et al. Increased levels of sIL-2R in the same study were in concordance with the degree of illness [28]. Activation markers showed positive correlation with AST and ALT, and negative correlation with platelets. Elevation of AST could be caused by acute renal disorders, but could also result from acute haemolytic anaemia or virus replication in the liver. Elevated levels of AST, ALT and γ-glutamyl transferase (γ-GT) are sensitive indicators of liver cell integrity and acute hepatic inflammation. Recently, Meng et al. [29] found that hantaviruses may be an important agent in acute hepatitis of unknown aetiology. Our findings that AST and ALT positively correlate with T cell activation markers indicate important liver involvement in HFRS pathogenesis.
We observed no changes in other lymphocyte populations such as NK cells (CD3−CD16+CD56+) and helper T cells (CD3+CD4+), although a significantly lower number of NK cells has been found during the first week of HFRS in comparison with the second week of the disease onset [9].
To test the possible activation of B cells in HFRS patients, we determined the expression of CD21 antigen, since its expression decreases upon B cell activation [30]. We observed that B lymphocytes were activated in seropositive patients, since they had a lower percentage of CD21+ B lymphocytes compared with seronegatives and healthy controls. This result emphasizes the role of B lymphocytes in the pathogenesis of HFRS.
In this study we also analysed the expression of naive (CD45RA) and memory (CD45RO) markers on CD4+ and CD8+ lymphocytes, and found an increased percentage of memory T cells with a consequently decreased percentage of naive T cells. The increase in triple-positive CD4+CD45RA+CD45RO+ and CD8+CD45RA+CD45RO+ lymphocyte subsets was especially pronounced. These results are in agreement with the observation that T cells express CD45RO very rapidly upon activation. Obviously, CD45RA expression is lost more slowly, so the cells co-express both isoforms for some time, presenting the transitional state of T lymphocytes after antigen (e.g. viral antigen) stimulation [31]. Positive correlation of T cells with co-expression of CD45RA and CD45RO markers and biochemical parameters such as AST, ALT, and α2-globulin, indicated that the process of memory cell development occurred at the same time as HFRS progression.
In conclusion, the observation of phenotypic changes, especially the early and late T cell activation markers, as well as memory cell development, could be useful parameters in the evaluation of HFRS course. The possibility that these parameters might be prognostic factors for the later development of a chronic sequel is the subject of our further study.
Acknowledgments
We would like to thank Dr J. W. LeDuc (CDC, Atlanta, GA) for his help in establishing the ELISA HFRS diagnostics in the Institute of Immunology, Zagreb. We wish to thank Dr J. Tomašić for preparing PRIST for total IgE and B. Dojnović for skillful technical assistance.
References
- 1.Schmaljohn C, Hjelle B. Hantaviruses. A global disease problem. Emerg Infect Dis. 1997;3:95–104. doi: 10.3201/eid0302.970202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nichol ST, Spiropoulou CF, Morzurov S, et al. Genetic identification of a hantavirus associated with an outbreak of acute respiratory illness. Science. 1993;262:914–7. doi: 10.1126/science.8235615. [DOI] [PubMed] [Google Scholar]
- 3.Xu ZK, Wang MX. The antigenic analysis of haemorrhagic fever with renal syndrome viruses in China by monoclonal antibodies. J Hyg Camb. 1986;97:369–75. doi: 10.1017/s0022172400065451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cosgriff TM, Lewis RM. Mechanisms of disease in hemorrhagic fever with renal syndrome. Kidney Int. 1991;40(Suppl. 35):72–79. [PubMed] [Google Scholar]
- 5.Settergren B, Ahlm C, Alexeyev O, Billheden J, Stegmayr B. Pathogenetic and clinical aspects of the renal involvement in hemorrhagic fever with renal syndrome. Renal Failure. 1997;19:1–14. doi: 10.3109/08860229709026255. [DOI] [PubMed] [Google Scholar]
- 6.LeDuc JW, Ksiazek TG, Rossi CA, Dalrymple JM. A retrospective analysis of sera collected by the hemorrhagic fever commission during the Korean conflict. J Infect Dis. 1990;162:1182–4. doi: 10.1093/infdis/162.5.1182. [DOI] [PubMed] [Google Scholar]
- 7.Markotić A, Šarćević A, Hlaća D. Significance of a rapid diagnostics of the hemorrhagic fever with renal syndrome (HFRS) during the war. Med Arch. 1994;48:109–11. [PubMed] [Google Scholar]
- 8.Xiao SY, Zhu B, Zhang M, Zhang T, Zheng Z, Xiang J. A sequential study of serum specific IgM antibody responses in patients with epidemic hemorrhagic fever and its relationship to the severity of illness. Chinese J Immunol. 1986;4:218–21. [Google Scholar]
- 9.Alexeyev OA, Linderholm M, Elgh F, Wadell G, Juto P, Tärnvik A. Increased plasma levels of soluble CD23 in hemorrhagic fever with renal syndrome. Relation to virus-specific IgE. Clin Exp Immunol. 1997;109:351–5. doi: 10.1046/j.1365-2249.1997.4641359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Turković B, Svoboda-Beusan I. Flow cytometric analysis of lymphocyte subsets in hemorrhagic fever with renal syndrome virus infection. Acta Virol. 1991;35:298–301. [PubMed] [Google Scholar]
- 11.Asada H, Tamura M, Kondo K, et al. Role of T lymphocyte subsets in protection and recovery from Hantaan virus infection in mice. J Gen Virol. 1987;68:1961–9. doi: 10.1099/0022-1317-68-7-1961. [DOI] [PubMed] [Google Scholar]
- 12.Linderholm M, Ahlm C, Settergren B, Waage A, Tarnvik A. Elevated plasma levels of tumor necrosis factor (TNF)-alfa, soluble TNF receptors, interleukin (IL)-6, and IL-10 in patients with hemorrhagic fever with renal syndrome. J Infect Dis. 1996;173:38–43. doi: 10.1093/infdis/173.1.38. [DOI] [PubMed] [Google Scholar]
- 13.Crabtree GR. Contingent genetic regulatory events in T lymphocyte activation. Science. 1989;243:355–61. doi: 10.1126/science.2783497. [DOI] [PubMed] [Google Scholar]
- 14.Markotić JW, LeDuc D, Hlaća, et al. Hantaviruses are a likely threat to NATO forces in Bosnia and Herzegovina and Croatia. Nature Med. 1996;2:269–70. doi: 10.1038/nm0396-269. [DOI] [PubMed] [Google Scholar]
- 15.Kuzman I, Markotić A, Turćinov D, Beus I. Outbreak of hemorrhagic fever with renal syndrome in Croatia in 1995. Lijeć Vjesn. 1997;119:311–5. [PubMed] [Google Scholar]
- 16.Gagro A, Rabatić S, Trešćec A, Dekaris D, Medar-Lasić M. Expression of lymphocytes FcɛRII/CD23 in allergic children undergoing hyposensitization. Int Arch Allergy Immunol. 1993;101:203–8. doi: 10.1159/000236520. [DOI] [PubMed] [Google Scholar]
- 17.Theodorsson-Norheim E. Kruskal–Wallis test: BASIC computer program to perform nonparametric one-way analysis of variance and multiple comparisons on ranks of several independent samples. Comput Method Program Biomed. 1986;23:57–62. doi: 10.1016/0169-2607(86)90081-7. [DOI] [PubMed] [Google Scholar]
- 18.Dekaris D, Sabioncello A, Mažuran R, Rabatić S, Svoboda-Beusan I, Raćunica NL, Tomašić J. Multiple changes of immunologic parameters in prisoners of war. Assessments after release from a camp in Manjaca, Bosnia. JAMA. 1993;270:595–9. [PubMed] [Google Scholar]
- 19.Rabatić A, Gagro M, Medar-Lasić CD21–CD23 ligand pair expression in children with allergic asthma. Clin Exp Immunol. 1993;94:337–40. doi: 10.1111/j.1365-2249.1993.tb03454.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Asada H, Tamura M, Kondo K, Dohi Y, Yamanishi K. Cell-mediated immunity to virus causing haemorrhagic fever with renal syndrome. Generation of cytotoxic T Lymphocytes. J Gen Virol. 1988;69:2179–88. doi: 10.1099/0022-1317-69-9-2179. [DOI] [PubMed] [Google Scholar]
- 21.Asada H, Balachandra K, Tamura M, Kondo K, Yamanishi K. Cross-reactive immunity among different serotypes of virus causing haemorrhagic fever with renal syndrome. J Gen Virol. 1989;70:819–25. doi: 10.1099/0022-1317-70-4-819. [DOI] [PubMed] [Google Scholar]
- 22.Nakamura T, Yanagihara R, Gibbs CJ, Amyx HL, Gajdušek DC. Differential susceptibility and resistance of immunocompetent and immunodeficient mice to fatal Hantaan virus infection. Arch Virol. 1985;86:109–20. doi: 10.1007/BF01314117. [DOI] [PubMed] [Google Scholar]
- 23.Nakamura T, Yanagihara R, Gibbs CJ, Gajdušek DC. Immune spleen cell-mediated protection against fatal Hantaan virus infection in infant mice. J Infect Dis. 1985;151:691–7. doi: 10.1093/infdis/151.4.691. [DOI] [PubMed] [Google Scholar]
- 24.Leist TP, Cobbold SP, Waldmann H, Aguet M, Zinkernagel R. Functional analysis of T lymphocyte subsets in antiviral host defense. J Immunol. 1987;138:2278–81. [PubMed] [Google Scholar]
- 25.Mehrotra PT, Wu D, Crim JA, Mostowski HS, Siegel JP. Effects of IL-12 on the generation of cytotoxic activity in human CD8+ T lymphocytes. J Immunol. 1993;151:2444–52. [PubMed] [Google Scholar]
- 26.Gordon J, Katira A, Strain AJ, Gillis S. Inhibition of interleukin 4-promoted CD23 production in human B lymphocytes by transforming growth factor-β, interferons or anti-CD19 antibody is overridden on engaging CD40. Eur J Immunol. 1991;21:1917–22. doi: 10.1002/eji.1830210821. [DOI] [PubMed] [Google Scholar]
- 27.Yodoi J, Hosoda M, Maeda Y, Sato S, Takami M, Kawabe T. Low affinity IgE receptors. Regulation and functional roles in cell activation. In: Chadwick D, Everd D, Whelan J, editors. IgE, mast cells and the allergic response. Chichester: John Wiley & Sons; 1989. pp. 133–48. [DOI] [PubMed] [Google Scholar]
- 28.Huang C, Jin B, Wang M, Li E, Sun C. Hemorrhagic fever with renal syndrome. Relationship between pathogenesis and cellular immunity. J Infect Dis. 1994;169:868–70. doi: 10.1093/infdis/169.4.868. [DOI] [PubMed] [Google Scholar]
- 29.Meng G, Lan Y, Nakagawa M, Maehara T, Mitani K, Tomiyama T, Che XG, Ohkubo A. High prevalence of hantavirus infection in a group of Chinese patients with acute hepatitis of unknown aetiology. J Viral Hepatitis. 1997;4:231–4. doi: 10.1046/j.1365-2893.1997.00140.x. [DOI] [PubMed] [Google Scholar]
- 30.Faeron DT. The CD19–CR2-TAPA-1 complex, CD45 and signaling by the antigen receptor of B-lymphocytes. Curr Opin Immunol. 1993;5:341–8. doi: 10.1016/0952-7915(93)90051-s. [DOI] [PubMed] [Google Scholar]
- 31.Akbar AN, Terry L, Timms A, Beverly PCL, Janossy G. Loss of CD45R and gain of UCHL-1 reactivity is a feature of primed T cells. J Immunol. 1988;140:2171–8. [PubMed] [Google Scholar]


