Abstract
With organ allografts considerable numbers of donor-type mononuclear cells are transferred to the recipient, leading to bilateral immunological interactions between donor and recipient lymphocytes. To study such bilateral immune reactions in detail, human two-way MLC were performed. In this model proliferation kinetics, patterns of activation, and survival of the two populations were analysed, and the relevance of initial cell subset composition, relative cell numbers, and the effect of immunosuppression on this co-culture were evaluated. It could be demonstrated that with an initial 50:50 ratio of two populations of allogeneic cells one population dominated after 21 days of co-culture in 78 out of 80 combinations (97%) tested; the other population decreased markedly after an initially stable phase of 6–7 days. With unequal starting conditions the larger population dominated when resting cells were used, but small populations of preactivated cells or separated CD8+ cells could also dominate. Depletion of CD16+ natural killer (NK) cells and of CD2− cells (B cell and monocytes) had no effect on domination. Addition of cyclosporin delayed or blocked the domination process while addition of IL-2 accelerated it. Disappearance of one population was associated with detection of apoptotic cells. The findings indicate that co-cultures of allogeneic mononuclear cells are generally not stable for more than 1 week, but lead to active elimination of one population. CD8+ cells and particularly preactivated cells seem to play the most important role in that process, while NK cells are of less importance. Cyclosporin can prolong survival of allogeneic cells in co-culture. These observations suggest that under the conditions of clinical organ transplantation even small amounts of immunocompetent donor cells transferred by the graft may persist for some time and may, thereby, have the chance to exert immunomodulatory functions.
Keywords: mixed leucocyte culture, two-way MLR
INTRODUCTION
Two-way MLC were first described in 1964 by Bain et al. [1] and by Bach & Hirschhorn [2] for measuring histocompatibility between two different individuals. Two years later the one-way MLC was introduced [3] and it has predominantly been used afterwards since it allows more detailed studies of immune responses against allogeneic and xenogeneic cells. In the one-way MLC proliferation of stimulator cells is typically inhibited either by irradiation or by pretreatment with anti-proliferative drugs like mitomycin C. These inactivated cells can still present alloantigens and produce cytokines, but proliferation is not possible, and these cells usually die after a few days in culture. Thus, by measuring proliferation following several days of co-culture, the response of only one population (‘responder cells’) is measured. In this one-way model, the stimulator cells are usually considered to represent the graft, i.e. the more or less passive target of the recipient's immunocompetent cells.
In contrast to this view, it could be demonstrated that donor organs contain considerable numbers not only of antigen-presenting leucocytes, but also of immunocompetent cells like T, B and natural killer (NK) lymphocytes, which are transferred to the recipient's body by organ transplantation [4,5]. Many of these cells leave the graft and can be detected in the peripheral circulation for several days, but they can also persist in tissues for longer periods [6,7]. The immunocompetence of these cells could be demonstrated in vitro and is also evident from a number of cases of graft-versus-host disease following clinical organ transplantation in vivo [8–10].
These observations demonstrate that the immune reactions occurring particularly in the early phase after organ transplantation are basically bilateral and are therefore not adequately represented by a standard one-way MLR. Thus, it was the aim of this study to analyse the course of bilateral immune reactions of allogeneic mononuclear cells using a two-way MLC in vitro system. Very early studies have already suggested that the two-way MLR may be qualitatively different from the corresponding one-way MLR [11]. In the present study it was therefore analysed how two populations of allogeneic cells interact during co-culture, how these cell populations develop over time, what initial conditions determine the outcome of the culture, and what the effect of immunosuppression on this interaction is. These studies could be a basis for further understanding of the complex immunological interactions occurring after organ transplantation.
MATERIALS AND METHODS
Cell preparations, antibodies, and culture media
Peripheral blood mononuclear cells (PBMC) were separated by Ficoll–Paque density gradient centrifugation from HLA-typed buffy coats obtained from healthy blood donors at the blood bank of the Medizinische Hochschule Hannover. Combinations of allogeneic cells used for the two-way MLC were selected on the basis of an HLA class 1 mismatch detectable by one of the available MoAbs; apart from that the combinations represented random matches of their HLA pattern.
For staining and cell separation various MoAbs were used. Antibodies against CD2 (Leu-5b), CD3 (Leu-4), CD4 (Leu-3a+3b), CD8 (Leu-2a), CD20 (Leu-16), and HLA-DR were obtained from Becton Dickinson (Heidelberg, Germany); against CD45RA, CD14 and CD56 from Serotec (Oxford, UK); and against CD45RO/UCHL1 and CD25/ACT-1 from Dako (Glostrup, Denmark). All antibodies were labelled either with FITC or PE. MoAbs against polymorphic HLA class I antigens were purified from culture supernatant of the hybridomas HB54, HB59, and HB122 recognizing HLA A2 and B17, B7 and B40, and A3, respectively. Hybridomas had been obtained from the American Type Culture Collection (ATCC, Rockville, MD). Antibodies against CD16 for panning of NK cells were obtained from culture supernatant of hybridoma 368H4SN kindly provided by R. E. Schmidt (Hannover, Germany).
Separation of PBMC into CD2+ and CD2− cells was performed by E-rosette formation with sheep erythrocytes. In brief, PBMC were mixed with neuraminidase (Behringwerke AG, Marburg, Germany)-treated sheep erythrocytes (BAG, Lich, Germany) and incubated at 37°C for 10 min, centrifuged (15 min, 200 g) and the cell sediment incubated on ice for another 30 min. Afterwards cells were resuspended in TC199 medium, underlayed with Ficoll–Hypaque (Pharmacia, Freiburg, Germany) and centrifuged at 400 g for 30 min. To obtain CD2+ cells sheep erythrocytes were removed by incubation with 7 ml lysis reagent (0.14 m NH4Cl, 0.25 mm EDTA, 5 mm KHCO3, pH 7.5) at 37°C for 5–10 min and subsequent washing; CD2− cells were harvested from the interface and washed.
Further depletion of cell populations was performed by antibody-mediated plastic adhesion (panning). To this end, 30 × 106 CD2+ cells were incubated with anti-CD4 or anti-CD8 MoAbs (at concentrations of 10 μg/ml) on ice for 30 min. Anti-CD16 antibody for panning proved to be optimal at a final 1:10 dilution of the culture supernatant and was used for the experiments at this dilution. After washing, 30 × 106 cells were incubated in goat anti-mouse (GAM)-coated Petri dishes at 4°C for 2 h. Non-binding cells were removed after shaking the dishes carefully. Control staining after panning of CD4+ cells showed that 80–85% were CD8+, after panning of CD8+ cells 90–95% were CD4+. Efficacy of depletion of NK cells by panning of CD16+ cells was controlled by staining for CD56; the content of these cells was reduced from a mean of 9.5% before panning to a mean of 0.8% after panning.
All cell cultures were performed in RPMI 1640 supplemented with 200 mml-glutamine, 50 mm 2-mercaptoethanol (2-ME), 20 mm HEPES buffer, 100 U/ml penicillin, 100 μg/ml streptomycin, and 10% heat-inactivated fetal calf serum (FCS) at 37°C in a humidified chamber containing 5% CO2. For some experiments, PBMC were prestimulated by 15 ng/ml anti-CD3 MoAb OKT3 (Muromonab-CD3; Ortho, Raritan, NJ). IL-2 (Biotest, Dreieich, Germany) was added to the medium of some cultures at a final concentration of 20 U/ml. Cyclosporin was provided by Novartis (Nürnberg, Germany); it was dissolved in ethanol, diluted with culture medium, and used at final concentrations between 50 and 400 ng/ml. Cyclosporin-containing medium was removed on day 2 and exchanged for fresh culture medium without cyclosporin.
Two-way MLC
Two-way MLC were set up using various types of cells, i.e. whole blood mononuclear cells, CD2+ cells, CD2+CD16− cells, CD2+ CD4− cells, CD2+ CD8− cells, or cells preactivated by OKT3 stimulation. A total number of 2 × 107 cells was cultivated in cell culture flasks in 20 ml of culture medium with or without 20 U/ml IL-2. Medium was exchanged when its colour changed to yellow, usually once or twice per week. Initial cell ratios between the two populations of allogeneic cells were variable, as indicated in the respective experiments. Cell counting error in the 50:50 setup was < 3%. Cell cultures were analysed for composition of cell populations and subsets at various time points using 21 days as end point.
For proliferation assays and determination of cell numbers after different periods of culture, 2 × 105 cells/well were cultured in 200 μl culture medium in triplicates using 96-well U-shaped microtitre plates. In the one-way MLC, 105 cells, ‘responder’ cells, were mixed with 105 irradiated (30 Gy) allogeneic ‘stimulator’ cells; in the two-way MLC, 105 cells of each of the two allogeneic populations were mixed. Following 2, 4, 6, 8, 10 and 14 days of culture 3H-thymidine (0.5 μCi/well, 5 Ci/mmol; NEN, Dreieich, Germany) was added and plates were incubated for 8 h. Cells were then harvested with an automatic cell harvester (Pharmacia LKB, Uppsala, Sweden). Activity was measured in a liquid scintillation counter (LKB Wallac, Turku, Finland). The data are given as mean ct/min of triplicates ± s.d.
Flow cytometry
The two allogeneic populations in each culture were differentiated by two-colour flow cytometry using MoAbs to polymorphic HLA class I epitopes and directly labelled MoAbs to various differentiation antigens. In brief, after incubation with a human immunoglobulin preparation for Fc receptor blocking, cell suspensions were first incubated with the respective anti-HLA class I antibodies at previously determined optimal staining concentrations at 4°C for 30 min. Secondary antibodies were FITC-coupled goat anti-mouse IgG. A second round of staining was then performed with directly labelled PE-coupled antibodies to various cell differentiation antigens at optimal staining concentrations or by propidium iodide (Sigma Chemicals, Deisenhofen, Germany). Cells incubated with irrelevant isotype-identical MoAbs were used as negative controls. For flow cytometric analysis, a gate was set to include all lymphocytes including blast cells based on forward and side scatter characteristics, and parameters of 20 000 events were accumulated into a list mode file.
To determine the degree of apoptosis in the culture, terminal deoxynucleotidyl transferase (TUNEL) staining was carried out. In brief, 2 × 106 cells were transferred into microtitre plates at different times after culture. After 30 min of fixation with 4% paraformaldehyde solution, cells were permeabilized with 0.1% Triton X-100 in 0.1% sodium citrate for 2 min on ice. Freshly prepared TUNEL reaction mixture (Boehringer, Mannheim, Germany) was added and incubation for 60 min at 37°C in a humidified atmosphere followed. Cells incubated with a reaction solution without terminal transferase were used as negative controls, and permeabilized cells in the presence of DNase I (Sigma) served as positive controls. By flow cytometry, a total number of 20 000 lymphocytes was accumulated into a list mode file, and the ratio of apoptotic cells was determined.
Statistical analysis
Statistical analysis was performed by Student's t-test. P < 0.05 was regarded as significant.
RESULTS
Functional characteristics of two-way MLC
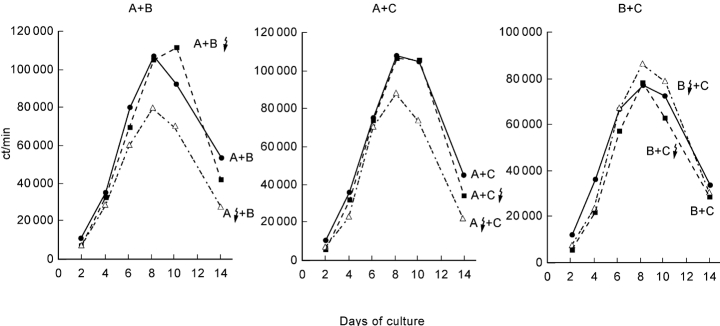
The kinetics of cell proliferation in the two-way MLC compared with the one-way MLC were similar. Maximum proliferation usually occurred on day 7–8 of the culture and decreased thereafter both in the absence and in the presence of exogenous IL-2. The extent of proliferation was usually comparable to that in each of the corresponding one-way MLC. There was no additive proliferative response in the two-way MLC, although the initial number of viable cells was twice as high as in the two one-way MLC. Representative examples of the proliferative responses in three combinations of two-way MLC (2 × 105 viable MNC) in comparison with the corresponding one-way MLC (105 viable MNC) are shown in Fig. 1.
Fig. 1.
Kinetics of proliferative response in two-way versus the corresponding one-way MLC in the presence of IL-2. Representative results from three different combinations of allogeneic cells are shown. Results are shown as mean ct/min of triplicates for different periods of culture.
Population dynamics in long-term two-way MLC
All of the allogeneic cell populations used had a random HLA match, the only bias being that combinations were selected that could be differentiated by one of the available MoAbs against HLA class I antigens. Starting with a 50:50 ratio of unseparated allogeneic mononuclear cells a major shift in the two cell populations could be observed after 3 weeks of culture in almost all cases. One of the two populations dominated while the other was strongly diminished or had disappeared in 78 out of 80 cultures studied (97.5%), particularly in the presence of IL-2 (Fig. 2). Only in two cases did the proportion of the two populations stay in equal balance for the entire co-cultivation period. Repeat assays with the cells from the same individuals gave identical results in seven out of seven combinations tested.
Fig. 2.
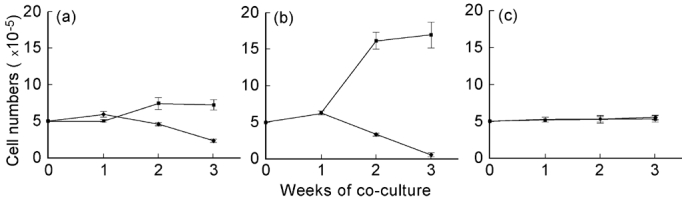
Development of cell numbers in two allogeneic populations during two-way MLR (a) with normal cell culture medium, (b) in the presence of 20 U/ml IL-2, and (c) in the presence of 400 ng/ml cyclosporin plus 20 U/ml IL-2. Cell populations had an initial ratio of 50:50 and were analysed by flow cytometry using MoAbs to polymorphic HLA class I antigens differing between the two populations.
Following the development of the two allogeneic cell populations over time, stable conditions were observed during the first 5–7 days of culture. Only after this period did the decrease of one population and the (relative and absolute) increase in the other begin. Numbers of viable cells in the two-way MLC over the 3 weeks of culture changed from initially 10 × 106 (= 2 × 105) cells to a total mean of 9.5 ± 0.9 × 106 cells under standard culture conditions and to a mean of 17.5 ± 1.8 × 106 cells after addition of exogenous IL-2 (Fig. 2).
When mononuclear cells from three to five different individuals (e.g. A, B, C and D) were used to analyse the outcome in the various combinations of co-cultures (e.g. A + B, A + C, A + D, B + C, B + D, and C + D), a hierarchy of domination could be recognized in most cases (e.g. C > A ≥ D > B).
Co-cultivating two populations of unseparated mononuclear cells at a starting ratio of 20:80, the larger population always dominated (10 out of 10 experiments) irrespective of which population had dominated when identical (i.e. 50:50) starting conditions were used. Figure 3 shows a representative example of the outcome of two-way MLC after 3 weeks using different starting conditions. With an initial cell ratio (A:B) of either 50:50 or 80:20 population A dominated over B completely after 3 weeks of culture; with a starting ratio of 20:80, however, B dominated after that period.
Fig. 3.
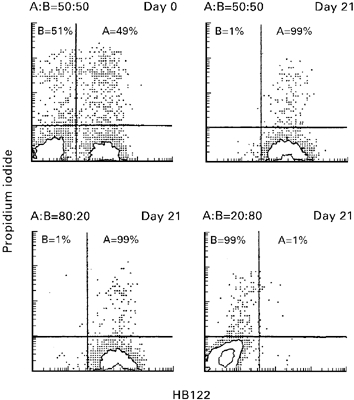
Effect of initial ratio of the two allogeneic populations (A, HLA-A3+; B, HLA-A3−) of CD2+ cells on population dynamics after 3 weeks. With an initial ratio on day 0 of 50:50 only population A survived after 3 weeks, while B disappeared. An initial ratio of 80:20 also led to domination of A, while an initial ratio of 20:80 led to a domination of B and disappearance of A after 3 weeks. Two-colour flow cytometric analysis was performed by staining with anti-HLA-A3-specific antibody H122 (abscissa) and propidium iodide (ordinate).
Effect of subpopulations and preactivation on population dynamics
Analysis of the subset composition of the populations in the two-way MLR revealed an association of initially higher numbers of CD8+ cells and of CD45RO+ cells (difference ≥ 5%) with dominance after co-culture. This association was observed in 10 out of 14 combinations studied for this parameter. However, in two out of the 14 cases domination also occurred with identical numbers of these cell subsets in both allogeneic populations. In another two out of the 14 combinations the population containing lower numbers of CD8+ cells dominated. The findings suggest that these cell populations may play an active role in the domination process, but that excess of these cell populations is not an absolute prerequisite for domination.
To study the role of the subpopulations in the domination process in more detail, depletion of various subpopulations from mononuclear cells was performed, and the effect of this depletion on the domination/elimination process was studied. Depletion of CD2− cells, i.e. mainly of B cells and monocytes/macrophages, by E-rosette formation with sheep erythrocytes did not influence the population changes at all. Moreover, depletion of NK cells from CD2+ cells by panning with an anti-CD16 antibody did not have any detectable effect. In contrast, depletion of either CD4+ or CD8+ lymphocytes from CD2+ cells was very critical. In these analyses, separated populations were not completely pure, but CD2+CD4− cells contained 80–85% CD8+ cells and CD2+CD8− cells were 90–95% CD4+. As shown in Table 1, population A dominated over B when CD2+ cells were used on both sides. Depletion of CD4+ cells from the ‘weaker’ population B as well as depletion of CD8+ cells from the ‘stronger’ population A switched the direction of population change, and cells from B finally dominated over A. Nine additional experiments gave identical results, indicating that the population containing markedly higher numbers of CD8+ cells eliminates the other. Using unequal starting conditions of 20:80 in the same allogeneic combination a depletion of CD8+ cells from the larger population led to domination of the smaller population (Table 1); this again demonstrates the functional importance of CD8+ cells for the domination/elimination effect in the co-culture.
Table 1.
Domination/elimination effects in two-way MLC over 21 days for various cell populations and starting conditions (relative cell numbers at days 0 and 21 for populations A and B, representative experiment)
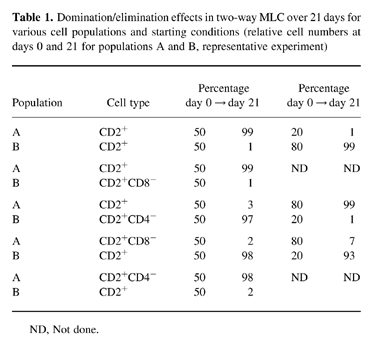
ND, Not done.
Incubation of mononuclear cells with 15 ng/ml OKT3 for 4 days prior to the two-way MLC caused a relative increase in the number of CD8+ cells to 41.0 ± 4.2%, expression of CD45RO to 73.5 ± 13.4%, of CD25 to 74.5 ± 9.1%, and of HLA-DR to 43.0 ± 4.2%, demonstrating marked T cell activation. These preactivated cells dominated freshly isolated mononuclear cells even when they made up only 5% of cells at the start of the two-way MLC (Fig. 4). This effect occurred irrespective of the direction of population change that was observed in the co-culture using resting cells and 50:50 starting conditions.
Fig. 4.
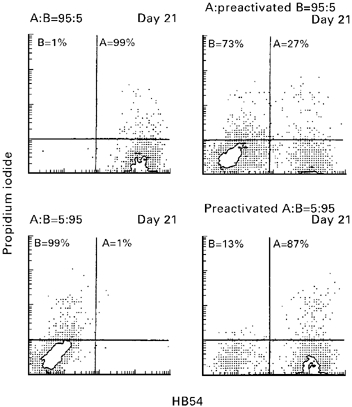
Population dynamics in two-way MLC using a population of preactivated cells. When resting CD2+ cells of A (HLA-A2+) and B (HLA-A2−) were used at relative ratios of 5:95 or 95:5 the larger population always dominated over the smaller one. In contrast, when cells preactivated by 4-day stimulation with 15 ng/ml OKT3 were use at a 5:95 ratio, those cells always dominated the larger population in both directions. Two-colour flow cytometric analysis was performed by staining with anti-HLA-A3-specific antibody H54 (abscissa) and propidium iodide (ordinate).
Influence of cyclosporin and IL-2 in the two-way MLC
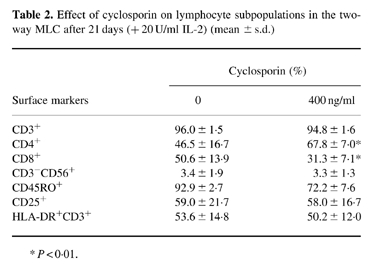
Addition of cyclosporin at concentrations between 50 and 400 ng/ml could block the domination/elimination process in the two-way MLC. These cyclosporin concentrations are in the range of the drug levels achieved in vivo after clinical organ transplantation; they are known to inhibit T cell activation and proliferation as well as development of cytotoxic cells markedly. Interestingly, the blocking effect of cyclosporin was also maintained in the presence of exogenous IL-2. In these cultures the 50:50 ratio of allogeneic cells remained stable over 3 weeks in eight out of nine combinations where domination was found in the absence of cyclosporin. Subset compositions after 3 weeks of co-culture in two-way MLC with and without cyclosporin and IL-2 are shown in Table 2. This shows that considerable cell activation had occurred under these conditions in terms of IL-2 receptor (CD25) expression, HLA-DR expression on T cells and expression of CD45RO. One major difference that could be observed in the presence of cyclosporin was a predominance of CD4 cells, whereas CD8 dominated in the absence of the drug.
Table 2.
Effect of cyclosporin on lymphocyte subpopulations in the two-way MLC after 21 days (+ 20 U/ml IL-2) (mean ± s.d.)
*P < 0.01.
Cell death and apoptosis in the two-way MLC
In the standard two-way MLC with a 50:50 ratio of allogeneic cells, staining by propidium iodide revealed a mean of 1.4% positive cells at the beginning of the culture. This number then increased to 25% on day 7, to 32% on day 14, and to 43% on day 21.
Analysis of apoptosis by TUNEL staining revealed a peak on day 7 (68% of all lymphocytes). In the presence of cyclosporin this peak disappeared (Fig. 5), suggesting marked inhibition of apoptosis under these conditions.
Fig. 5.
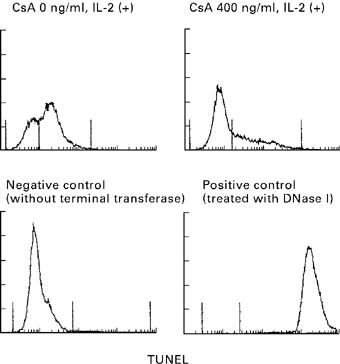
Apoptosis in two-way MLC in the presence and absence of cyclosporin. In the standard two-way MLC supplemented with IL-2 a considerable number of cells showing terminal deoxynucleotidyl transferase (TUNEL) staining could be detected on day 7 of culture; this staining was markedly diminished in the culture containing cyclosporin (CsA).
DISCUSSION
In contrast to the straightforward setup of a one-way MLR with one (rather passive) stimulator and one active responder cell population, the interactions occurring in a two-way setting are much more complex. This is already suggested by the observation that the proliferative response in the two-way MLR is not just a summation of two one-way MLR. Although the number of viable immunocompetent cells in the two-way culture was twice as high as in the corresponding one-way cultures, the proliferative response was similar to that of the corresponding one-way MLR. This suggests that the bilateral interactions interfere with activation of the two populations. This might be due to cytokine effects, to cytotoxic effector functions of alloreactive T cells and NK cells on both sides, to a different extent of apoptosis in the two populations, or to a combination of these mechanisms.
A central finding of the study was that under standard conditions human two-way mixed leucocyte cultures are unstable over time. With very few exceptions, one of the two populations markedly decreased or disappeared during 3–4 weeks of co-culture, while the other population survived and proliferated strongly. A similar observation has recently been described for two-way MLR of cattle PBMC; cells resulting from the culture after 3 weeks were predominantly of one an animal [12]. Although details of the domination/elimination process were not analysed in that study, these findings suggest that considerable population shifts may be a general phenomenon under the conditions of the two-way MLC.
Reciprocal studies of one-way MLR have shown that the immune reactions occurring in the two directions usually differ in strength [3]. Therefore, it is very likely that the bilateral interactions in the two-way culture are also unequal in their relative intensity, which could explain the development of a disequilibrium. Unequal strength of the proliferative response in the corresponding one-way MLC was also observed in our experiments, but the findings were not generally predictive of the outcome of the cultures observed under two-way conditions. An inferior extent of proliferative response, however, does not preclude a dominant cytotoxic response. Since the relative numbers of cytotoxic precursor cells for the respective alloantigens in the two populations have not been studied, it could well be that there would have been a better correlation for this parameter.
Domination of one population and elimination of the other was markedly accelerated by addition of exogenous IL-2 to the two-way culture. In contrast, cultures remained in equilibrium for the whole period when cyclosporin was added. Since cyclosporin is known to interfere with T cell activation, particularly by blocking transcription of the gene for IL-2 [13], this cytokine seems to be critical for the mechanisms associated with the domination/elimination process. Remarkably, the stabilizing effect of cyclosporin also persisted in the presence of exogenous IL-2, suggesting that a cyclosporin effect separate from blocking transcription and production of IL-2 seems to be involved.
Stabilization of the co-cultures in the presence of cyclosporin could be explained by two mechanisms. One could be an interference with the development of cytotoxic cells in the dominating population; this is suggested by a predominant activation and expansion of CD4+ lymphocytes in both cultures in the presence of cyclosporin. The other mechanism could be inhibition of apoptosis in the population that would have been eliminated. Cyclosporin is able to block activation-associated apoptosis of lymphocytes [14] and markedly decreased apoptosis could also be found in the two-way cultures in the presence of cyclosporin. Apoptosis, therefore, appears to be an important mechanism for the development of imbalances between the two populations under two-way MLR conditions.
CD8+ cells also seem to play an important role in the elimination process. The population containing higher numbers of CD8+ cells usually dominated, and while depletion of CD8+ cells frequently led to elimination of the respective population. In contrast, depletion of CD16+ NK cells, like depletion of CD2− cells, from either population did not affect the domination process at all. This suggests that the domination/elimination process in the two-way MLC is mainly based on the activity of cytotoxic T lymphocytes, while NK cells and other cells in the culture seem to be of much less relevance. That NK cells play no major role in this system is also suggested by the kinetics of the elimination process. The cultures are stable for the first 5–7 days, and the domination/elimination process starts during the second week of culture. This is well compatible with the kinetics of elimination by cytotoxic T lymphocytes, but not by NK cells, which kill much more rapidly. The marked decrease of NK cells that occurred in the cultures by the second week, i.e. when elimination started, also argues strongly against a contribution of NK cells.
Thus, the mechanisms by which allogeneic mononuclear cells are eliminated in our in vitro culture model seem to differ from the mechanisms that have been shown to be responsible for elimination of allogeneic haematopoietic cells in vivo. After injection of allogeneic haematopoietic cells in vivo, elimination from the circulation occurs much more rapidly, i.e. usually within several hours to a few days [15,16]. Several studies, particularly in rodent models, suggest that this elimination process in vivo depends on NK cells. Expression of allogeneic MHC class I antigens on the allogeneic haematopoietic cells leads to activation of the recipient's NK cells by inadequate triggering of killing-inhibiting receptors [17,18]. It has also been demonstrated in rodent models, however, that survival of allogeneic haematopoietic cells can be considerably prolonged by treatment with tacrolimus, a drug which strongly interferes with IL-2 production—similar to cyclosporin [19]. Since this treatment does not lead to a marked inhibition of NK cell activity, it is very likely that cytotoxic T lymphocytes also play an important role in elimination in vivo. This effect is also in agreement with our in vitro observations where cell survival in co-culture was markedly prolonged by cyclosporin. Moreover, persistence of donor lymphocytes after liver transplantation appeared markedly enhanced in a patient shortly after bone marrow transplantation that had normal NK function but still inadequate cytotoxic T cell function at the time of liver transplantation [6,10]. Nevertheless, the much more rapid elimination of allogeneic haematopoietic cells in the human in vivo suggests that different mechanisms may be involved in vivo compared with the in vitro model. It must be kept in mind, hovever, that disappearance of injected allogeneic cells from the circulation in vivo does necessarily indicate their destruction. Instead, they may just have left the blood stream and migrated into certain tissues.
The typical in vivo situation after injection or transfer of allogeneic cells is not adequately described by a 50:50 ratio of allogeneic cells in the two-way MLC. In the clinical situation, there is always a imbalance of cell numbers in favour of the recipient's cells. After unequal starting conditions in our in vitro model, the larger population dominated the smaller one—but only when resting unseparated cells or CD2+ cells were used. The domination of the larger population could be reversed if the smaller population consisted predominantly of CD8+ cells or—even more impressive—if the cells had been preactivated. Particularly under the latter conditions, 5% of cells in the co-culture were sufficient to dominate over 95% of (resting) allogeneic mononuclear cells. Again, this supports the hypothesis that elimination of allogeneic cells in this culture model is an active process. Furthermore, it clearly demonstrates that under certain conditions small numbers of immunocompetent cells may have a considerable functional relevance against an excessive number of allogeneic mononuclear cells.
In the clinical transplant situation where allogeneic mononuclear cells are generally transferred to the recipient by a graft, three parameters have to be considered in order to estimate potentially resulting interactions of donor- and recipient-type lymphocytes: first, the number of immunocompetent donor-type cells transferred to the recipient is usually markedly lower than the number of respective cells in the recipient; the numbers are, however, highly variable and may constitute up to 1010 cells in the case of liver or lung transplantation [4,5]; second, passenger cells in donor organs are not resting blood mononuclear cells, but represent a selected population of preactivated (HLA-DR+) and mainly CD8+ and CD45RO+ T lymphocytes [4,5]; and third, the cellular interactions after transplantation occur in the setting of immunosuppression interfering with IL-2 production, i.e. in the presence of either cyclosporin or tacrolimus. Thus, several of the parameters present in the setting of clinical organ transplantation favour prolonged survival of the passenger cells and may thereby enable functional effects of passenger lymphocytes on the recipient's immune system [20–23]. To what extent two-way interactions between allogeneic immunocompetent cells early after organ transplantation are also important for the development of long-term microchimerism, a phenomenon frequently observed after solid organ transplantation, [24–26] remains speculative.
In conclusion, the two-way MLC represents a useful tool to evaluate bilateral interactions between allogeneic immunocompetent cells under various conditions. Although the interactions in vivo will be much more complex than those observed in vitro, the culture model can help to determine parameters that would allow donor lymphocytes to survive in an allogeneic environment and that might enable an immunoregulatory effect of such cells in allograft recipients.
Acknowledgments
This work was supported by Deutsche Forschungsgemeinschaft, Sonderforschungsbereich 265 (project B5). T.S. was a research fellow supported by a grant from the Japanese Ministry of Education.
References
- 1.Bain B, Vas MR, Lowenstein L. The development of large immature mononuclear cells in mixed leukocyte cultures. Blood. 1964;23:108–16. [PubMed] [Google Scholar]
- 2.Bach F, Hirschhorn K. Lymphocyte interaction: a potential histocompatibility test in vitro. Science. 1964;143:813–4. doi: 10.1126/science.143.3608.813. [DOI] [PubMed] [Google Scholar]
- 3.Bach FH, Voynow NK. One-way stimulation in mixed leukocyte cultures. Science. 1966;153:545–7. doi: 10.1126/science.153.3735.545. [DOI] [PubMed] [Google Scholar]
- 4.Schlitt HJ, Raddatz G, Steinhoff G, Wonigeit K, Pichlmayr R. Passenger lymphocytes in human liver allografts and their potential role after transplantation. Transplantation. 1993;56:951–5. doi: 10.1097/00007890-199310000-00033. [DOI] [PubMed] [Google Scholar]
- 5.Richter N, Raddatz G, Steinhoff G, Schäfers HJ, Schlitt HJ. Transmission of donor lymphocytes in clinical lung transplantation. Transplant Int. 1994;7:414–9. [PubMed] [Google Scholar]
- 6.Schlitt HJ, Kanehiro H, Raddatz G, et al. Persistence of donor lymphocytes in liver allograft recipients. Transplantation. 1993;56:1001–7. doi: 10.1097/00007890-199310000-00042. [DOI] [PubMed] [Google Scholar]
- 7.Richter N, Raddatz G, Graeter T, Schäfers HJ, Schlitt HJ. Allogeneic lymphocyte chimerism in lung allograft recipients. Transplant Immunol. 1995;3:74–80. doi: 10.1016/0966-3274(95)80010-7. [DOI] [PubMed] [Google Scholar]
- 8.Burdick JF, Vogelsang GB, Smith WJ, et al. Severe graft-versus-host disease in a liver-transplant recipient. N Engl J Med. 1988;318:689–91. doi: 10.1056/NEJM198803173181107. [DOI] [PubMed] [Google Scholar]
- 9.Jamieson NV, Joysey V, Friend PJ, et al. Graft-versus-host disease in solid organ transplantation. Transplant Int. 1991;4:67–71. doi: 10.1007/BF00336399. [DOI] [PubMed] [Google Scholar]
- 10.Schlitt HJ, Tischler HJ, Ringe B, Maschek H, Dietrich E, Kuse E, Pichlmayr R, Link H. Allogeneic liver transplantation for venoocclusive disease after bone marrow transplantation— clinical and immunological considerations. Bone Marrow Transplant. 1995;16:473–8. [PubMed] [Google Scholar]
- 11.Lawler SD, Ukaejiofo EO, Reeves BR. Interaction of maternal and neonatal cells in mixed-lymphocyte cultures. Lancet. 1975;ii:1185–7. doi: 10.1016/s0140-6736(75)92663-x. [DOI] [PubMed] [Google Scholar]
- 12.Harms JS, Splitter GA. CD8+ lymphocytes that kill allogeneic and xenogeneic major histocompatibility complex class I targets. Hum Immunol. 1995;44:50–57. doi: 10.1016/0198-8859(95)00061-8. [DOI] [PubMed] [Google Scholar]
- 13.Borel JF, Baumann G, Chapman I, Donatsch P, Fahr A, Mueller EA, Vigouret JM. In vivo pharmakological effects of cyclosporin and some analogues. Adv Pharmacol. 1996;35:115–246. doi: 10.1016/s1054-3589(08)60276-8. [DOI] [PubMed] [Google Scholar]
- 14.Labalette M, Queyrel V, Masy E, Noel C, Pruvot FR, Dessaint JP. Implications of cyclosporine in up-regulation of bcl-2 expression and maintenance of CD8 lymphocytosis in cytomegalovirus-infected allograft recipients. Transplantation. 1995;59:1714–23. doi: 10.1097/00007890-199506270-00013. [DOI] [PubMed] [Google Scholar]
- 15.Heslop BF, McNeilage LJ. Natural cytotoxicity: early killing of allogeneic lymphocytes in rats. Immunol Rev. 1979;73:35–51. doi: 10.1111/j.1600-065x.1983.tb01077.x. [DOI] [PubMed] [Google Scholar]
- 16.Rolstad B, Fossum S, Bazin H, Kimber I, Marshall J, Sparshott SM, Ford WL. The rapid rejection of allogeneic lymphocytes by a non-adaptive, cell-mediated mechanism (NK activity) Immunol. 1985;54:127–38. [PMC free article] [PubMed] [Google Scholar]
- 17.Rolstad B, Wonigeit K, Vaage JT. Alloreactive rat natural killer (NK) cells in vivo and in vitro: the role for the major histocompatibility complex. In: Rolstad B, editor. Natural immunity to normal hemopoietic cells. Boca Raton: CRC Press; 1994. pp. 99–149. [Google Scholar]
- 18.Rolstad B, Vaage JT, Naper C, Lambracht D, Wonigeit K, Joly E, Butcher GW. Positive and negative MHC class I recognition by rat NK cells. Immunol Rev. 1997;155:91–104. doi: 10.1111/j.1600-065x.1997.tb00942.x. [DOI] [PubMed] [Google Scholar]
- 19.Iyengar AR, Bonham CA, Antonysamy MA, et al. Striking augmentation of hematopoietic cell chimerism in noncytoablated allogeneic bone marrow recipients by FLT3 ligand and tacrolimus. Transplantation. 1997;63:1193–9. doi: 10.1097/00007890-199705150-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martin DR, Sheng-Tanner X, Miller RG. Rapid and long-term changes to host cytotoxic T lymphocyte precursors reactive to donor antigens caused by intravenous injection of histoincompatible lymphocytes. Transplantation. 1992;54:125–9. doi: 10.1097/00007890-199207000-00022. [DOI] [PubMed] [Google Scholar]
- 21.Thomas JM, Verbanac KM, Carver FM, Kasten-Jolly J, Haisch CE, Gross U, Smith PJ. Veto cells in transplantation tolerance. Clin Transplant. 1994;8:195–203. [PubMed] [Google Scholar]
- 22.Miller RG. An immunological suppressor cell inactivating cytotoxic T-lymphocyte precursor cells recognizing it. Nature. 1980;287:544–6. doi: 10.1038/287544a0. [DOI] [PubMed] [Google Scholar]
- 23.Raddatz G, Deiwick A, Sato T, Schlitt HJ. Inhibition of cytotoxic alloreactivity by human allogeneic mononuclear cells: evidence for veto function of CD2+ cells. Immunol. 1998;94:101–8. doi: 10.1046/j.1365-2567.1998.00480.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Starzl TE, Demetris AJ, Trucco M, et al. Cell migration and chimerism after whole-organ transplantation: the basis of graft acceptance. Hepatology. 1993;17:1127–52. [PMC free article] [PubMed] [Google Scholar]
- 25.Schlitt HJ, Hundrieser J, Ringe B, Pichlmayr R. Donor-type microchimerism associated with rejection eight years after liver transplantation. N Engl J Med. 1994;330:646–7. doi: 10.1056/NEJM199403033300919. [DOI] [PubMed] [Google Scholar]
- 26.Schlitt HJ, Hundrieser J, Hisanaga M, Uthoff K, Karck M, Wahlers T, Wonigeit K, Pichlmayr R. Patterns of donor-type microchimerism after heart transplantation. Lancet. 1994;343:1469–71. doi: 10.1016/s0140-6736(94)92584-4. [DOI] [PubMed] [Google Scholar]