Abstract
Studies performed in mice together with the demonstration of increased levels of heart-specific autoantibodies, cytokines and cytokine receptors in sera from cardiomyopathy (CMP) patients argued for a pathogenic role of autoimmune mechanisms in CMP. This study was designed to analyse the presence of IgG anti-heart antibodies in sera from patients suffering from hypertrophic and dilatative forms of CMP as well as from patients with ischaemic heart disease and healthy individuals. Patients' sera were analysed for IgG reactivity to Western-blotted extracts prepared from human epithelial and endothelial cells, heart and skeletal muscle specimens as well as from Streptococcus pyogenes. The IgG subclass (IgG1–4) reactivity to purified human cardiac myosin was analysed by ELISA. While sera from CMP patients and healthy individuals displayed comparable IgG reactivity to a variety of human proteins, cardiac myosin represented the prominent antigen detected strongly and preferentially by sera from CMP patients. Pronounced IgG anti-cardiac myosin reactivity was frequently found in sera from patients with dilatative CMP and reduced ventricular function. ELISA analyses revealed a prominent IgG2/IgG3 anti-cardiac myosin reactivity in CMP sera, indicating a preferential Th1-like immune response. Elevated anti-cytomegalovirus, anti-enterovirus IgG titres as well as IgG reactivity to nitrocellulose-blotted S. pyogenes proteins were also frequently observed in the group of CMP patients. If further work can support the hypothesis that autoreactivity to cardiac myosin represents a pathogenic factor in CMP, specific immunomodulation of this Th1- towards a Th2-like immune response may represent a promising therapeutic strategy for CMP.
Keywords: cardiomyopathy, autoantibody, cardiac myosin, Th1, autoimmunity
INTRODUCTION
Cardiomyopathy (CMP) represents a prevalent cause of progressive heart disease leading to heart failure [1]. An estimated 20 000 new cases of idiopathic dilated cardiomyopathy (DCMP) are diagnosed annually in the USA and the frequency of familial DCMP is about 30% [2]. Factors thought to be involved in the pathogenesis of CMP include (i) familial and genetic predisposition [3–6], (ii) alterations in Ca2+ handling and β-receptor density [7,8], and (iii) autoimmune mechanisms [9–16].
Support for the concept that immunological mechanisms may be involved in the pathogenesis of certain forms of CMP comes from experimental animal models and data obtained from patients. It could be shown that infection with certain viruses and immunization with cardiac myosin or immunization with cardiac C-protein of genetically predisposed mouse strains results in CMP [17–21] and that also genetically resistant mice can be rendered susceptible to myocarditis by co-treatment with cytokines (i.e. IL-1, tumour necrosis factor (TNF)) [22,23]. Murine cardiac myosin-induced myocarditis represents an organ-specific autoimmune disease mediated by CD4+ T cells that recognize myosin-derived peptides in association with MHC class II molecules and thus trigger the production of autoantibodies [24].
Evidence for the involvement of autoimmune mechanisms in CMP of man comes from (i) the detection of autoantibodies against various heart antigens (e.g. cardiac myosin [14,25], β1-adrenoreceptor [26], acetylcholine receptor-2 [27], mitochondrial membrane ATP carrier [28], heat shock protein 60 [29]) in sera of CMP patients and that such antibodies might even be used as markers for disease severity [30]; (ii) the demonstration of elevated levels of cytokines [31,32] and cytokine receptors [33] in sera from myocarditis and CMP patients; (iii) the fact that autoantibodies may impair cardiac functions [28]; and (iv) the demonstration that depletion of autoantibodies by immunoapheresis resulted in significant clinical improvement in CMP patients [34,35].
In the present study we searched, by immunoblotting, for the presence of IgG autoantibodies directed against human cell line-, heart and skeletal muscle-derived antigens in sera from children, juvenile and adult CMP patients, patients with ischaemic (coronary) heart disease as well as in sera from healthy individuals. Antibody responses to human proteins were compared with those against bacterial (Streptococcus pyogenes) proteins as well as with viruses and analysed in view of cardiac function. In order to gain evidence whether autoimmunity against heart antigens (i.e. cardiac myosin) resembles features of a Th-1 or Th-2 immune response, the IgG subclass (IgG1–4) reactivity to human cardiac myosin, the prominent autoantigen detected by CMP sera, was analysed in detail.
MATERIALS AND METHODS
Demographic and clinical characterization of patients
In the present study we investigated sera from a group of 28 young CMP patients (children/juveniles: 14 females, 14 males; mean age 12 years), a group of eight adult CMP patients (adults: one female, seven males; mean age 51 years) and, for control purposes, a group of nine patients with ischaemic heart disease (coronary heart disease, infarct) (ischaemic patients: eight males, one female; mean age 57.1 years) and 10 healthy individuals (healthy persons: seven females, three males; mean age 33.2 years) (Table 1). The diagnosis of dilated and hypertrophic CMP was based on the criteria of the World Health Organization [36] and included typical echocardiographic/Doppler-echocardiographic measurements. The group of children/juvenile CMP patients comprised 20 patients with hypertrophic and eight patients with DCMP. According to the New York Heart Association (NYHA) criteria, 20 patients of this group were class I, six class II and two class III. Diagnostic procedures in the children/juveniles included echocardiography and Doppler-echocardiography. The thickness of the interventricular septum in the patients with hypertrophic CMP ranged between 5 and 30 mm, the left ventricular end-systolic and end-diastolic dimensions were between 10 and 40 mm and 26 and 64 mm, respectively (Table 1). Among the dilated CMP patients the left ventricular end-systolic and end-diastolic dimensions were between 28 and 49 mm and 37 and 61 mm, respectively, and the left ventricular ejection fraction ranged between 9.7% and 33.3% (Table 1).
Table 1.
Demographic and clinical characterization of individuals (continued on next page)
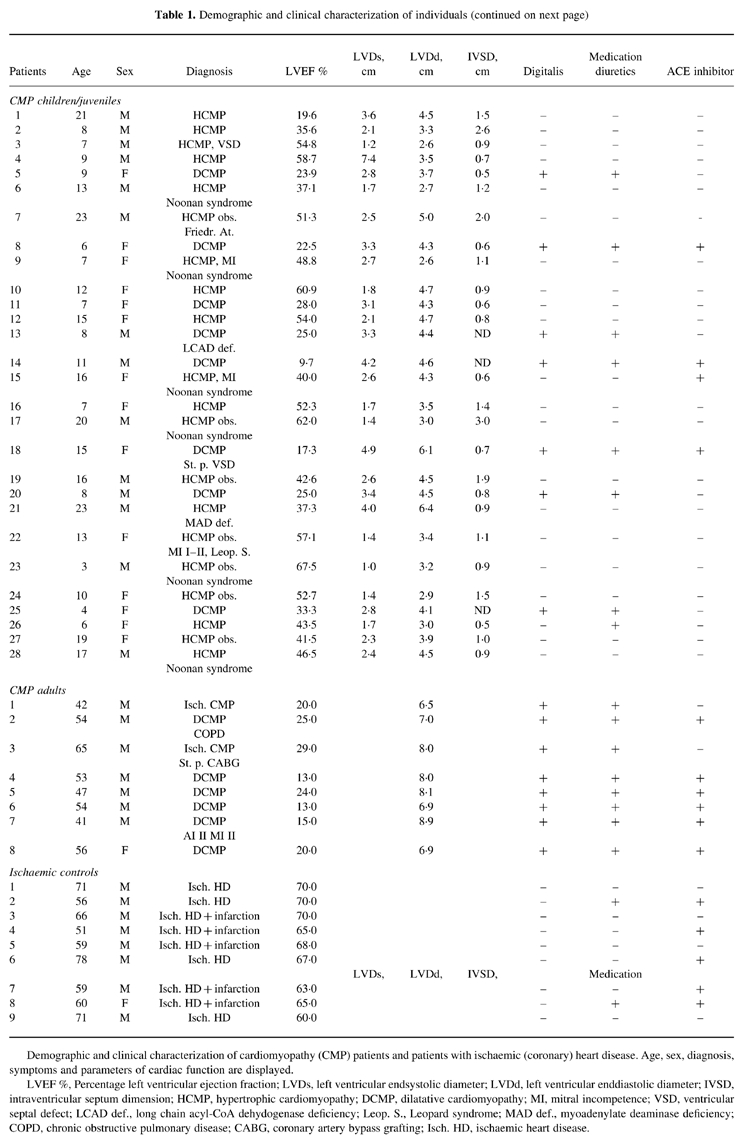
Demographic and clinical characterization of cardiomyopathy (CMP) patients and patients with ischaemic (coronary) heart disease. Age, sex, diagnosis, symptoms and parameters of cardiac function are displayed.LVEF %, Percentage left ventricular ejection fraction; LVDs, left ventricular endsystolic diameter; LVDd, left ventricular enddiastolic diameter; IVSD, intraventricular septum dimension; HCMP, hypertrophic cardiomyopathy; DCMP, dilatative cardiomyopathy; MI, mitral incompetence; VSD, ventricular septal defect; LCAD def., long chain acyl-CoA dehydogenase deficiency; Leop. S., Leopard syndrome; MAD def., myoadenylate deaminase deficiency; COPD, chronic obstructive pulmonary disease; CABG, coronary artery bypass grafting; Isch. HD, ischaemic heart disease.
The group of adult CMP patients (one female, seven males) consisted of six patients with dilated, and two patients with ischaemic CMP. One of the latter two had undergone aortocoronary bypass operation (Table 1). Diagnostic procedures in the adult group included left heart catheterization/ventriculography, coronary arteriography and two-dimensional echocardiography. Five of the adult patients were classified NYHA class III and the three others class IV. The left ventricular ejection fraction in the adult group ranged between 13% and 29% (Table 1). The type of medication (digitalis, diuretics, angiotensin-converting enzyme (ACE) inhibitors) taken by the two groups of patients at the time when clinical and laboratory parameters were determined is displayed in Table 1.
Bacteria, cell lines, tissue specimens and protein extracts
Streptococcus pyogenes strain CCUG (Culture Collection University Götheborg) 514 was cultivated in brain-heart infusion (BHI) broth (Oxoid, Basingstoke, UK) under aerobic conditions at 37°C overnight. Human umbilical vein endothelial cells (HUVEC) were cultured as described previously [37]. The human epithelial cell line A431 derived from an epidermoid mammary carcinoma was obtained from American Type Culture Collection (ATCC, Rockville, MD). Heart and skeletal muscle specimens were from patients undergoing heart transplantation. All cells were washed twice in PBS to remove exogenous proteins and cellular pellets containing approx. 5 × 106 cells each, were lysed in SDS sample buffer [38] (1 ml/cell pellet) and boiled for 10 min. Tissue specimens were immediately frozen in liquid nitrogen, homogenized in SDS lysis buffer using an ultraturrax (Ika, Heidelberg, Germany) and boiled for 10 min. Insoluble particles were removed by centrifugation and protein extracts were stored at −70°C until use. Protein extracts were analysed regarding protein amount and quality by SDS–PAGE [39] and coomassie blue staining [40]. The absence of culture medium-derived bovine proteins from S. pyogenes extracts was confirmed by the lack of reactivity of rabbit antisera to actin, albumin and myosin (Biogenesis, Poole, UK) to the nitrocellulose-blotted extracts. Purified human cardiac myosin was purchased from Biogenesis.
SDS–PAGE, immunoblotting, immunoblot inhibition
According to analytical SDS–PAGE and coomassie staining, comparable amounts of protein extracts were separated by 10% preparative SDS–PAGE (20 cm width) [39]. Proteins were then blotted onto nitrocellulose membranes (Schleicher & Schuell, Dassel, Germany) [41]. Membranes were incubated twice for 5 min and once for 30 min in 50 mm sodium phosphate buffer pH 7.4, containing 0.5% w/v bovine serum albumin (BSA), 0.5% v/v Tween 20, and 0.05% w/v NaN3. Strips were then incubated overnight with patients' sera (1:1000 diluted in this buffer) at 4°C, washed as described above and incubated overnight with 1:2000 diluted rabbit anti-human IgG antibodies (Dako, Glostrup, Denmark) at 4°C. Bound rabbit immunoglobulin was detected with a 1:2000 diluted 125I-labelled donkey anti-rabbit immunoglobulin antiserum (Amersham, Aylesbury, UK) by overnight incubation at room temperature. Strips were washed as above, dried, and exposed to Kodak X-OMAT S films at −70°C using intensifying screens (Kodak, Heidelberg, Germany).
In order to confirm the immunological identity of the 200-kD IgG-reactive band in nitrocellulose-blotted human heart muscle extract with cardiac myosin, 1:1000 diluted serum from a reactive CMP patient was preincubated with 20 μg purified cardiac myosin or, for control purposes, with 20 μg BSA overnight at 4°C. The preabsorbed serum samples were then exposed to nitrocellulose-blotted human heart muscle extract and bound human IgG antibodies were detected as described above.
Virus serology
Serum IgM and IgG antibodies to cytomegalovirus (CMV), measles and mumps virus were measured by ELISA (Behring, Marburg, Germany). The determination of IgM and IgG anti-parvovirus and anti-enterovirus antibodies was performed using ELISA assays from MRL Diagnostics (Cypress, CA) and from Genzyme Virotech GmbH (Rüsselsheim, Germany), respectively. All determinations were carried out as duplicates according to the manufacturers' instructions.
Determination of total serum IgG levels; IgG anti-cardiac myosin subclass ELISA
Total serum IgG levels were determined in all serum samples using a Behring nephelometric analyser according to the manufacturer's instructions and are displayed in mg/dl. ELISA plates (Nunc Maxisorb, Roskilde, Denmark) were coated with purified cardiac myosin dissolved in 0.1 m sodium carbonate buffer pH 9.6 at a concentration of 10 μg/ml overnight at 4°C. Plates were washed with PBS containing 0.05% v/v Tween 20, 0.5% w/v BSA three times and blocked with PBS containing 1% w/v BSA for 2 h at 37°C. Plates were then washed, incubated with 1:100 in PBS 0.05% v/v–Tween 20 0.5% w/v–BSA-diluted sera from CMP patients, and for control purposes, with sera from individuals without blot-detectable anti-cardiac myosin IgG antibodies overnight at 4°C, washed and exposed to 1:1000 diluted mouse anti-human IgG1–4 MoAbs (Pharmingen, San Diego, CA) overnight at 4°C. Bound mouse antibodies were detected with a 1:2000 diluted horseradish peroxidase (HRP)-labelled rabbit anti-mouse immunoglobulin antiserum (Amersham) for 1 h at 4°C. Plates were washed and 2,2′-azino-bis-(3-ethylbenzthiazoline-6-sulphonic acid) solution (Amersham) was added. After incubation for approx. 30 min, the reaction was stopped by addition of 0.32% w/v NaF. The optical density (OD) was determined in an ELISA reader (Dynatech, Denkendorf, Germany). All determinations were performed in duplicates. The results displayed in Table 2 represent the mean OD determined for each CMP serum minus background levels (i.e. mean OD measured in sera from three healthy individuals without blot-detectable anti-cardiac myosin IgG antibodies).
Table 2.
Serological characterization of individuals (continued on next page)
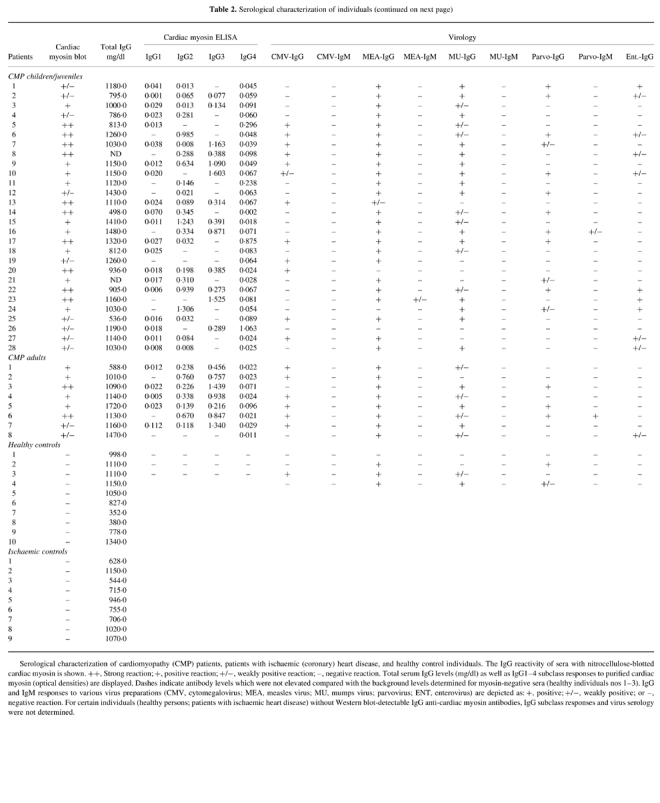
Serological characterization of cardiomyopathy (CMP) patients, patients with ischaemic (coronary) heart disease, and healthy control individuals. The IgG reactivity of sera with nitrocellulose-blotted cardiac myosin is shown. ++, Strong reaction; +, positive reaction; +/−, weakly positive reaction; –, negative reaction. Total serum IgG levels (mg/dl) as well as IgG1–4 subclass responses to purified cardiac myosin (optical densities) are displayed. Dashes indicate antibody levels which were not elevated compared with the background levels determined for myosin-negative sera (healthy individuals nos 1–3). IgG and IgM responses to various virus preparations (CMV, cytomegalovirus; MEA, measles virus; MU, mumps virus; parvovirus; ENT, enterovirus) are depicted as: +, positive; +/−, weakly positive; or –, negative reaction. For certain individuals (healthy persons; patients with ischaemic heart disease) without Western blot-detectable IgG anti-cardiac myosin antibodies, IgG subclass responses and virus serology were not determined.
RESULTS
SDS–PAGE analysis of heart muscle, skeletal muscle, epithelial cell, and endothelial cell-derived protein extracts
SDS–PAGE analysis followed by coomassie blue staining of protein extracts from human heart, skeletal muscle, endothelial and epithelial cells revealed similarities between heart and skeletal muscle as well as between endothelial and epithelial cell extracts (Fig. 1). Heart and skeletal muscle contained prominent bands of approx. 200 kD and 43 kD molecular weight, which by testing with specific antibodies were identified as myosin and actin, respectively (data not shown). The pattern of proteins expressed in endothelial and epithelial cell extracts was different from muscle tissue specimens. Prominent bands of 90 kD and 46 kD were detected in epithelial and endothelial cells, whereas myosin and actin did not constitute major components in these extracts (Fig. 1).
Fig. 1.
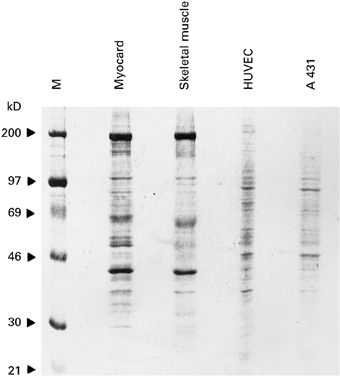
Coomassie blue-stained SDS–PAGE containing human cellular (epithelial cells: A 431; endothelial cells: HUVEC) and tissue-derived (heart, skeletal muscle) protein extracts. M represents the molecular weight marker. Molecular weights are displayed in kD on the left.
CMP patients can be discriminated from patients with coronary heart disease and healthy individuals on the basis of IgG reactivity to cardiac myosin
By Western blotting we searched for the presence of IgG antibodies to human myocard-derived proteins in sera of 36 CMP patients, of nine patients suffering from coronary heart disease and 10 healthy individuals. We found that sera from CMP patients as well as from healthy individuals contained IgG antibodies against a variety of epithelial and endothelial cell-derived proteins ranging between 15 and 200 kD (Fig. 2a,b). CMP patients and healthy persons could not be discriminated on the basis of serum IgG reactivity to certain epithelial or endothelial cell-derived proteins. For example, a 69-kD band was recognized by sera from children/juvenile CMP patients (nos 6, 11, 15–18, 28), by sera from adult CMP patients (nos 2, 7, 8) and by sera from healthy persons (nos 4, 7) in epithelial as well as endothelial cell extracts (Fig. 2a,b). In contrast, only sera from CMP patients, but not from patients with coronary heart disease or healthy individuals, displayed significant IgG reactivity to a 200-kD moiety presumably representing cardiac myosin (Fig. 2c, large arrow). Although several sera from CMP patients (children/juvenile sera: 5, 6, 13–15, 17, 20–22; adults sera: 1, 3 and 6) displayed pronounced IgG reactivity to skeletal muscle myosin at 200 kD (Fig. 2d), cardiac myosin was more frequently and intensively recognized than skeletal muscle myosin. Our contention that the 200-kD moiety detected by IgG from CMP patients in heart muscle extract represents cardiac myosin was supported by the demonstration that purified cardiac myosin, but not BSA, inhibited serum IgG binding of a CMP serum to this band (Fig. 3). The finding that cardiac myosin was more frequently and intensively detected than skeletal muscle myosin indicates that IgG anti-myosin antibodies of CMP patients recognize preferentially cardiac myosin-specific epitopes. Prominent immunoreactive bands of 46 kD in myocard and 46 and 65 kD in skeletal muscle extracts were also observed in the buffer controls (Fig. 2c,d, lanes B). These immunoreactivities probably resulted from the binding of the secondary or tertiary antiserum to human IgG antibodies/antibody fragments present in the tissue specimens.
Fig. 2.
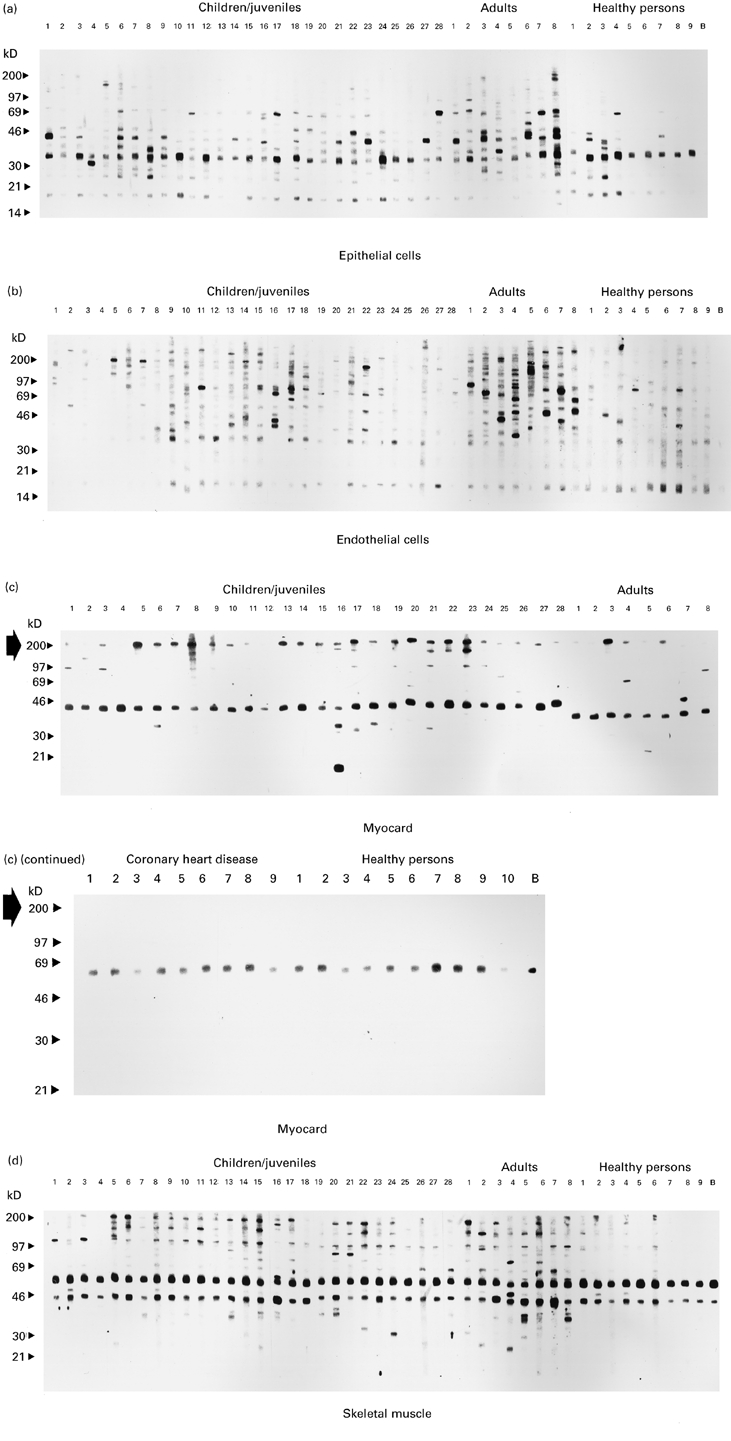
IgG reactivity to nitrocellulose-blotted human epithelial (a), endothelial (b), myocard (c) and skeletal muscle (d) protein extracts. Epithelial, endothelial, and skeletal muscle extracts were exposed to sera from cardiomyopathy (CMP) patients (children/juveniles, lanes 1–28; adults, lanes 1–8) and from healthy individuals (lanes 1–9). Myocard extracts (c) were probed with the same sera and in addition with nine sera from patients with coronary heart disease and one additional healthy person. Lanes B represent the buffer control without addition of serum. Molecular weights are displayed in kD. The large arrow in (c) indicates the position of anti-myosin immunoreactivity.
Fig. 3.
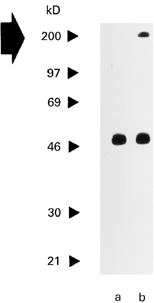
Purified cardiac muscle myosin inhibits serum IgG reactivity of a cardiomyopathy (CMP) patient to Western-blotted heart muscle-derived myosin. Serum from a CMP patient which was preabsorbed with purified cardiac myosin (lane a) or bovine serum albumin (BSA) (lane b) was exposed to nitrocellulose-blotted human heart muscle extract. Molecular weights are displayed on the left. The large arrow indicates the position of cardiac myosin.
CMP patients frequently display IgG reactivity to S. pyogenes and viral proteins
Cross-reactivity of antibodies with streptococcal, enterovirus-derived proteins and human cardiac myosin has been reported previously [42–44]. The cytotoxic effect of such antibodies that cross-reacted with bacterial, viral antigens and cardiac myosin was proposed as a possible mechanism for autoimmune heart disease [42]. We found that sera from CMP patients frequently displayed IgG reactivity to nitrocellulose-blotted streptococcal proteins (Fig. 4). Bands ranging from 40 to 97 kD, a 30-kD and a 14-kD moiety were preferentially recognized by serum IgG from CMP patients. Thirteen of the 36 CMP sera reacted with the 14-kD species, eight bound to the 30-kD moiety and 24 sera displayed pronounced IgG reactivity to the 40–97-kD bands (Fig. 4). Sera from the healthy persons showed weak (40–97 kD) or no (14 kD, 30 kD) reactivity to nitrocellulose-blotted Streptococcus proteins (Fig. 4).
Fig. 4.
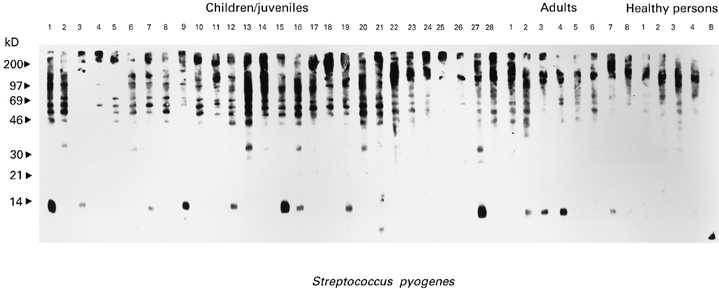
IgG reactivity of sera from cardiomyopathy (CMP) patients (children/juveniles: lanes 1–28, adults: lanes 1–8) and from healthy individuals (lanes 1–4) with nitrocellulose-blotted Streptococcus pyogenus protein extracts. Lane B represents the buffer control without addition of serum. Molecular weights (kD) are displayed on the left.
Eighteen of the 36 CMP sera containing anti-human cardiac myosin antibodies contained elevated IgG anti-CMV titres and 11/36 sera displayed significant IgG levels to enterovirus. In contrast, only one of the four sera from healthy individuals displayed IgG reactivity to CMV and no serum contained significantly elevated anti-enterovirus IgG titres.
CMP patients display a preferential IgG2/IgG3 subclass reactivity to cardiac myosin
While cardiac myosin has been described as autoantigen in CMP patients before [25], nothing was known concerning what subclass human anti-cardiac myosin IgG autoantibodies belong to. The IgG subclass reactivity pattern to a given antigen is of interest, as it reflects the type of underlying T cell response (Th1/Th2 immune response) towards this moiety (e.g. elevated IgG2/IgG3 levels point to a Th1 response) [45]. We therefore tested sera from the 36 CMP patients which we had studied by immunoblotting for IgG subclass reactivity to purified human cardiac myosin by ELISA. Anti-cardiac myosin antibody levels were measured at a serum dilution of 1:100 to allow detection of meaningful antibody titres. Elevated (OD > 0.1) IgG2 and/or IgG3 anti-cardiac myosin antibody titres were measured in 25/36 of the CMP sera. One CMP serum contained elevated IgG1 levels and only 4/36 CMP sera displayed pronounced (OD > 0.1) IgG4 reactivity to cardiac myosin. The number of sera containing significantly elevated IgG2/3 versus IgG4 anti-cardiac myosin antibody levels clearly indicates a preferential Th1-like anti-cardiac myosin immune response in CMP patients.
Pronounced IgG and IgG subclass reactivity to cardiac myosin is frequently found in DCMP patients with reduced ventricular function
IgG reactivity to cardiac myosin as measured by Western blotting and ELISA was observed in patients suffering from dilated, hypertrophic and ischaemic forms of CMP, but not in healthy individuals (Table 2). Seven out of the 28 CMP patients from the group of children and juveniles displayed weak IgG reactivity to blotted and low (OD < 0.1) titres to ELISA plate-bound cardiac myosin. Six of these weakly reacting patients (Tables 1 and 2: nos 1, 2, 12, 19, 27, 28) suffered from hypertrophic cardiomyopathy (HCMP) and one patient (Tables 1 and 2: no. 25) from DCMP with good ventricular function (LVEF: 33.3%). The other seven DCMP patients (Tables 1 and 2: nos 5, 8, 11, 13, 14, 18, 20) mounted significant IgG reactivity either to nitrocellulose-blotted or ELISA plate-bound cardiac myosin and had a strongly reduced ventricular function (Tables 1 and 2). Among the group of adult CMP patients 5/6 DCMP patients with strongly reduced ventricular function (Tables 1 and 2: nos 2, 4, 5, 6, 7) displayed pronounced IgG reactivity to cardiac myosin. In summary, these data indicate that pronounced IgG anti-cardiac myosin reactivity was found frequently in sera from DCMP patients with poor ventricular function.
DISCUSSION
It has been reported that patients suffering from certain forms of CMP (e.g. DCMP) display IgG reactivity against a variety of human proteins (cardiac myosin [14,25], β1-adrenoreceptor [26], acetylcholine receptor-2 [27], mitochondrial membrane ATP carrier [28], heat shock protein 60 [29]). In the present study we investigated sera from 36 patients suffering from various forms of CMP, from nine patients with coronary heart disease and from 10 healthy persons for the presence of IgG autoantibodies to nitrocellulose-blotted human heart muscle extracts. Exposure of sera to epithelial, endothelial cells, skeletal and heart muscle-derived proteins revealed that sera from CMP patients strongly and preferentially displayed IgG reactivity to cardiac myosin and only occasionally bound to other heart muscle proteins of various molecular weights. CMP patients could be discriminated from patients with coronary heart disease and healthy individuals tested only on the basis of IgG anti-cardiac myosin reactivity. Our contention that CMP patients mount specific IgG reactivity to cardiac myosin was supported by two findings: (i) elevated total serum IgG levels were not a prerequisite for serum IgG recognition of nitrocellulose-blotted cardiac myosin; (ii) purified cardiac heart muscle myosin, but not BSA, inhibited serum IgG binding of a CMP patient to nitrocellulose-blotted heart muscle-derived myosin. Pronounced IgG anti-cardiac myosin reactivity was frequently observed in sera from DCMP patients with strongly reduced ventricular function. The tissue specificity of anti-cardiac myosin autoantibodies was supported by two other observations: (i) sera from CMP patients and healthy individuals could not be discriminated on the basis of their IgG reactivity to endothelial or epithelial proteins but only on the basis of IgG anti-cardiac myosin binding; and (ii) more CMP sera reacted with cardiac myosin than with skeletal muscle myosin, indicating that heart muscle myosin-specific epitopes may be the primary immunogen.
There are several possibilities for how autoreactivity to cardiac myosin might be induced. Data from experimental animal models [15,24] as well as in vitro experiments [42–44] suggest viral and bacterial infections as possible trigger factors for CMP. Indeed, we observed elevated anti-enterovirus and anti-CMV titres as well as IgG reactivity to certain streptococcal proteins preferentially among the CMP patients.
Although our data clearly indicate that IgG anti-cardiac myosin antibody responses are confined to patients who have experienced cardiac muscle damage and that pronounced IgG reactivity is frequently found in DCMP patients with strongly impaired ventricular function, data from experimental animal models have argued against a pathogenic role of anti-cardiac myosin antibodies. In this context it was shown that the transfer of anti-cardiac myosin autoantibodies in mice did not cause myocarditis, thus indicating that CD4+ autoreactive T cells rather than autoantibodies contribute to immune-mediated tissue damage [24,46]. By contrast, recent work in man points to a pathogenic role of anti-cardiac myosin antibodies by the demonstration (i) of lack of peripheral T cell responses to cardiac myosin in patients suffering from idiopathic dilated cardiomyopathy [47], (ii) that treatment of CMP patients with intravenously applied immunoglobulin improved clinical symptoms [48] and that depletion of autoantibodies from CMP patients led to a substantial improvement of clinical parameters [34].
One possible answer to the question how myosin, being primarily an intracellular protein, can be reached by autoantibodies, comes from recent work demonstrating that autoantibodies may penetrate into living cells [49,50]. Regarding the mode of antibody-mediated tissue damage, several mechanisms are conceivable. Anti-myosin antibodies may either possess cytotoxic activity, as has been reported for antibodies that cross-reacted between myosin and streptococcal proteins [42], and/or if directed to portions of myosin that are required for contractile functions may influence heart muscle motor functions, as has been exemplified by the description of anti-cardiac myosin hinge region-specific antibodies [51].
Evidence has recently accumulated that organ-specific forms of autoimmunity may be discriminated on the basis of the T cell activation pattern which can involve Th1-like or Th2-like T cells which either secrete interferon-gamma (IFN-γ), TNF-β or IL-4, IL-5, IL-10, IL-13, respectively [52–55]. The phenotype of a preferential Th1- or Th2-like immune response is also reflected by the corresponding antibody subclass reactivity in serum [45,52]. A preferential IgG2/IgG3 antibody profile is indicative of a Th1-like immune response, whereas increased IgG4 levels are associated with a Th2-like immune reaction. This discrimination has recently gained attention in the field of autoimmunity because several studies performed in experimental animal systems suggest that many organ-specific forms of autoimmunity resemble Th1 features and that control of autoimmune disease may be achieved by procedures that regulate the relative contribution of Th1/Th2 CD4+ T cells to an autoimmune response. Notably, there is good evidence that induction of self-antigen-specific Th2 cells can prevent autoimmune disease [52,53]. In the present study we clearly demonstrate that sera from CMP patients display a preferential IgG2/IgG3 antibody response to purified cardiac myosin which would support the concept that CMP resembles features of a Th1-like autoimmune disease. It still remains to be answered if certain of these antibodies may exert pathogenic effects or reflect the activation of Th1-prone autoreactive T cells capable of inducing heart muscle damage. If future studies further support the hypothesis that autoreactivity to cardiac myosin contributes to the initiation and/or perpetuation of disease, and if longitudinal studies indicate that CMP patients with a preferential Th2-like anti-cardiac myosin immune response show a better clinical course, antigen-specific modulation of the Th1-like response towards a Th2 response or perhaps the induction of tolerance/anergy to this very same antigen may be considered as a promising therapeutical strategy.
Acknowledgments
This study was supported in part by grant F0506 of the Austrian Science Foundation and by a grant from Pharmacia & Upjohn, Uppsala, Sweden. We thank Dietrich Kraft for his support during the studies.
References
- 1.Hein S, Schaper J. Pathogenesis of dilated cardiomyopathy and heart failure: insights from cell morphology and biology. Curr Opin Cardiol. 1996;11:293–301. doi: 10.1097/00001573-199605000-00010. [DOI] [PubMed] [Google Scholar]
- 2.Kuhn H, Gietzen F, Beer G, Pethig K. Epidemiology of idiopathic dilated cardiomyopathy. In: Figulla HR, Kandolf R, McManus B, editors. Idiopathic dilated cardiomyopathy: cellular and molecular mechanisms, clinical consequences. Berlin: Springer-Verlag; 1993. pp. 13–25. [Google Scholar]
- 3.Durand JB, Bachinski LL, Bieling LC, et al. Localization of a gene responsible for familial dilated cardiomyopathy to chromosome 1q32. Circulation. 1995;92:3387–9. doi: 10.1161/01.cir.92.12.3387. [DOI] [PubMed] [Google Scholar]
- 4.Tanigawa G, Jarcho JA, Kass S, et al. A molecular basis for familial hypertrophic cardiomyopathy: an α/β cardiac myosin heavy chain hybrid gene. Cell. 1990;62:991–8. doi: 10.1016/0092-8674(90)90273-h. [DOI] [PubMed] [Google Scholar]
- 5.Geisterfer-Lowrance AAT, Kass S, Tanigawa G, et al. A molecular basis for familial hypertrophic cardiomyopathy: a β cardiac myosin heavy chain gene missense mutation. Cell. 1990;62:999–1006. doi: 10.1016/0092-8674(90)90274-i. [DOI] [PubMed] [Google Scholar]
- 6.Sata M, Ikebe M. Functional analysis of the mutations in the human cardiac β-myosin that are responsible for familial hypertrophic cardiomyopathy. J Clin Invest. 1996;98:2866–73. doi: 10.1172/JCI119115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pieske B, Kretschmann B, Meyer M, et al. Alterations in intracellular calcium handling associated with the inverse force-frequency relation in human dilated cardiomyopathy. Circulation. 1995;92:1169–78. doi: 10.1161/01.cir.92.5.1169. [DOI] [PubMed] [Google Scholar]
- 8.Böhm M, Lohse MJ. Quantification of β-adrenoreceptors and β-adrenoreceptor kinase on protein and mRNA levels in heart failure. Eur Heart J. 1994;15:30–34. doi: 10.1093/eurheartj/15.suppl_d.30. [DOI] [PubMed] [Google Scholar]
- 9.Herskowitz A, Neumann DA, Ansari AA. Concepts of autoimmunity applied to idiopathic dilated cardiomyopathy. J Am Coll Cardiol. 1993;22:1385–8. doi: 10.1016/0735-1097(93)90547-e. [DOI] [PubMed] [Google Scholar]
- 10.Caforio ALP, Keeling PJ, Zachara E, et al. Evidence from familial studies for autoimmunity in dilated cardiomyopathy. Lancet. 1994;344:773–7. doi: 10.1016/s0140-6736(94)92339-6. [DOI] [PubMed] [Google Scholar]
- 11.Magnusson Y, Mallukat G, Waagstein F, et al. Autoimmunity in idiopathic dilated cardiomyopathy. Circulation. 1994;89:2760–7. doi: 10.1161/01.cir.89.6.2760. [DOI] [PubMed] [Google Scholar]
- 12.Caforio ALP. Role of autoimmunity in dilated cardiomyopathy. Br Heart J. 1994;72(Suppl):30–34. doi: 10.1136/hrt.72.6_suppl.s30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goldman JH, McKenna WJ. Immunopathogenesis of dilated cardiomyopathies. Curr Opin Cardiol. 1995;10:306–11. doi: 10.1097/00001573-199505000-00012. [DOI] [PubMed] [Google Scholar]
- 14.Caforio ALP, Goldman JH, Haven AJ, et al. Evidence for autoimmunity to myosin and other heart-specific autoantigens in patients with dilated cardiomyopathy and their relatives. Int J Cardiol. 1996;54:157–63. doi: 10.1016/0167-5273(96)02593-4. [DOI] [PubMed] [Google Scholar]
- 15.Penninger JM, Neu N, Bachmaier K. A Genetic map of autoimmune heart disease. The Immunologist. 1996;4/4:131–41. [Google Scholar]
- 16.Penninger JM, Pummerer C, Liu P, et al. Cellular and molecular mechanisms of murine autoimmune myocarditis. APMIS. 1997;105:1–13. doi: 10.1111/j.1699-0463.1997.tb00532.x. [DOI] [PubMed] [Google Scholar]
- 17.O'Donoghue HL, Lawson CM, Reed WD. Autoantibodies to cardiac myosin in mouse cytomegalovirus myocarditis. Immunology. 1990;71:20–28. [PMC free article] [PubMed] [Google Scholar]
- 18.Neumann DA, Rose NR, Ansari AA, et al. Induction of multiple heart autoantibodies in mice with coxsackievirus B3- and cardiac myosin-induced autoimmune myocarditis. J Immunol. 1994;152:343–50. [PubMed] [Google Scholar]
- 19.Penninger JM, Neu N, Timms E, et al. Induction of experimental autoimmune myocarditis in mice lacking CD3 or CD8 molecules. J Exp Med. 1993;178:1837–42. doi: 10.1084/jem.178.5.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Donermeyer DL, Beisel KW, Allen PM, et al. Myocarditis-inducing epitope of myosin binds constitutively and stably to I-Ak on antigen-presenting cells in the heart. J Exp Med. 1995;182:1291–300. doi: 10.1084/jem.182.5.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kasahara H, Itoh M, Sugiyama T, et al. Autoimmune myocarditis induced in mice by cardiac C-protein. Cloning of complementary DNA encoding murine cardiac C-protein and partial characterization of the antigenic peptides. J Clin Invest. 1994;94:1026–36. doi: 10.1172/JCI117416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huber SA, Polgar J, Schultheiss P, et al. Augmentation of pathogenesis of Coxsackievirus B3 infections in mice by exogenous administration interleukin-1 and interleukin-2. J Virol. 1994;68:195–206. doi: 10.1128/jvi.68.1.195-206.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamada T, Matsumori A, Sasayama S. Therapeutic effect of anti-tumor necrosis factor-alpha antibody on the murine model of viral myocarditis induced by encephalomyocarditis virus. Circulation. 1994;89:846–51. doi: 10.1161/01.cir.89.2.846. [DOI] [PubMed] [Google Scholar]
- 24.Rose NR, Hill SL. Autoimmune myocarditis. Int J Cardiol. 1996;54:171–5. doi: 10.1016/0167-5273(96)02595-8. [DOI] [PubMed] [Google Scholar]
- 25.Caforio ALP, Grazzini M, Mann JM, et al. Identification of α- and β-cardiac myosin heavy chain isoforms as major autoantigens in dilated cardiomyopathy. Circulation. 1992;85:1734–42. doi: 10.1161/01.cir.85.5.1734. [DOI] [PubMed] [Google Scholar]
- 26.Magnusson Y, Marullo S, Hoyer S, et al. Mapping of a functional autoimmune epitope on the β1-adrenergic receptor in patients with idiopathic dilated cardiomyopathy. J Clin Invest. 1990;86:1658–63. doi: 10.1172/JCI114888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fu LX, Magnusson Y, Bergh CH, et al. Localization of a functional autoimmune epitope on the muscarinic acetylcholine receptor-2 in patients with idiopathic dilated cardiomyopathy. J Clin Invest. 1993;91:1964–8. doi: 10.1172/JCI116416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schulze K, Becker BF, Schauer R, et al. Antibodies to ADP-ATP carrier—an autoantigen in myocarditis and dilated cardiomyopathy—impair cardiac function. Circulation. 1990;81:959–69. doi: 10.1161/01.cir.81.3.959. [DOI] [PubMed] [Google Scholar]
- 29.Latif N, Baker CS, Dunn MJ, et al. Frequency and specificity of antiheart antibodies in patients with dilated cardiomyopathy detected using SDS–PAGE and Western blotting. J Am Coll Cardiol. 1993;22:1378–84. doi: 10.1016/0735-1097(93)90546-d. [DOI] [PubMed] [Google Scholar]
- 30.Maisch B. Anticardiac antibodies in hypertrophic cardiomyopathy as a marker of severity. Postgrad Med J. 1992;68(Suppl. 1):29–35. [PubMed] [Google Scholar]
- 31.Matsumori A, Yamada T, Suzuki H, et al. Increased circulating cytokines in patients with myocarditis and cardiomyopathy. Br Heart J. 1994;72:561–6. doi: 10.1136/hrt.72.6.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matsumori A. Cytokines in myocarditis and cardiomyopathies. Curr Opin Cardiol. 1996;11:302–9. doi: 10.1097/00001573-199605000-00011. [DOI] [PubMed] [Google Scholar]
- 33.Limas CJ, Goldenberg IF, Limas C. Soluble interleukin-2 receptor levels in patients with dilated cardiomyopathy. Correlation with disease severity and cardiac autoantibodies. Circulation. 1995;91:631–4. doi: 10.1161/01.cir.91.3.631. [DOI] [PubMed] [Google Scholar]
- 34.Dörffel WV, Felix SB, Wallukat G, et al. Short-term hemodynamic effects of immunoadsorption in dilated cardiomyopathy. Circulation. 1997;95:1994–7. doi: 10.1161/01.cir.95.8.1994. [DOI] [PubMed] [Google Scholar]
- 35.Wallukat G, Reinke P, Dörffel WV, et al. Removal of autoantibodies in dilated cardiomyopathy by immunoadsorption. Int J Cardiol. 1996;54:191–5. doi: 10.1016/0167-5273(96)02598-3. [DOI] [PubMed] [Google Scholar]
- 36.Brandenburg RO, Chazov E, Cherian G, et al. Report of the WHO/ISFC task force on definition and classification of cardiomyopathies. Circulation. 1981;64:437A–438A. [Google Scholar]
- 37.Jaffe EA, Nachman RL, Becker CG, et al. Culture of human endothelial cells derived from umbilical veins. J Clin Invest. 1973;52:2745–51. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–5. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 39.Fling SP, Gregerson DS. Peptide and protein molecular weight determination by electrophoresis using a high molarity Tris buffer system without urea. Anal Biochem. 1986;155:83–88. doi: 10.1016/0003-2697(86)90228-9. [DOI] [PubMed] [Google Scholar]
- 40.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 41.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cunningham MW, Antone SM, Gulizia JM, et al. Cytotoxic and viral neutralizing antibodies crossreact with streptococcal M protein, enteroviruses, and human cardiac myosin. Proc Natl Acad Sci USA. 1992;89:1320–4. doi: 10.1073/pnas.89.4.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kil KS, Cunningham MW, Barnett LA. Cloning and sequence analysis of a gene encoding a 67-kilodalton myosin-cross-reactive antigen of Streptococcus pyogenes reveals its similarity with class II major histocompatibility antigens. Infect Immun. 1994;62:2440–9. doi: 10.1128/iai.62.6.2440-2449.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Keeling PJ, Tracy S. Link between enteroviruses and dilated cardiomyopathy: serological and molecular data. Br Heart J. 1994;72(Suppl.):25–29. doi: 10.1136/hrt.72.6_suppl.s25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.German T, Bongartz M, Dlugonska H, et al. Interleukin-12 profoundly up-regulates the synthesis of antigen-specific complement-fixing IgG2a, IgG2b and IgG3 antibody subclasses in vivo. Eur J Immunol. 1995;25:823–9. doi: 10.1002/eji.1830250329. [DOI] [PubMed] [Google Scholar]
- 46.Neu N, Ploier B, Ofner C. Cardiac myosin-induced myocarditis: heart autoantibodies are not involved in the induction of the disease. J Immunol. 1990;145:4094–100. [PubMed] [Google Scholar]
- 47.Bromelow KV, Souberbielle B, Alavi A, et al. Lack of T cell response to cardiac myosin and a reduced response to PPD in patients with idiopathic dilated cardiomyopathy. J Autoimmun. 1997;10:219–27. doi: 10.1006/jaut.1996.0119. [DOI] [PubMed] [Google Scholar]
- 48.McNamara DM, Rosenblum WD, Janosko KM, et al. Intravenous immune globulin in the therapy of myocarditis and acute cardiomyopathy. Circulation. 1997;95:2476–8. doi: 10.1161/01.cir.95.11.2476. [DOI] [PubMed] [Google Scholar]
- 49.Alarcon-Segovia D, Ruiz-Arguelles A, Llorente L. Broken dogma: penetration of autoantibodies into living cells. Immunol Today. 1996;17:163–4. doi: 10.1016/s0167-5699(96)90258-3. [DOI] [PubMed] [Google Scholar]
- 50.Yanase K, Smith RM, Jarett L, et al. Receptor-mediated cellular entry of nuclear anti-DNA antibodies via myosin 1. J Clin Invest. 1997;100:25–31. doi: 10.1172/JCI119517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Margossian SS, Krueger JW, Sellers JR, et al. Influence of the cardiac myosin hinge region on contractile activity. Proc Natl Acad Sci USA. 1991;88:4941–5. doi: 10.1073/pnas.88.11.4941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Charlton B, Lafferty KJ. The Th1/Th2 balance in autoimmunity. Curr Opin Immunol. 1995;7:793–8. doi: 10.1016/0952-7915(95)80050-6. [DOI] [PubMed] [Google Scholar]
- 53.Nicholson LB, Kuchroo VK. Manipulation of the Th1/Th2 balance in autoimmune disease. Autoimmunity. 1996;8:837–42. doi: 10.1016/s0952-7915(96)80013-6. [DOI] [PubMed] [Google Scholar]
- 54.Romagnani S. The Th1/Th2 paradigm. Immunol Today. 1997;18:263–6. doi: 10.1016/s0167-5699(97)80019-9. [DOI] [PubMed] [Google Scholar]
- 55.Adorini L, Sinigaglia F. Pathogenesis and immunotherapy of autoimmune diseases. Immunol Today. 1997;18:209–11. doi: 10.1016/s0167-5699(97)01031-1. [DOI] [PubMed] [Google Scholar]


