Abstract
This study presents data on more than 300 RA and allergic patients analysed for their serum levels of anti-immunoglobulin isotype autoantibodies and IgE. We observed high levels of IgE in sera of RA and allergic patients. Interestingly, we measured significantly higher specific IgE levels against Alternaria but not against nine other allergens in the RA compared with the allergic group. As expected, anti-IgG autoantibodies (rheumatoid factors (RF)) of different isotypes were detected in sera from RA patients only. However, we found increased titres of complexed anti-IgE autoantibodies in all RF+ groups and in the allergic group. These findings may explain why despite elevated IgE levels a decreased prevalence of allergic diseases in RA patients has been observed.
Keywords: rheumatoid arthritis, rheumatoid factor, IgE, anti-IgE, allergy
INTRODUCTION
RA is a widely distributed disease of chronic autoimmune inflammation in which the pathogenic mechanism is still unclear. The development of RA seems to result from a series of multifactorial events with the involvement of both T and B cell-dependent pathways [1]. Specific autoantibodies directed against antigenic determinants on the Fc fragment of IgG molecules have been described and are called rheumatoid factors (RF). However, their role in the pathogenesis of RA is still not established. The determination of RF is routinely performed for clinical diagnosis of RA and is one of 10 criteria defined by the American Rheumatology Association (ARA) [2]. Initial RF positivity is a strong prognostic marker for later joint damage [3]. Even the measurement of diverse isotypes of RF might be of importance in the clinical evaluation of RA [4]. Together with the number of swollen joints and the erosion score, IgM-RF has an accuracy of predicting outcome of RA of 70–80% [4]. IgA-RF is associated with increased disease activity, radiological progression and prevalence of extra-articular manifestation [5], whereas IgG-RF is suspected of being associated with vasculitis [6].
Other anti-isotype antibodies have previously been reported to play an important role in immunoregulation and may be also in the pathogenesis of atopic disease. Notably, anti-IgE antibodies have been detected in patients with atopic dermatitis, rhinitis, asthma and hay fever [7,8]. In vivo [9] and in vitro [10,11] analysis has shown that these natural anti-IgE antibodies are able to up- or down-regulate the effects and the synthesis of IgE. Such anti-IgE antibodies with the capacity of down-regulating IgE are currently being tested in clinical trials for passive immunization against IgE-mediated diseases [12]. Another approach envisages the use of peptide mimotopes of these particular down-regulating anti-IgE antibodies as a means of active immunization [13].
High titres of total IgE have already been measured in sera from patients with RA [14,15], but clinical trials showed no increased prevalence of atopy in RA patients [16,17] or even a decreased prevalence of atopy in RA [18]. The only known specificity described for IgE in RA is against cartilage collagen [19] and against Fc (IgE-RF), which is also related to extra-articular rheumatoid vasculitis [20]. However, a potential role for IgE in RA has not been clearly established.
In this study we compared sera from patients with rheumatoid and allergic disease. We found high levels of IgE in sera of RA patients and elevated titres of complexed anti-IgE. Compared with the allergic group, sera of RA patients showed an increased specific IgE titre against one allergen (Alternaria) but not against nine other frequently encountered allergens.
MATERIALS AND METHODS
Reagents
Human IgG-Fc fragment was purchased at ANAWA Trading SA (Wangen ZH, Switzerland). BSW17 and Le27 are murine IgG1 anti-IgE MoAbs, which have been produced in our laboratory [21]. IgE JW8 [22] and SUS11 [23] were produced in our laboratory. HP6017 is an anti-human IgG MoAb, purchased at ATCC (CRL-1753; Manassas, VA). 4F4 was a kind gift from Dr M. P. Samoilovich and Dr V. B. Klimovich (Hybridoma Technology Lab., Central Research Institute for Roentgeno-Radiology, St Petersburg, Russia). Sheep anti-human λ (APO 17) and κ (APO 18) peroxidase were ordered from The Binding Site (Birmingham, UK). A substrate solution of 16% of 4-chloro-1-napthol (Fluka Chemie, Buchs, Switzerland; stock solution 3 mg/ml in methanol) in 0.1% H2O2 (30%) in PBS was used. PBS–C is a solution of 1.5 mg/ml casein (Fluka; 9000-71-9) in PBS. Nitrocellulose was ordered from Millipore Corp. (HAWG304F0; Bedford, MA).
Serum samples
Sera from 140 patients (Table 1) with a clinical diagnosis of RA were kindly provided by the Department of Rheumatology, Inselspital, Bern, Switzerland. Sera were sorted into different groups according to their RF content. The cut-off for IgA- (17.5 U) and IgG-RF (1.8 ng/ml) was determined by calculating the mean + 1 s.d. of the following groups: control, allergic, high IgE. For IgM-RF we used the international standard (20 U). Seronegative spondyloarthropathy is a group of chronic inflammatory diseases, which are all associated with the HLA-B27 gene locus and classically all have no detectable RF [24]. Our spondyloarthropathy group, also provided by the Department of Rheumatology, contained 47 sera from patients with psoriatic arthritis (n = 19), ankylosing spondylitis (n = 14), Reiter's disease (n = 8) and reactive arthritis (n = 6). The allergic group (n = 51) consisted of sera collected in the Institute of Immunology and Allergy. All sera had a total IgE > 200 U and high reactivity for at least one allergen measured in a standard radioallergosorbent test. In contrast, sera with IgE < 120 U and no reactivity for any allergen-specific IgE were used as controls (n = 44). Eight sera with IgE levels > 200 U (439 ± 222 U (mean ± s.d.)) but no specific reaction to any standard allergen were grouped as high IgE.
Table 1.
Patients' data and rheumatoid factors
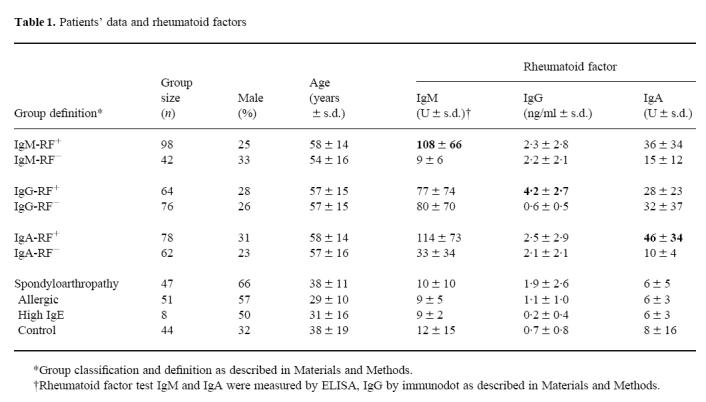
Determination of IgG-, IgM- and IgA-RF
IgG-RF were measured in a dot immunobinding assay according to the method of Derer et al. [25]. One microlitre of human IgG-Fc fragment (50 μg/ml) was dotted in duplicate on nitrocellulose. After drying the nitrocellulose was blocked with PBS–C for 1 h in order to prevent non-specific protein absorption and thereafter cut into strips. The strips were incubated with serum (1:20 in PBS–C) overnight with gentle shaking and then washed three times with PBS. Subsequently strips were incubated with sheep anti-human λ/κ peroxidase antibodies at a dilution of 1:1000 for 4 h. After three washings with PBS, the strips were developed with substrate solution of chloronaphthol and hydrogen peroxide. The optical density (OD) of the dots was measured using a densitometer (Gretag Ltd, Regensdorf, Switzerland). For quantification a polyclonal human IgG (Sandoglobulin) was used as a standard. IgM- and IgA-RF were measured by ELISA (ImmuLisa; Immco Diagnostics, Buffalo, NY). The results are expressed as U.
Measurement of free and complexed anti-IgE
The same assay as described above for IgG-RF was used to detect free anti-IgE. JW8 (27 μg/ml) and SUS11 (8 μg/ml) were dotted, blocked and incubated with sera (1:20 in PBS–C) as described previously. After washing, the strips were developed with peroxidase-conjugated anti-IgG MoAb (HP6017 1:1000 in PBS–C). For detecting complexed anti-IgE, BSW17 (500 μg/ml) and Le27 (500 μg/ml) were used as described by Vassella et al. [26]. Sera were diluted 1:20 in PBS–C. Strips were developed with sheep anti-human λ/κ peroxidase antibodies.
Measurement of total and allergen-specific IgE
Quantitative measurement for IgE and allergen-specific IgE was performed using a commercial dot assay kindly provided by Dr M. Derer (Gerimmun, Fribourg, Switzerland). BSW17-Pox was used as the developing antibody. Allergen-specific IgE was measured against the following 10 allergens (indoor, Dermatophagoides pteronyssinus, D. farinae, cat, dog, Alternaria; outdoor, mixture of six grasses: rye, birch, mugwort, ribwort). Serum samples for detecting specific IgE were diluted 1:5 and developed using a mixture of BSW17-, Le27-and 4F4-Pox at a dilution of 1:500.
Statistical analysis
We used the unpaired Student's t-test and linear regression analyses to determine the Pearson correlation coefficient from Sigma Plot, Version 2 (Jandel Corp., San Rafael, CA).
RESULTS
Determination of IgM-, IgG- and IgA-RF
We compared sera from rheumatic and allergic patients, as in both types of diseases anti-isotype autoantibodies are involved. As an index for disease in the former group we measured RF, which is one of the most characteristic laboratory parameters in RA. We used a commercial ELISA for detecting IgM- and IgA-RF. To determine IgG-RF an immunodot assay was performed as described previously. Classically RA is divided into seropositive or -negative according to IgM-RF titres. When we analysed the RA group for this parameter 30% of the sera contained no IgM-RF (Table 1). However, 50% of these IgM-RF− sera contained IgG-RF and 26% IgA-RF (data not shown). Looking at all three isotypes, only 15 of 140 (11%) were still RF−. These data indicate that IgM-RF measurement alone might be insufficient to classify either seropositive or seronegative RA. When looking at all 140 RA sera there was no correlation between IgM- and IgG-RF (r = 0.2) and a weak correlation between IgM- and IgA-RF (r = 0.6). Therefore we analysed RA sera for all further experiments divided into positive and negative groups for all three isotypes. As expected, the spondyloarthropathy group (SAP) showed low levels of IgA- and IgM-RF and only slightly elevated IgG-RF titres. In the allergic, the high IgE and in the control groups we found only background levels of RF.
Determination of free and complexed anti-IgE
Another disease in which anti-immunoglobulin autoantibodies are involved is allergy. IgG anti-IgE were shown to play an important role in the regulation of IgE. Therefore we also analysed the sera of RA patients for the presence of free and complexed anti-IgE using an immunodot assay. We found no significant differences in the level of free anti-IgE between all the seropositive and -negative groups (Fig. 1a). Also no difference to the control group was found. Free anti-IgE titres determined in the sera of allergic patients were only slightly higher than those of the control group. In the seronegative SAP, there was a significant elevation of free anti-IgE detected by SUS11 (P = 0.006) and by JW8 (P = 0.012).
Fig. 1.
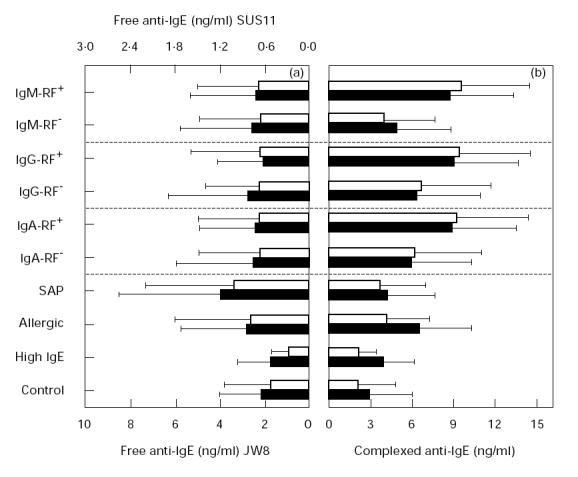
Determination of free and complexed anti-IgE. (a) Free anti-IgE measured by two different preparations of IgE: SUS11 (□) and JW8 (▪). (b) Complexed anti-IgE measured by BSW17 (□) and Le27 (▪). Values displayed represent mean ± s.d.
In contrast, complexed anti-IgE (Fig. 1b) was markedly elevated in sera from patients suffering from RA compared with the control group. Nevertheless, high individual variations were observed as indicated by the large s.d. Interestingly, the RF+ groups all showed higher values than the RF−. This difference was most pronounced and statistically significant for the IgM-RF+ group compared with the corresponding negative group (P < 0.001). The serum levels of complexed anti-IgE were also significantly higher in allergic patients compared with controls.
In summary, sera from RA patients showed no increase of free anti-IgE but significantly elevated levels of complexed anti-IgE, and these increased titres were even more pronounced in all RF+ groups.
Quantification of total and allergen-specific IgE
Next we assessed whether elevated titres of complexed anti-IgE in RA patients were also accompanied by high levels of IgE. For this purpose, IgE was measured by a commercial kit as described in Materials and Methods. We observed increased total IgE levels in sera of RA patients (Fig. 2a). Out of 140 sera 51 (36%) had IgE titres > 200 U. Furthermore, IgE levels were significantly different in the IgA-RF+ (P = 0.0004) and IgM-RF+ (P = 0.0036) compared with the corresponding negative groups (Fig. 2a). This correlates with the data obtained for complexed anti-IgE. In contrast, no difference in total IgE was observed between the IgG-RF+ and IgG-RF− group, but both groups showed increased titres compared with the control. As expected, sera from allergic patients had the highest IgE values.
Fig. 2.
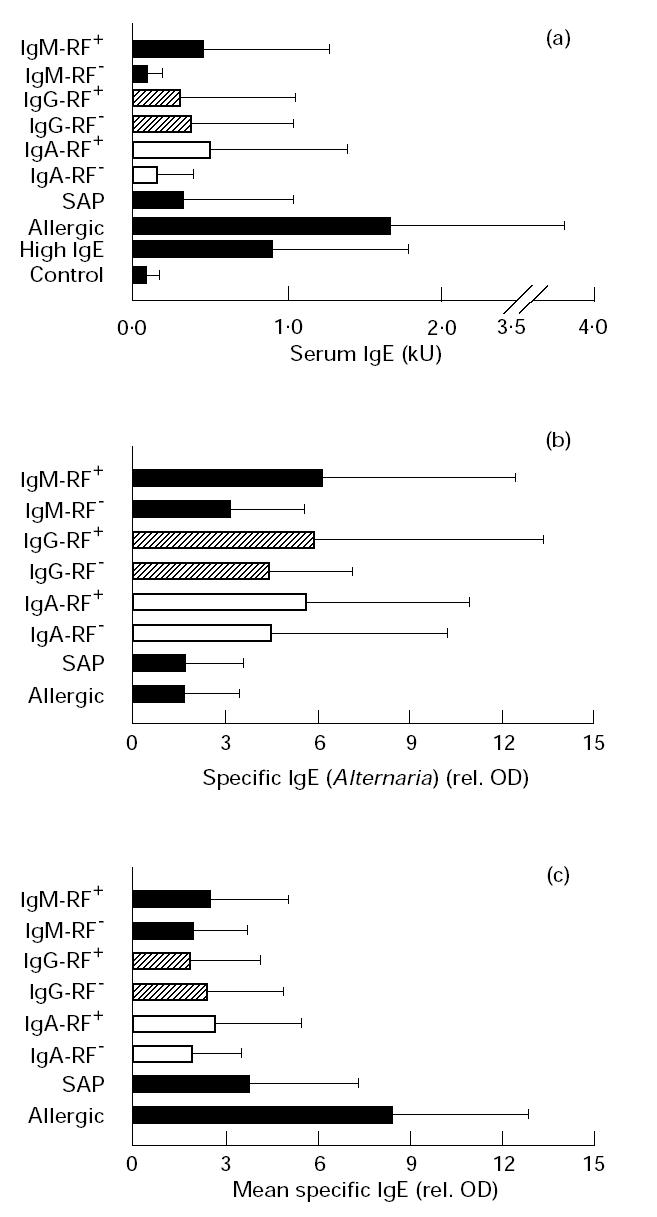
Quantification of total and allergen-specific IgE. (a) Total serum IgE (kU). (b) Alternaria-specific IgE (rel. optical density (OD)). (c) IgE specific for nine allergens measured by immunodot assay (rel. OD). Results are expressed as a mean value of all nine allergens and for each patient group.
To analyse further the high total IgE in sera of RA patients, we investigated whether allergen-specific IgE could account for the increased levels of total IgE. For this purpose we measured allergen-specific IgE against 10 standard indoor/outdoor allergens. Surprisingly, we observed a significant elevation of Alternaria-specific IgE in RA compared with the allergic group and a significant difference between IgM-RF+ and IgM-RF− sera (P = 0.006) against this allergen (Fig. 2b). This difference was also noted in the IgG-RF and IgA-RF groups, but was not so marked. No elevation of allergen-specific IgE in RA patients against any of the other allergens tested was observed (Fig. 2c).
DISCUSSION
Anti-immunoglobulin autoantibodies have been described in both RA and allergies. Comparing sera from allergic and rheumatic patients we found elevated amounts of IgE in sera from RA patients and high levels of complexed anti-IgE in all RF+ groups. In addition, specific IgE to Alternaria was increased in the RF+ groups compared with the allergic group.
An age-matched comparison of RA and allergic sera is not possible because the average age of onset of disease in the RA group is in the fourth and fifth decades of life and in childhood to late adolescence in the allergic patients. Additionally, previous studies have shown a declining IgE level in older people [27]. The high levels of IgE found in the relatively older RA group are even more surprising given these previous studies.
We noted low levels of free anti-IgE in RA patients. This finding can be explained by the fact that most free anti-IgE is probably bound to IgE and thus hidden in complexes. Indeed, we have found increased amounts of complexed anti-IgE in sera from RA patients. It was previously reported by de Clerck et al. [28] that the levels of complexed anti-IgE and IgE can be overestimated or even false positive due to cross-reaction of RF with the catching antibody (BSW17 and Le27 in our case). To investigate this we analysed the correlation of complexed anti-IgE and IgE with RF. We observed only a weak positive correlation for these parameters (data not shown). Furthermore, it was suggested in the same paper [27] that the use of monoclonal instead of polyclonal catching antibodies would greatly diminish the probability of a cross-reaction of RF with the capturing antibody. This issue is dealt with in this study by the use of two MoAbs. Therefore a cross-reaction of RF with the capturing antibody as a major source of error is unlikely.
No obvious explanation for the elevated IgE levels can be given. Other investigators interpreted similar observations in parallel with other immunoglobulins and as a general immunological hyperactivity [14]. Indeed there is evidence that not only total IgE but also total IgG and IgA serum levels are increased in RA patients apart from IgM [29]. It is well known that IL-4 is important in the isotype switching to IgE and that the CD40/CD40L interaction is needed for efficient activation of B and T cells [30]. Enhanced expression of CD40 by human synovial fibroblasts isolated from RA patients [31] and CD40L by human synovial T cells [32] have been reported. In an animal model it was even shown that collagen-induced arthritis was inhibited by an antibody to CD40L [33]. In contrast, no evidence for elevated levels of IL-4 or IL-13 in RA has been described. On the contrary, predominant cytokines in RA are of the proinflammatory Th1-type, such as IL-1, IL-6, IL-8 and tumour necrosis factor-alpha (TNF-α) [34]. It has been shown that TNF-α alone cannot induce IgE but acts synergistically with IL-4 in the induction of IgE [35].
Findings from Gruber et al. [36] suggest that IgE might play a role in the pathogenesis of RA. They showed anti-IgE-dependent release of histamine from synovial mast cells, contributing to joint inflammation. In another study [15] elevated IgE levels in RA patients were reported but without correlation to rheumatoid nodules, extra-articular manifestations or RF. Furthermore IgE–anti-IgE complexes were shown to have a positive correlation to active disease (swollen joints) [37].
Interestingly, the presence of large amounts of IgE does not lead to an enhanced prevalence of allergic disorders in RA patients. On the contrary, even a decreased incidence of allergies in RA patients has been reported recently [18]. Our finding that not only IgE but also anti-IgE antibodies are elevated in RA patients may in part explain this paradox. These anti-IgE antibodies may interfere with binding of IgE and thus prevent sensitization of basophils and mast cells. Similarly it has been found that patients treated with anti-IgE antibodies had increased levels of IgE–anti-IgE complexes and thereby the half-life of serum IgE was increased [12].
Elevated levels of IgE specific for the allergen of Alternaria in RA are now reported for the first time. Different groups [38–41] suggested a possible allergenic/antigenic stimulus to induce, drive or facilitate the process of RA. However, further studies are needed to elucidate an eventual role for Alternaria in the pathogenesis in RA.
Acknowledgments
We thank Vreni Ramseyer and Esther Schnegg for their excellent technical assistance. We are grateful to Dr Michael Derer for his generous gift of material. We also thank Immco Diagnostics (Buffalo, NY) and Ruwag Diagnostics (Zürich) for providing the IgM- and IgA-RF ELISA kits. This work was supported by grant no. 3100-052469/97 of the Swiss National Foundation.
REFERENCES
- 1.Goronzy JJ, Weyand CM. T and B cell-dependent pathways in rheumatoid arthritis. Curr Opin Rheumatol. 1995;7:214–21. doi: 10.1097/00002281-199505000-00010. [DOI] [PubMed] [Google Scholar]
- 2.Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 3.Paimela L, Palasuo T, Leirisalo-Repo M, Helve T, Aho K. Prognostic value of quantitative measurements of rheumatoid factors in early rheumatoid arthritis. Br J Rheumatol. 1995;34:1146–50. doi: 10.1093/rheumatology/34.12.1146. [DOI] [PubMed] [Google Scholar]
- 4.Van Zeben D, Hazes JM, Zwinderman AH, Vandenbroucke JP, Breedveld FC. Factors predicting outcome of rheumatoid arthritis: results of a follow-up study. J Rheumatol. 1993;20:1288–96. [PubMed] [Google Scholar]
- 5.Houssien DA, Jonsson T, Davies E, Scott DL. Clinical significance of IgA rheumatoid factor subclasses in rheumatoid arthritis. J Rheumatol. 1997;24:2119–22. [PubMed] [Google Scholar]
- 6.Mannik M, Nardella FA. IgG rheumatoid factors and self-association of these antibodies. Clin Rheum Dis. 1985;11:551–72. [PubMed] [Google Scholar]
- 7.Marone G, Casolaro V, Paganelli R, Quinti I. IgG anti-IgE from atopic dermatitis induces mediator release from basophils and mast cells. J Invest Dermatol. 1989;93:246–52. doi: 10.1111/1523-1747.ep12277582. [DOI] [PubMed] [Google Scholar]
- 8.Ritter C, Battig M, Kraemer R, Stadler BM. IgE hidden in immune complexes with anti-IgE autoantibodies in children with asthma. J Allergy Clin Immunol. 1991;88:793–801. doi: 10.1016/0091-6749(91)90187-s. [DOI] [PubMed] [Google Scholar]
- 9.Haba S, Nisonoff A. Role of antibody and T cells in the long-term inhibition of IgE synthesis. Proc Natl Acad Sci USA. 1994;91:604–8. doi: 10.1073/pnas.91.2.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stadler BM, Rudolf MP, Zuercher AW, Miescher S, Vogel M. Anti-IgE in allergic sensitization. Immunol Cell Biol. 1996;74:195–200. doi: 10.1038/icb.1996.27. [DOI] [PubMed] [Google Scholar]
- 11.Stämpfli MR, Miescher S, Aebischer I, Zuercher AW, Stadler BM. Inhibition of human IgE synthesis by anti-IgE antibodies requires divalent recognition. Eur J Immunol. 1994;24:2161–7. doi: 10.1002/eji.1830240934. [DOI] [PubMed] [Google Scholar]
- 12.Corne J, Djukanovic R, Thomas L, et al. The effect of intravenous administration of a chimeric anti-IgE antibody on serum IgE levels in atopic subjects: efficacy, safety and pharmacokinetics. J Clin Invest. 1997;99:879–87. doi: 10.1172/JCI119252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rudolf MP, Vogel M, Kricek F, et al. Epitope-specific antibody response to IgE by mimotope immunization. J Immunol. 1998;160:3315–21. [PubMed] [Google Scholar]
- 14.Grennan DM, Palmer DG. Serum IgE concentration in rheumatoid arthritis: lack of correlation with gold toxicity. Br Med J. 1979;2:1477–8. doi: 10.1136/bmj.2.6203.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hunder GG, Gleich GJ. Immunoglobulin E (IgE) levels in serum and synovial fluid in rheumatoid arthritis. Arthritis Rheum. 1974;17:955–63. doi: 10.1002/art.1780170606. [DOI] [PubMed] [Google Scholar]
- 16.O'Driscoll BR, Milburn HJ, Kemeny DM, Cochrane GM, Panayi GS. Atopy and rheumatoid arthritis. Clin Allergy. 1985;15:547–53. doi: 10.1111/j.1365-2222.1985.tb02308.x. [DOI] [PubMed] [Google Scholar]
- 17.Hasan WU, Keaney NP, Holland CD, Kelly CA. Bronchial reactivity and airflow obstruction in rheumatoid arthritis. Ann Rheum Dis. 1994;53:511–4. doi: 10.1136/ard.53.8.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Allanore Y, Hilliquin P, Coste Renoux M, Menkès CJ. Decreased prevalence of atopy in rheumatoid arthritis. Lancet. 1998;351:497. doi: 10.1016/s0140-6736(05)78684-0. [DOI] [PubMed] [Google Scholar]
- 19.Bartholomew JS, Evanson JM, Wooley DE. Serum IgE anti-cartilage collagen antibodies in rheumatoid patients. Rheumatol Int. 1991;11:37–40. doi: 10.1007/BF00290249. [DOI] [PubMed] [Google Scholar]
- 20.Mizushima Y, Shoji Y, Hoshi K, Kiyokawa S. Detection and significance of IgE rheumatoid factors. J Rheumatol. 1984;11:22–26. [PubMed] [Google Scholar]
- 21.Knutti-Muller JM, Stadler BM, Magnusson CM, de Weck AL. Human IgE synthesis in vitro. Detection with monoclonal antibodies. Allergy. 1986;41:457–67. doi: 10.1111/j.1398-9995.1986.tb00327.x. [DOI] [PubMed] [Google Scholar]
- 22.Neuberger MS, Williams GT, Mitchell EB, Jouhal SS, Flanagan JG, Rabbitts TH. A hapten-specific chimaeric IgE antibody with human physiological effector function. Nature. 1985;314:268–70. doi: 10.1038/314268a0. [DOI] [PubMed] [Google Scholar]
- 23.Zuercher AW, Lang AB, Aebischer I, Miescher S, Stadler BM. IgE-producing hybridomas established after B-cell culture in the CD40 system. Immunol Letters. 1995;46:49–57. doi: 10.1016/0165-2478(95)00014-v. [DOI] [PubMed] [Google Scholar]
- 24.Arnett FC. Seronegative spondylarthropathies. Bull Rheum Dis. 1987;37:1–12. [PubMed] [Google Scholar]
- 25.Derer MM, Miescher S, Johansson B, Frost H, Gordon J. Application of the dot immunobinding assay to allergy diagnosis. J Allergy Clin Immunol. 1984;74:85–92. doi: 10.1016/0091-6749(84)90093-9. [DOI] [PubMed] [Google Scholar]
- 26.Vassella CC, de Weck AL, Stadler BM. Influence of IgE fragments on IgE determination. Int Arch Allergy Appl Immunol. 1990;92:272–80. doi: 10.1159/000235189. [DOI] [PubMed] [Google Scholar]
- 27.Klink M, Cline MG, Halonen M, Burrows B. Problems in defining normal limits for serum IgE. J Allergy Clin Immunol. 1990;85:440–4. doi: 10.1016/0091-6749(90)90153-u. [DOI] [PubMed] [Google Scholar]
- 28.De Clerck LS, Gigase PL, Bridts CH, Stevens WJ. Role of IgM-rheumatoid factor interference in the determination of total serum IgE and IgE-containing circulating immune complexes. Clin Exp Immunol. 1988;72:32–36. [PMC free article] [PubMed] [Google Scholar]
- 29.Al-Balaghi S, Strom H, Moller E. High incidence of spontaneous Ig-producing lymphocytes in peripheral blood and synovial fluid of patients with active seropositive rheumatoid arthritis. Scand J Immunol. 1982;16:69–76. doi: 10.1111/j.1365-3083.1982.tb00700.x. [DOI] [PubMed] [Google Scholar]
- 30.Sutton BJ, Gould HJ. The human IgE network. Nature. 1993;366:421–8. doi: 10.1038/366421a0. [DOI] [PubMed] [Google Scholar]
- 31.Rissoan MC, van Kooten C, Chomarat P, Galibert L, Durand I, Thivolet-Bejui F, Miossec P, Banchereau J. The functional CD40 antigen of fibroblasts may contribute to the proliferation of rheumatoid synovium. Clin Exp Immunol. 1996;106:481–90. doi: 10.1046/j.1365-2249.1996.d01-858.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.MacDonald KP, Nishioka Y, Lipsky PE, Thomas R. Functional CD40 ligand is expressed by T cells in rheumatoid arthritis. J Clin Invest. 1997;100:2404–14. doi: 10.1172/JCI119781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Durie FH, Fava RA, Foy FM, Aruffo A, Ledbetter JA, Noelle RJ. Prevention of collagen-induced arthritis with an antibody to gp39, the ligand for CD40. Science. 1993;261:1328–30. doi: 10.1126/science.7689748. [DOI] [PubMed] [Google Scholar]
- 34.Feldmann M, Brennan FM, Maini RN. Role of cytokines in rheumatoid arthritis. Annu Rev Immunol. 1996;14:397–440. doi: 10.1146/annurev.immunol.14.1.397. [DOI] [PubMed] [Google Scholar]
- 35.Gauchat JF, Aversa G, Gascan H, de Vries JE. Modulation of IL-4 induced germline epsilon RNA synthesis in human B cells by tumor necrosis factor-alpha, anti-CD40 monoclonal antibodies or transforming growth factor-beta correlates with levels of IgE production. Int Immunol. 1992;4:397–406. doi: 10.1093/intimm/4.3.397. [DOI] [PubMed] [Google Scholar]
- 36.Gruber B, Pozuansky M, Boss E, Partin J, Gorevic P, Kaplan AP. Characterization and functional studies of rheumatoid synovial mast cells. Arthritis Rheum. 1986;29:944–55. doi: 10.1002/art.1780290802. [DOI] [PubMed] [Google Scholar]
- 37.De Clerck LS, Struyf NJ, Bridts CH, et al. Humoral immunity and composition of immune complexes in patients with rheumatoid arthritis, with special reference to IgE-containing immune complexes. Clin Exp Rheumatol. 1989;7:485–92. [PubMed] [Google Scholar]
- 38.Posnett DN, Edinger J. When do microbes stimulate rheumatoid factor? J Exp Med. 1997;185:1721–3. doi: 10.1084/jem.185.10.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McCullock J, Lydyard PM, Rook GAW. Rheumatoid arthritis: how well do the theories fit the evidence? Clin Exp Immunol. 1993;92:1–6. doi: 10.1111/j.1365-2249.1993.tb05938.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Olee T, Lu EW, Huary F, Soto-Gil RW, Deftos M, Kozin F, Carson DA, Chen PP. Genetic analysis of self-associating immunoglobulin G rheumatoid factor from two rheumatoid synovia implicates an antigen-driven response. J Exp Med. 1992;175:831–42. doi: 10.1084/jem.175.3.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Silman AJ. Is rheumatoid arthritis an infectious disease? Brit Med J. 1991;303:524–786. doi: 10.1136/bmj.303.6796.200. [DOI] [PMC free article] [PubMed] [Google Scholar]


