Abstract
IL- 12 is the prominent inducer of Th1 responses in humans and in the mouse. CD40 ligand (CD40L) plays important roles in regulation of immune responses, including T cell-dependent activation of B cells and cytokine production by monocytes and dendritic cells. The present study examined the influences of IL-12 on the CD40L expression of activated human CD4+ T cells. IL-12 enhanced CD40L expression on CD4+ T cells stimulated with immobilized anti-CD3 in the complete absence of accessory cells, whereas IL-4 and IL-10 decreased it. Exogenous interferon-gamma (IFN-γ) did not increase CD40L expression on immobilized anti-CD3 stimulated CD4+ T cells at any time up to 168 h of culture. The IL-12-induced enhancement of CD40L expression on anti-CD3 activated CD4+ T cells was not influenced in the presence of a metalloproteinase inhibitor KB8301, which up-regulated CD40L expression by preventing the processing of membrane-bound CD40L, or B cells, which down-regulated CD40L expression by receptor-mediated endocytosis. These results indicate that IL-12 enhances the CD40L expression of activated CD4+ T cells independently of the IFN-γ production. The data thus suggest that Th1 responses induced by IL-12 might play an important role in the regulation of humoral immune responses through up-regulated CD40L expression.
Keywords: Th1/Th2, cytokines, cell surface molecules, anti-CD3, FACS
INTRODUCTION
Human T cells have been shown to play a critical role in the regulation of humoral immune responses [1]. Thus, it has been demonstrated that the establishment of interactions between B cells and activated CD4+ T cells provides all of the signals necessary for proliferation and differentiation of B cells [2]. Apart from the cytokines produced by activated CD4+ T cells, the direct cellular interactions between B cells and activated CD4+ T cells have been demonstrated to be essential for B cell activation [2]. The ligand for CD40, CD40L (CD154), a 33–35-kD member of the tumour necrosis factor (TNF) family, has been identified on activated CD4+ T cells [3]. A number of studies have disclosed that CD40–CD40L interactions play a critical role in T cell-dependent B cell activation, proliferation, and differentiation [4–6]. Moreover, it has recently been shown that during T cell–B cell collaboration, CD40–CD40L interactions exert bidirectional effects on B cell immunoglobulin production, depending on the state of activation of B cells and on the extent of CD40 ligation [7,8]. Thus, the regulation of the expression of CD40L on CD4+ T cells is crucial for the outcome of humoral immune responses.
In response to pathogens, naive CD4+ T cells have been found to differentiate into effector Th1 and Th2 cells [9–11]. Th1 cells produce IL-2 and interferon-gamma (IFN-γ), which are involved in cell-mediated immune responses, whereas Th2 cells secrete mainly IL-4, IL-5, IL-10 and IL-13, which are involved in humoral immune responses [11,12]. A previous report showed that a murine Th1 clone expressed comparable levels of CD40L to those observed in a murine Th2 clone [13]. However, it was also disclosed that the activated Th1 clones expressed approximately 23-fold greater levels of CD40L compared with anti-CD3 activated CD4+ splenic T cells [13]. Since a T cell clone was established through repeated stimulation and selection, it was possible that the expression of CD40L on the T cell clones might be influenced by the procedures of the repeated stimulation and selection rather than the pattern of Th1 and Th2. Thus, it remains unclear whether there are any significant differences in the expression of CD40L between Th1 responses and Th2 responses.
IL-12 is a heterodimeric cytokine produced by activated monocytes, macrophages, and B cells [14–16]. One of the most important activities of IL-12 in immune systems is to induce the production of IFN-γ, not only by natural killer (NK) cells but also by T cells [16]. Recently, accumulating evidence has indicated that IL- 12 has the prominent capacity to induce the differentiation of naive CD4+ T cells into Th1 cells [17]. Consistently, anti-IL-12 has been shown to decrease IFN-γ production, and to induce a Th2-like cytokine response in vivo (reviewed in [18]). The current study were therefore undertaken to explore the influences of IL-12 on CD40L expression of activated human CD4+ T cells, utilizing a system with immobilized anti-CD3, 64.1, which permits stimulation of all peripheral blood T cells in the complete absence of accessory cells. This system therefore enables us to evaluate the effects of exogenous IL-12 on CD4+ T cells without any effects of endogenous IL-12 produced by accessory cells or B cells.
MATERIALS AND METHODS
MoAbs and reagents
A variety of T cell-specific MoAbs was used, including 64.1 (a gift of Dr P. E. Lipsky, University of Texas Southwestern Medical Center, Dallas, TX), an IgG2a MoAb directed at the CD3 molecule on mature T cells, and a murine anti-CD8 MoAb B9.11 (Immunotech, Marseille, France). A variety of miscellaneous MoAbs was also used, including IOT2a (Immunotech), an IgG2b MoAb directed at monomorphic HLA-DR determinants; FITC-conjugated or unconjugated anti-CD40L (a murine IgG1 MoAb, clone 24-31) (Ancell, Bayport, MN); PE-conjugated anti-CD4 (a murine IgG1 MoAb, 13B8.2) (Immunotech); FITC- and PE-conjugated control mouse IgG1 (Dako, Glostrup, Denmark); and a murine IgG1 control MoAb MOPC21 (Cappel Labs, West Chester, PA). Recombinant human IL-12 was purchased from R&D Systems (Minneapolis, MN). Recombinant human IFN-γ was a generous gift of Shionogi Pharmaceutical Co. (Osaka, Japan). Recombinant human IL-4 and IL-10 were purchased from Pepro Tech, Inc. (Rocky Hill, NJ). A metalloproteinase inhibitor KB8301, [4-(N-hydroxyamino)-2R-isobutyl-3S-methylsuccinyl]-L-3-(5,6,7,8-tetrahydro-1-naphthyl) alanine-N-methylamide (mol. wt 418), which strongly inhibits MMP1, MMP2, MMP3 and MMP9 (IC50; 0.3–0.6 nm) [19], was a generous gift of Kanebo Ltd (Osaka, Japan). KB8301 was dissolved in DMSO, and added to cultures at a final concentration of 10 μm with 0.01% DMSO.
Culture medium
RPMI 1640 medium (Life Technologies, Inc., Grand Island, NY) supplemented with penicillin G 100 U/ml, streptomycin 100 μg/ml, l-glutamine 0.3 mg/ml, and 10% fetal bovine serum (FBS; Life Technologies) was used for all cultures.
Cell preparation
Peripheral blood mononuclear cells (PBMC) were obtained from healthy adult volunteers by centrifugation of heparinized venous blood over sodium diatrizoate-Ficoll gradients (Histopaque; Sigma Chemical Co., St Louis, MO). PBMC were depleted of monocytes and NK cells by incubation with 5 mml-leucine methyl ester HCl (Sigma) in serum-free RPMI 1640, as described elsewhere [20]. The treated cell population was further separated into T cells and B cells by rosetting with neuraminidase-treated sheep erythrocytes as previously described [5]. The resultant population of B cells contained < 1% T cells as determined by staining with OKT3 and OKT11 pan T cell MoAb (American Type Culture Collection (ATCC), Rockville, MD), followed by analysis by flow cytometry. The cells were additionally characterized as containing > 90% CD20+ (B1; Coulter Immunology, Hialeah, FL) B cells, < 1% CD14+ (IOM2; Immunotech) monocytes, and no CD16+ (Leu-11b; Becton Dickinson, Mountain View, CA) NK cells. Purified CD4+ T cells were prepared from the total T cell population by negative selection, using a panning technique to deplete contaminating HLA-DR+ cells and CD8+ T cells, as previously indicated [5]. The CD4+ T cell population obtained in this manner contained < 2% CD8+ T cells and > 96% CD4+ T cells.
Cell culture techniques for induction of expression of CD40L
Anti-CD3 MoAb, 64.1, was diluted in RPMI 1640 (2 μg/ml), and 50 μl were placed in each well of 96-well flat-bottomed microtitre plates (no. 3596; Costar, Cambridge, MA) and incubated at room temperature for 1 h [21]. The wells were then washed once with culture medium to remove non-adherent MoAb before the cells were added. Purified CD4+ T cells (2 × 105/well) were cultured in wells with immobilized anti-CD3 in the presence or absence of various cytokines. In some experiments, purified CD4+ T cells (2 × 105/well) and B cells (2.5 × 104/well) were cultured from the initiation of incubation in wells with immobilized anti-CD3 in the presence or absence of IL-12 (10 ng/ml). The cells were incubated for 1–7 days at 37°C in a humidified atmosphere of 5% CO2 and 95% air.
Immunofluorescence staining of cell surface markers and analysis by flow cytometry
After incubation, the cells were washed three times with PBS containing 2% normal human AB serum and 0.1% sodium azide (staining buffer). The cells were reacted in suspension by incubating for 30 min at 4°C with saturating concentrations of either FITC- or PE-conjugated MoAb. After the cells were washed three times with staining buffer, the cells were fixed with 1% paraformaldehyde in PBS pH 7.4 for > 5 min at room temperature. In some experiments the cells were washed once with cold PBS and fixed with ethanol cooled to −20°C for 5 min prior to staining. The cells were analysed using an EPICS XL flow cytometer (Coulter) equipped with an argon-ion laser at 488 nm, as previously described [22]. The flow cytometer was calibrated with Immuno-Check (Coulter) by a computer program. EPICS XL software was used to generate the plots. A gating on the forward and side scatter measurement was used to identify viable lymphocytes. The data generated by flow cytometry, based on 5000 lymphocytes defined by the scatter gates, were collected on single-parameter histograms or dual-parameter scattergrams. In conjunction with logarithmic amplifiers, the scattergrams encompassed a 4-decade range of fluorescence intensity. The percentages of cells staining positively for each MoAb were determined by integration of cells above a specified fluorescence channel, calculated in relation to isotype-matched control MoAb. Density of staining was expressed as the change in mean fluorescence intensity (MFI) for staining with the MoAb of interest calculated by subtracting the MFI of staining with the control MoAb.
Measurement of IFN-γ
IFN-γ contents in the supernatants were assessed using a solid-phase ELISA as previously described [23]. The detection limit of the assay was approximately 5.0 U/ml of IFN-γ. The assay was specific for natural and recombinant human IFN-γ.
RESULTS
IL-12 enhances the expression of CD40L on immobilized anti-CD3 activated CD4+ T cells
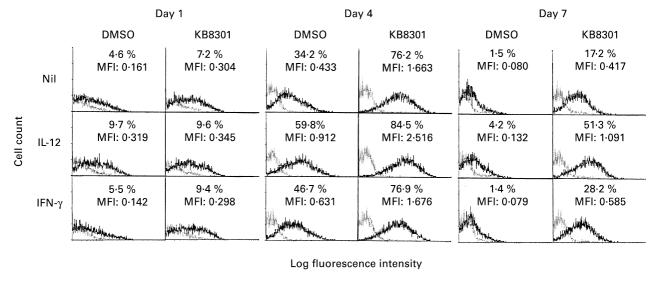
It has been demonstrated that membrane-bound Fas ligand as well as TNF-α is cleaved by metalloproteinases [24]. Thus, a metalloproteinase inhibitor KB8301 has been shown to markedly enhance the expression of Fas ligand on the cell surface [24]. Initial experiments therefore examined whether KB8301 also enhances the expression of CD40L on immobilized anti-CD3 activated CD4+ T cells, since CD40L as well as Fas ligand belongs to the TNF family. As can be seen in Fig. 1, KB8301 (10 μm) enhanced the expression of CD40L on anti-CD3 activated CD4+ T cells, suggesting that membrane-bound CD40L might also be cleaved by metalloproteinases, as is the case with Fas ligand. Moreover, IL-12 markedly enhanced the expression of CD40L on CD4+ T cells activated with immobilized anti-CD3 for 96 h irrespective of the presence of KB8301. As can be seen in Fig. 2, IL-12 significantly enhanced the expression of CD40L, which is reflected by the changes in MFI as described in Materials and Methods, on anti-CD3 stimulated CD4+ T cells from 15 normal healthy individuals, although there was some variation in the degree of enhancement between individuals. Time kinetics experiments showed that the expression of CD40L as well as its enhancement by IL-12 was most remarkable after 96 h of stimulation of CD4+ T cells with immobilized anti-CD3, whereas the enhancement of CD40L expression of IL-12 was very modest after 24 h of stimulation of CD4+ T cells (Fig. 3). Consistent with previous reports [16], IL-12 also enhanced IFN-γ production of anti-CD3 stimulated CD4+ T cells. Of note, IL-12 still increased IFN-γ production of anti-CD3 stimulated CD4+ T cells between 96 h and 168 h of culture, during which period CD40L expression of such CD4+ T cells markedly declined even in the presence of IL-12. It should be noted, however, that IL-12 still comparably up-regulated the expression of CD40L at 168 h of culture. These results indicate that IL- 12 up-regulates the expression of CD40L as well as the production of IFN-γ of immobilized anti-CD3 activated CD4+ T cells, presumably through induction of terminal differentiation into Th1 cells. The data also suggest that expression of CD40L and production of IFN-γ might be differently regulated depending on the stage of activation.
Fig. 1.
Enhancement of expression of CD40L on immobilized anti-CD3-activated CD4+ T cells by IL-12. CD4+ T cells (2 × 105/well) were cultured in wells with immobilized anti-CD3 (64.1, 100 ng/well) in the presence or absence of a metalloproteinase inhibitor KB8301 (10 μm) with or without IL-12 (10 ng/ml). After 96 h of incubation, the cells were harvested and stained with FITC-conjugated anti-CD40L MoAb or control MoAb and PE-conjugated anti-CD4 MoAb. Immunofluorescent staining, fixation, and analysis were performed as described in Materials and Methods. Mean fluorescence intensity (MFI) for staining with FITC-conjugated MoAb is indicated. The data are representative of three different experiments.
Fig. 2.
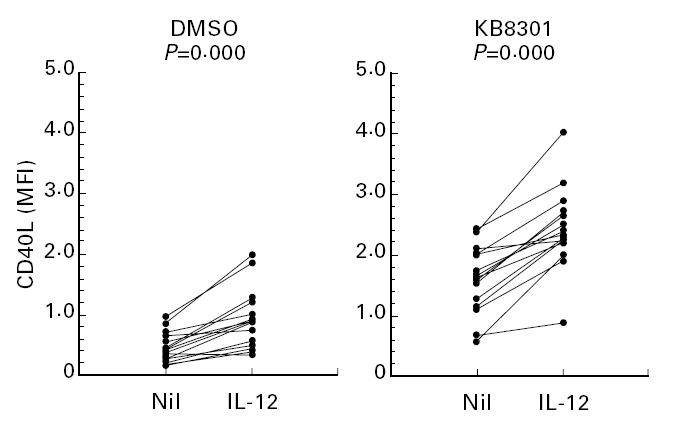
Enhancement of expression of CD40L on immobilized anti-CD3 activated CD4+ T cells by IL-12. CD4+ T cells (2 × 105/well) from 15 normal healthy individuals were cultured in wells with immobilized anti-CD3 (64.1, 100 ng/well) in the presence or absence of a metalloproteinase inhibitor KB8301 (10 μm) with or without IL-12 (10 ng/ml). After 96 h of incubation, the cells were harvested and stained with FITC-conjugated anti-CD40L MoAb or control MoAb and PE-conjugated anti-CD4 MoAb. Immunofluorescent staining, fixation, and analysis were performed as described in Materials and Methods. Mean fluorescence intensity (MFI) for staining with CD40L on cells gated for positive CD4 staining was calculated and displayed. Statistical significance was evaluated by Wilcoxon signed rank test.
Fig. 3.
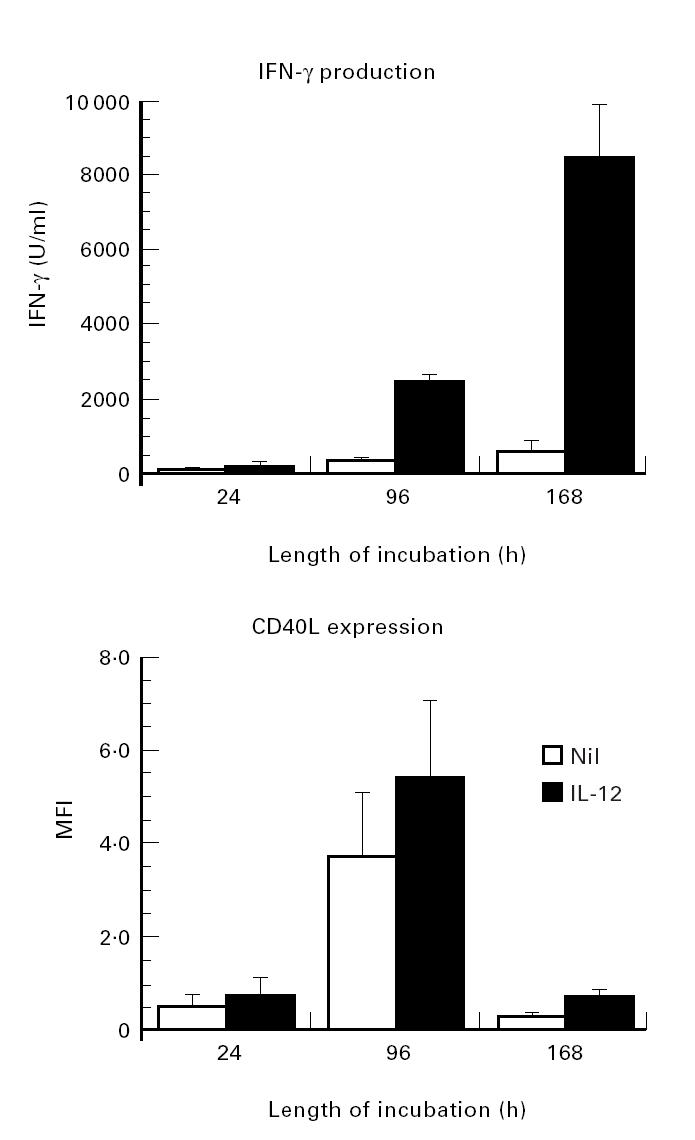
Differential kinetics of IL-12-mediated enhancement of IFN-γ production and expression of CD40L on CD4+ T cells stimulated with immobilized MoAb to CD3. CD4+ T cells (2 × 105/well) were cultured in wells with immobilized anti-CD3 (64.1, 100 ng/well) in the presence of a metalloproteinase inhibitor KB8301 (10 μm). After various periods of incubation, the supernatants were assayed for IFN-γ contents by ELISA, and the cells were reacted with control IgG1 or with anti-CD40L MoAb followed by staining with FITC-conjugated goat anti-mouse immunoglobulin and were then analysed on flow cytometry. Mean fluorescence intensity (MFI) of specific staining for CD40L was determined by subtracting the MFI of staining with the control MoAb. Data are expressed as the mean ± s.d. of three different experiments.
Effect of IL-4 and IL-10 on the expression of CD40L on immobilized anti-CD3 activated CD4+ T cells
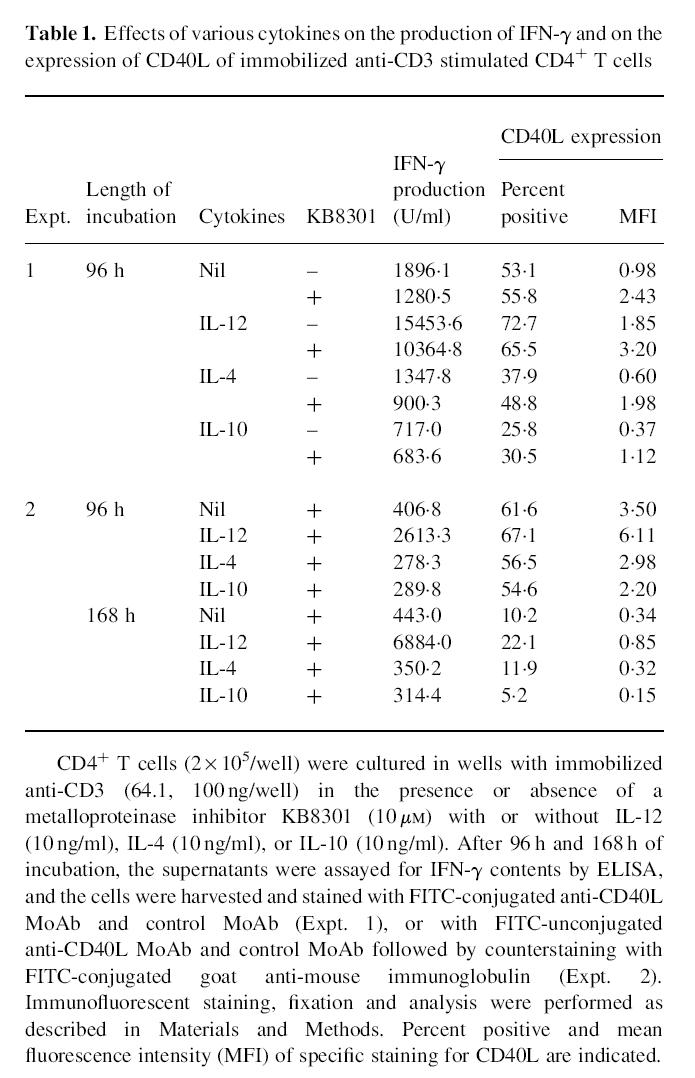
It has been shown that IL-12 and IL-4 directly induce human neonatal CD4+ T cells into Th1 and Th2 subsets, respectively [25]. In addition, IL-10 has been found to inhibit the differentiation of naive CD4+ T cells into Th1 cells [26–28]. The next experiments therefore examined the effects of IL-4 and IL-10 on the expression of CD40L on anti-CD3 stimulated CD4+ T cells to explore whether the expression of CD40L might be also related to the modulation of Th cell differentiation. As shown in Table 1, both IL-4 and IL-10 significantly decreased IFN-γ production as well as the CD40L expression of immobilized anti-CD3 activated CD4+ T cells, irrespective of the presence of KB8301 at 96 h of stimulation. The suppressive effects of IL-4 and IL-10 on CD40L expression and on IFN-γ production were still observed up to 168 h of culture. The results are therefore consistent with the hypothesis that the regulation of CD40L expression might be closely associated with the terminal differentiation of Th1 and Th2 subsets.
Table 1.
Effects of various cytokines on the production of IFN-γ and on the expression of CD40L of immobilized anti-CD3 stimulated CD4+ T cells
IFN-γ does not enhance the expression of CD40L on immobilized anti-CD3 activated CD4+ T cells
IL-12 enhanced IFN-γ production as well as the expression of CD40L on CD4+ T cells activated with immobilized anti-CD3 for 96 h, whereas IL-4 and IL-10 decreased both of them. It was thus possible that the expression of CD40L on anti-CD3 activated CD4+ T cells might be regulated directly by IFN-γ, at least within 96 h from the initiation of cultures. The next experiments therefore examined the effects of IFN-γ on the expression of CD40L on anti-CD3 activated CD4+ T cells. As shown in Fig. 4, IFN-γ did not enhance the expression of CD40L on CD4+ T cells activated with immobilized anti-CD3 after 24 h, 96 h, or 168 h of stimulation. Moreover, anti-IFN-γ did not decrease the CD40L expression of CD4+ T cells stimulated with immobilized anti-CD3 and IL-12, although it neutralized IFN-γ activity in the supernatants (data not shown). These results indicate that the enhancement of CD40L expression on immobilized anti-CD3 activated CD4+ T cells by IL-12 does not involve the direct action of IFN-γ.
Fig. 4.
Differential effects of IL-12 and IFN-γ on the expression of CD40L on CD4+ T cells stimulated with immobilized MoAb to CD3. CD4+ T cells (2 × 105/well) were cultured in wells with immobilized anti-CD3 (64.1, 100 ng/well) in the presence or absence of a metalloproteinase inhibitor KB8301 (10 μm) with or without IL-12 (10 ng/ml) or IFN-γ (5000 U/ml). After various periods of incubation, the cells were harvested and reacted with FITC-conjugated anti-CD40L MoAb (solid line), or control MoAb (dotted line). Immunofluorescent staining, fixation and analysis were performed as described in Materials and Methods. Percent positive and mean fluorescence intensity (MFI) for specific CD40L staining are indicated.
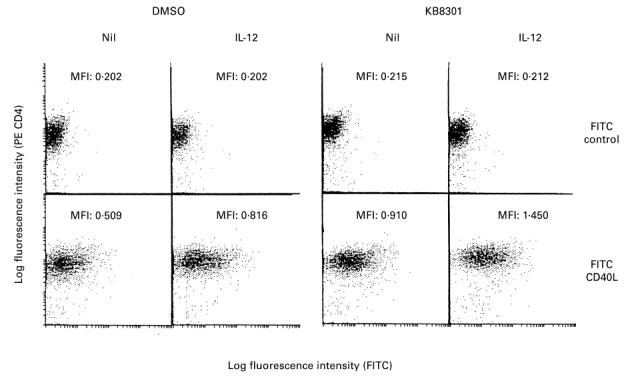
B cells do not abrogate the enhancing effects of IL- 12 on the expression of CD40L on immobilized anti-CD3 activated CD4+ T cells
Previous studies have shown that B cells down-regulate the expression of CD40L on activated CD4+ T cells [29]. The next experiments examined whether B cells might also influence the enhancing effects of IL-12 on the expression of CD40L on immobilized anti-CD3 activated CD4+ T cells. As can be seen in Fig. 5a, addition of B cells markedly decreased the expression of CD40L on the surface of CD4+ T cells stimulated with immobilized anti-CD3 for 96 h, whereas it enhanced IFN-γ production. Thus, no substantial expression of CD40L was observed on activated CD4+ T cells stimulated with immobilized anti-CD3 and B cells even with IL-12 in the absence of KB8301. However, in the presence of KB8301, where CD40L expression was slightly increased, IL-12 still enhanced the expression of CD40L on activated CD4+ T cells cultured with B cells as effectively as that on those cultured without B cells. In the presence of B cells, IL-12 enhanced the IFN-γ production of CD4+ T cells. Of note, IL-12 also enhanced the intracytoplasmic expression of CD40L by CD4+ T cells activated with immobilized anti-CD3 in the absence or presence of B cells (Fig. 5b). These results indicate that IL-12 as well as B cells up-regulate the intracytoplasmic expression of CD40L by anti-CD3 activated CD4+ T cells.
Fig. 5.
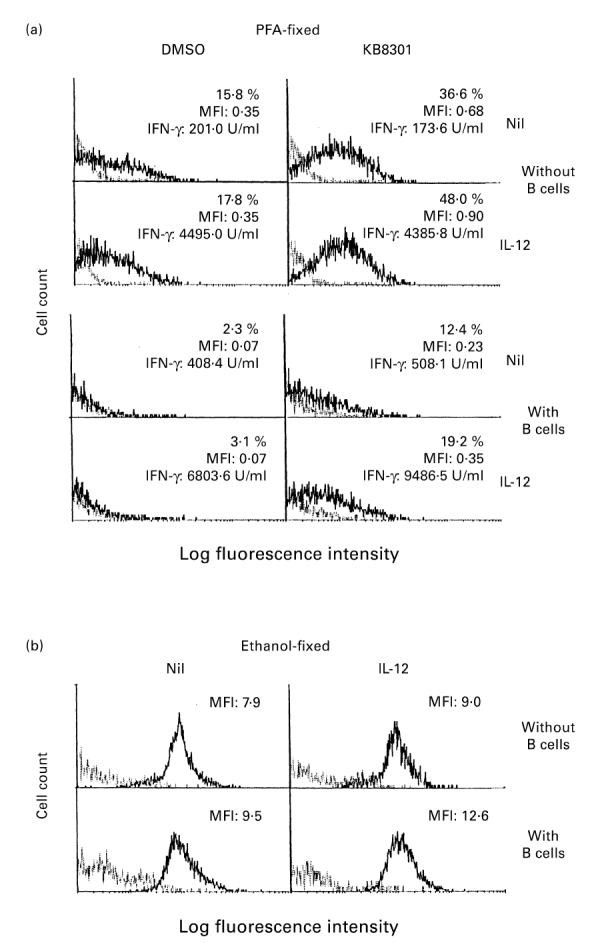
Effect of IL-12 on the expression of CD40L on CD4+ T cells stimulated with immobilized MoAb to CD3: effect of B cells. CD4+ T cells (2 × 105/well) were cultured in wells with immobilized anti-CD3 (64.1, 100 ng/well) in the presence or absence of a metalloproteinase inhibitor KB8301 (10 μm). B cells (2.5 × 104/well) and IL-12 (10 ng/ml) were added from the initiation of cultures where indicated. After 96 h of incubation, the supernatants were assayed for IFN-γ content by ELISA, and the cells were harvested and stained with PE-conjugated anti-CD4 MoAb and FITC-conjugated anti-CD40L MoAb (solid line), or control MoAb (dotted line), followed by fixation with paraformaldehyde (PFA) (a). Alternatively, the cells were fixed with ethanol cooled to −20°C prior to staining (b). The cells were then analysed by flow cytometry. The staining for CD40L or control MoAb on cells gated for positive CD4 staining is displayed. Percent positive as well as mean fluorescence intensity (MFI) for specific CD40L staining were calculated as described in Materials and Methods. The data are representative of three different experiments.
CD40–CD40L interactions have been shown to play a pivotal role in T cell-dependent B cell activation [4–8]. In fact, IL-12 enhanced the capacity of immobilized anti-CD3 activated CD4+ T cells to stimulate resting B cells to express CD25, whereas IL-10 decreased it (data not shown). By contrast, compared with CD4+ T cells activated with immobilized anti-CD3 alone, CD4+ T cells activated with immobilized anti-CD3 and IL-12 displayed stronger suppression of immunoglobulin production by B cells activated with immobilized anti-CD3 stimulated mitomycin C-treated CD4+ T cells [8] (data not shown). These results indicate that the changes of CD40L expression on anti-CD3 stimulated CD4+ T cells driven by IL-12 or IL-10 parallel their capacity to activate resting B cells and to suppress previously activated B cells. The data thus suggest that the shifts toward Th1/Th2 responses driven by IL-12 or IL-10 might also play an important role in the regulation of humoral immune responses.
DISCUSSION
The current studies examined in detail the kinetics and regulation of the expression of CD40L in immobilized anti-CD3 stimulated CD4+ T cells. As has been demonstrated in the case of Fas ligand [24], a metalloproteinase inhibitor KB8301 significantly enhanced CD40L expression on anti-CD3 activated CD4+ T cells. It is therefore possible that CD40L on activated CD4+ T cells might be cleaved into soluble forms by metalloproteinases. However, it should be noted that the inhibition of the processing of Fas ligand as well as CD40L required much higher concentrations (10 μm) of KB8301 than those which inhibit the activities of MMP1, MMP2, MMP3 and MMP9 (IC50; 0.3–0.6 nm) [19,24]. It is therefore plausible that KB8301 might enhance the expression of CD40L and Fas ligand by a mechanism distinct from inhibitions of metalloproteinases. Further studies would be necessary to elucidate the precise mechanism of the inhibition of processing of Fas ligand and CD40L by KB8301. Regardless of the mechanism of action of KB8301, IL- 12 comparably up-regulated the expression of CD40L on immobilized anti-CD3 activated CD4+ T cells in the presence or absence of KB8301. The results therefore suggest that the up-regulation of CD40L expression on activated CD4+ T cells by IL-12 might be independent of the action of KB8301.
Recently, considerable attention has been paid to the ability of IL-12 to induce the differentiation of naive CD4+ T cells into IFN-γ-producing Th1 cells [17,18,24]. By contrast, it has also been demonstrated that IL-4 promotes the differentiation of Th2 cells [30]. Thus, the balance between IL-12 and IL-4 is considered to be important for the decision between Th1 response or Th2 response. Moreover, IL-10 has been found to inhibit the differentiation of Th1 cells [26]. A previous study showed that in murine systems a Th1 clone expressed CD40L comparably to a Th2 clone, while Th1 clones expressed much more CD40L than activated CD4+ spleen T cells [13]. Since cloned T cells might have received selection bias, it remained unclear whether Th1 cells and Th2 cells express comparable levels of CD40L. The current study, however, disclosed that IL- 12 up-regulated IFN-γ production and CD40L expression in anti-CD3 stimulated CD4+ T cells, whereas IL-4 as well as IL-10 down-regulated both of them. It is therefore suggested that CD40L expression might be up-regulated in Th1 responses, but rather down-regulated in Th2 responses. It has been generally believed that the ability of IL-10 to inhibit Th1 responses might be due to the suppression of IL-12 production by antigen-presenting cells (APC) [26]. However, it should be noted that in the present study, IL-10 down-regulated the IFN-γ production and CD40L expression of immobilized anti-CD3 activated CD4+ T cells in the complete absence of APC. The data therefore indicate that IL-10 can also directly act on CD4+ T cells to inhibit Th1 responses.
IFN-γ production was significantly correlated with the CD40L expression of CD4+ T cells stimulated with immobilized anti-CD3 in the presence of various cytokines. However, IFN-γ itself did not enhance the expression of CD40L on anti-CD3 activated CD4+ T cells in the present study. Moreover, anti-IFN-γ did not decrease the CD40L expression of CD4+ T cells stimulated with immobilized anti-CD3 and IL-12, although it neutralized IFN-γ activity in the supernatants. Finally, it should be noted that the expression of CD40L on CD4+ T cells was markedly down-regulated even in the presence of IL-12 between 96 h and 168 h of cultures, during which period IFN-γ production was still markedly up-regulated by IL-12. These results indicate that the IL-12 mediated up-regulation of CD40L expression of anti-CD3 stimulated CD4+ T cells is not mediated by the action of IFN-γ. In fact, a previous study also showed that expression of CD40L was inhibited by IFN-γ on activated Th1, Th2, and CD4+ T cells in murine systems [13]. However, IFN-γ did not decrease CD40L expression of CD4+ T cells stimulated with immobilized anti-CD3 for as long as 168 h. It is therefore suggested that the effect of IFN-γ on CD40L expression of CD4+ T cells might be different in human systems from that in murine systems.
Interactions between CD40 and CD40L play a pivotal role in T cell-dependent B cell activation [4–6]. In this study, CD40L expression has been shown to be up-regulated in Th1 responses, but rather down-regulated in Th2 responses, indicating that Th1 cells provide more prominent signals for initial activation of resting B cells than Th2 cells. In fact, the current study has shown that the changes of CD40L expression on anti-CD3 stimulated CD4+ T cells driven by IL-12 or IL-10 parallel their capacity to activate resting B cells to express CD25. Of interest, recent studies have indicated that CD40–CD40L interactions exert bidirectional effects in the B cell responses depending on the state of activation of B cells and on the extent of CD40 ligation [7,8]. Thus, higher saturation of CD40 with CD40L inhibits the growth and immunoglobulin secretion of human B cell hybridomas [31] as well as those of peripheral blood B cells activated by anti-CD3 stimulated CD4+ T cells [7,8]. In fact, IL-12 enhanced the capacity of CD4+ T cells activated with immobilized anti-CD3 to suppress immunoglobulin production by activated B cells. In this regard, the up-regulation of CD40L expression in Th1 responses might be a safeguard to avoid the over-production of antigen-non-specific immunoglobulins, including autoantibodies. Collectively, it is plausible that the balances between Th1 and Th2 might influence the outcome of humoral immune responses through regulation of the extent of CD40 ligation on activated B cells.
B cells down-regulate CD40L expression of activated CD4+ T cells through receptor-mediated endocytosis followed by lysosomal degradation [29]. It has been reported that proteolytic cleavage did not account for the down-modulation of CD40L by B cells [29]. Consistently, a metalloproteinase inhibitor KB8301 did not reverse the down-modulation of CD40L in the present study. Nor did IL-12 reverse the B cell-mediated down-modulation of CD40L, although IL-12 increased CD40L expression even under the influence of B cells as effectively as in the absence of B cells. In addition, IL-12 significantly enhanced the intracytoplasmic expression of CD40L. These results strongly suggest that IL-12 might up-regulate the synthesis of CD40L without affecting either its receptor-mediated endocytosis or cleavage by metalloproteinases. The conclusion that IL-12 enhances CD40L synthesis in activated CD4+ T cells also well accounts for the observation that the IL- 12-mediated up-regulation of CD40L expression was not observed as early as at 24 h of stimulation, but was evident at 96 h of stimulation. Of interest, B cells also increased the IFN-γ production as well as the intracytoplasmic expression of CD40L by activated CD4+ T cells in the presence or absence of IL-12. It is possible that B cells might up-regulate the production of IFN-γ and CD40L through cytokines they produce, such as IL-12, or through direct cellular interactions, such as CD80–CD28 interactions, although further studies are required to delineate the precise mechanisms.
Acknowledgments
The author wishes to thank Tamiko Yanagida and Haremi Watanabe for technical assistance and Chise Kawashima for preparing the illustrations and the manuscript. This work was supported by the Program for Promotion of Fundamental Studies in Health Sciences of the Organization for Drug ADR Relief, R&D Promotion and Product Re view of Japan.
REFERENCES
- 1.Thomas Y, Rogozinski L, Chess L. Relationship between human T cell functional heterogeneity and human T cell surface molecules. Immunol Rev. 1983;74:113–28. doi: 10.1111/j.1600-065x.1983.tb01086.x. [DOI] [PubMed] [Google Scholar]
- 2.Hirohata S, Jelinek DF, Lipsky PE. T cell-dependent activation of B cell proliferation and differentiation by immobilized monoclonal antibodies to CD3. J Immunol. 1988;140:3736–-44. [PubMed] [Google Scholar]
- 3.Hollenbaugh D, Grosmaire LS, Kullas CD, et al. The human T cell antigen gp39, a member of the TNF gene family, is a ligand for the CD40 receptor: expression of a soluble form of gp39 with B cell co-stimulatory activity. EMBO J. 1992;11:4313–21. doi: 10.1002/j.1460-2075.1992.tb05530.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Banchereau J, Rousset F. Growing human B lymphocytes in the CD40 system. Nature. 1991;353:678–9. doi: 10.1038/353678a0. [DOI] [PubMed] [Google Scholar]
- 5.Jabara HH, Fu SM, Geha RS, Vercelli D. CD40 and IgE: synergism between anti-CD40 monoclonal antibody and interleukin 4 in the induction of IgE synthesis by highly purified human B cells. J Exp Med. 1990;172:1861–4. doi: 10.1084/jem.172.6.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nishioka Y, Lipsky PE. The role of CD40–CD40 ligand interaction in human T cell–B cell collaboration. J Immunol. 1994;153:1027–36. [PubMed] [Google Scholar]
- 7.Miyashita T, McIlraith MJ, Grammer AC, Miura Y, Attrep JF, Shimaoka Y, Lipsky PE. Bidirectional regulation of human B cell responses by CD40–CD40 ligand interactions. J Immunol. 1997;158:4620–33. [PubMed] [Google Scholar]
- 8.Hirohata S. The role of CD40–CD40 ligand interactions in suppression of human B cell responsiveness by CD4+ T cells. Cell Immunol. 1997;182:20–28. doi: 10.1006/cimm.1997.1209. [DOI] [PubMed] [Google Scholar]
- 9.Mosmann TR, Cherwinski H, Bond M, Giedlin MA, Coffman RL. Two-types of mouse helper T cell clone. I. Definition according to profile of lymphokine activities and secreted proteins. J Immunol. 1986;136:2348–57. [PubMed] [Google Scholar]
- 10.Wierenga EA, Snoek M, de Groot C, Chrétien I, Bos JD, Jansen HM, Kapsenberg M. Evidence for compartmentalization of functional subsets of CD4+ T lymphocytes in atopic patients. J Immunol. 1990;144:4651–6. [PubMed] [Google Scholar]
- 11.Mosmann TR, Coffman RL. TH1 and TH2 cells: different, patterns of lymphokine secretion lead to different functional properties. Ann Rev Immunol. 1989;7:145–73. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 12.Kamogawa Y, Minasi LA, Carding SR, Bottomly K, Flavell RA. The relationship of IL-4- and IFN-γ-producing T cells studied by lineage ablation of IL-4-producing cells. Cell. 1993;75:985–95. doi: 10.1016/0092-8674(93)90542-x. [DOI] [PubMed] [Google Scholar]
- 13.Roy M, Waldschmidt T, Aruffo A, Ledbetter JA, Noelle RJ. The regulation of the expression of gp39, the CD40 ligand, on normal and cloned CD4+ T cells. J Immunol. 1993;151:2497–510. [PubMed] [Google Scholar]
- 14.Wolf SF, Temple PA, Kobayashi M, et al. Cloning of cDNA for natural killer cell stimulatory factor, a heterodimeric cytokine with multiple biologic effects on T and natural killer cells. J Immunol. 1991;146:3074–81. [PubMed] [Google Scholar]
- 15.Gubler U, Chua AO, Schoenhaut DS, et al. Coexpression of two distinct genes is required to generate secreted bioactive cytotoxic lymphocyte maturation factor. Proc Natl Acad Sci USA. 1991;88:4143–7. doi: 10.1073/pnas.88.10.4143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trinchieri G. Interleukin-12 and its role in the generation of Thl cells. Immunol Today. 1993;14:335–8. doi: 10.1016/0167-5699(93)90230-I. [DOI] [PubMed] [Google Scholar]
- 17.Abbas AK, Murphy KM, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383:787–93. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- 18.Briscoe DM, Henault LE, Geehan C, Alexander SI, Lichtman AH. Human endothelial cell costimulation of T cell IFN-γ production. J Immunol. 1997;159:3247–56. [PubMed] [Google Scholar]
- 19.Yamamoto M, Tsujishita H, Hori N, Ohishi Y, Inoue S, Ikeda S, Okada Y. Inhibition of membrane-type 1 matrix metalloproteinase by hydroxamate inhibitors: an examination of the subsite pocket. J Med Chem. 1998;41:1209–17. doi: 10.1021/jm970404a. [DOI] [PubMed] [Google Scholar]
- 20.Thiele DL, Kurosaka M, Lipsky PE. Phenotype of the accessory cell necessary for mitogen-stimulated T and B cell responses in human peripheral blood: delineation by its sensitivity to the lysosomotrophic agent l-leucine methyl ester. J Immunol. 1983;131:2282–90. [PubMed] [Google Scholar]
- 21.Geppert TD, Lipsky PE. Accessory cell independent proliferation of human T4 cells stimulated by immobilized monoclonal antibodies to CD3. J Immunol. 1987;138:1660–6. [PubMed] [Google Scholar]
- 22.Hirohata S, Yanagida T. Inhibition of expression of cyclin A in human B cells by an immunosuppressant Mizoribine. J Immunol. 1995;155:5175–83. [PubMed] [Google Scholar]
- 23.Hirohata S, Oka H. Modulation of T cell production of interferon-γ by human monocytes: effect of engagement of CD14 on monocytes. Cell Immunol. 1993;151:270–82. doi: 10.1006/cimm.1993.1237. [DOI] [PubMed] [Google Scholar]
- 24.Kayagaki N, Kawasaki A, Ebata T, et al. Metalloproteinase-mediated release of human Fas ligand. J Exp Med. 1995;182:1777–83. doi: 10.1084/jem.182.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sornasse T,, Larenas PV, Davis KA, de Vries JE, Yssel H. Differentiation and stability of T helper 1 and 2 cells derived from naive human neonatal CD4+ T cells, analyzed at the single-cell level. J Exp Med. 1996;184:473–83. doi: 10.1084/jem.184.2.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Libraty DH, Airan LE, Uyemura K, Jullien D, Spellberg B, Rea TH, Modlin RL. Interferon-γ differentially regulates interleukin-12 and interleukin-10 production in leprosy. J Clin Invest. 1997;99:336–41. doi: 10.1172/JCI119162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.D'Andrea A, Aste-Amezaga M, Valiante NM, Ma X, Kubin M, Trinchieri G. Interleukin-10 inhibits human lymphocyte IFN-γ production by suppressing natural killer cell stimulatory factor/interleukin-12 synthesis in accessory cells. J Exp Med. 1993;178:1041–8. doi: 10.1084/jem.178.3.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fiorentino DF, Zlotnik A, Mosmann TR, Howard M, O'Garra A. IL-10 inhibits cytokine production by activated macrophages. J Immunol. 1991;147:3815–24. [PubMed] [Google Scholar]
- 29.Yellin MJ, Sippel K, Inghirami G, et al. CD40 molecules induce down-modulation and endocytosis of T cell surface T cell–B cell activating molecule/CD40-L. Potential role in regulating helper effector function. J Immunol. 1994;152:598–608. [PubMed] [Google Scholar]
- 30.Mosmann TR, Sad S. The expanding universe of T-cell subsets: Th1, Th2 and more. Immunol Today. 1996;17:138–46. doi: 10.1016/0167-5699(96)80606-2. [DOI] [PubMed] [Google Scholar]
- 31.Bergman MC, Attrep JF, Grammer AC, Lipsky PE. Ligation of CD40 influences the function of human Ig-secreting B cell hybridomas both positively and negatively. J Immunol. 1996;156:3118–32. [PubMed] [Google Scholar]