Abstract
Although B cell activation and subsequent immunoglobulin production are the immunopathological features of chronic inflammatory periodontal disease, in situ expression of costimulatory molecules in humoral immunity has not been investigated. In the present study we examined the expression of CD40, CD40 ligand (CD40L), CD80, CD86, CD28 and cytolytic T lymphocyte-associated antigen-4 (CTLA-4) on lymphocytes immunohistochemically. Cryostat sections were prepared from the gingival tissue samples of 14 patients with moderate to advanced adult periodontitis. In vitro kinetics of the expression of CD40L and CTLA-4 by peripheral blood T cells and that of CD80 and CD86 by peripheral blood B cells were also investigated by flow cytometry. Positive percentage expression of CD40L, CD28 and CTLA-4, and CD40, CD80 and CD86 was calculated for the number of CD3+ and CD19+ cells, respectively. Flow cytometric analysis demonstrated that the expression of CD40L and CTLA-4 on T cells, and CD80 and CD86 on B cells of peripheral blood was up-regulated upon activation. While most T cells and B cells expressed CD28, and CD80 and CD86, respectively, in gingival tissues, the expression of CD40L and CTLA-4 was lower but highly variable between specimens. Furthermore, these two molecules seemed to be expressed reciprocally in the lesion. As both CD40L and CTLA-4 expression are induced transiently by stimulation, variability in the expression of the molecules may reflect immunological activities and participation in the regulation of B cell activation of the lesion.
Keywords: costimulatory molecules, immunohistochemistry, flow cytometry, periodontitis
INTRODUCTION
Periodontitis lesions contain large numbers of B lymphocytes and plasma cells together with significant numbers of T lymphocytes [1,2]. It has been demonstrated that most T cells infiltrating periodontitis lesions are CD45RO- [3,4] and HLA-DR-expressing [5] memory/activated phenotypes. Furthermore, their cytokine profile shifts towards type 2 in periodontitis lesions where IL-4 and IL-6 are predominant [6,7]. As it is likely that both antigen-specific and polyclonal activation of B cells occurs locally [8] and that regulation of B cell activation is mediated by T cells [9], periodontitis lesion-infiltrating T cells are thought to play important roles by producing B cell activating cytokines during the development of the lesion. Moreover, the proportion of cytokine-producing cells varies among patients, suggesting that although their clinical characteristics are similar, activation stages of the infiltrating T cells differ from patient to patient or from site to site.
Taken together, immunological findings may reflect an actual disease activity which can not be detected by ordinary clinical diagnostic parameters. T cell-dependent B cell activation and differentiation require both cell–cell contact as well as cytokines derived from activated T cells. Various receptor–ligand pairs are thought to be important in contact-dependent T cell–B cell collaboration. Although the intercellular adhesion molecule-1 (ICAM-1)/leucocyte function-associated antigen-1 (LFA-1) pathway has been extensively studied [10–12], it has become evident that the most potent costimulatory signalling pathways comprise the CD28/B7 (CD80 and CD86) [13] and CD40/CD40L (CD154) systems [14]. CD28, which is expressed constitutively on the surface of all CD4+ cells and on about 50% of CD8+ cells, is up-regulated following activation [15]. However, cytolytic T lymphocyte-associated antigen-4 (CTLA-4; CD152), another ligand for B7 molecules, is not constitutively expressed on T cells. It is up-regulated following T cell activation [16]. Whereas the CD28–B7 interactions promote T cell activation, engagement of CTLA-4 by B7 appears to inhibit T cell activation [17].
While CD40 is widely expressed on various types of cells including B cells and fibroblasts, both of which are major components of inflamed gingival tissues, CD40L is expressed mainly on activated T cells. Many in vitro studies have demonstrated that ligation of CD40 induces B cell activation, resulting in the proliferation and secretion of immunoglobulin, as well as immunoglobulin heavy chain recombination in the presence of appropriate cytokines [14].
To enhance understanding of the immune response in chronic inflammatory periodontal disease, analysis of costimulatory molecules would further elucidate B cell regulation by T cells. In the present study therefore we investigate the in situ expression and distribution of costimulatory molecules in periodontitis lesions by immunohistochemical methods.
PATIENTS AND METHODS
Patients and biopsies
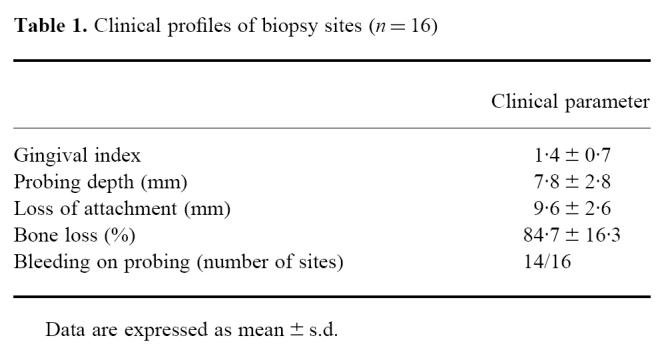
Fourteen patients with moderate to advanced adult periodontitis (AP) referred to the Periodontal Clinic of Niigata University Dental Hospital took part in this study (age 30–64 years, mean 46.9 years). Two specimens were taken from each of two patients (n = 16). Gingival biopsies were obtained at the time of periodontal surgery or at extraction of severely involved teeth after completion of initial therapy, which included motivation, oral hygiene instruction, scaling and root planing. Clinical assessments of the sampling sites are shown in Table 1. Informed consent was obtained from all patients.
Table 1.
Clinical profiles of biopsy sites (n = 16)
The biopsies were taken by making two parallel vertical incisions approx. 2 mm apart connected by a horizontal incision approx. 1 mm below the base of the pockets. The tissue was immediately embedded in OCT compound (Miles Inc., Elkhart, IN), quenched in liquid nitrogen and stored at −70°C until use.
Serial cryostat sections (6 μm in thickness) were cut from the central part of each specimen in a plane parallel to the long axis of the teeth and orientated so that the pocket epithelium, oral epithelium and connective tissues were present in the same section. Then sections were air-dried, fixed in equal parts of chloroform/acetone for 5 min, and stored at −20°C.
Immunohistochemistry
Monoclonal anti-CD28 (Nichirei, Tokyo, Japan), anti-CTLA-4 (Pharmingen, San Diego, CA), anti-CD80 (Immunotec, Marseille, France), anti-CD86 (Ancell, Bayport, MN), anti-CD40 and anti-CD40L (Serotec, Oxford, UK) were used for single staining by an alkaline-phosphatase anti-alkaline-phosphatase (APAAP) method. Monoclonal anti-CD3 and anti-CD19 (Dako, Glostrup, Denmark) were used for double staining by combining an avidin-biotin-immunoperoxidase (ABC-PO) method and an APAAP method to identify T cells and B cells. In some specimens, double stainings of CD28 and CD80, CD28 and CD86, CTLA-4 and CD80, CTLA-4 and CD86, and CD40L and CD40 were also carried out.
After rehydration in 0.05% Tris-buffered saline (TBS, pH 7.6) and blocking with normal rabbit serum (Dako), the sections were incubated with primary MoAb at a predetermined dilution, followed by rabbit anti-mouse immunoglobulins (Dako) and finally with monoclonal mouse APAAP (Dako). Colour was developed with an alkaline phosphatase substrate III kit (Vector, Burlingame, CA). For double staining, the sections were first incubated with monoclonal anti-CD3 as first primary MoAb at a predetermined dilution, followed by biotinylated horse anti-mouse IgG (Vector) and finally with ABC-PO. After colour development using 0.005% 3,3-diaminobenzidine in Tris–HCl buffer pH 7.2 containing 0.01% hydrogen peroxide, an APAAP method using monoclonal anti-CD19 as a second primary MoAb followed. Incubation for 30 min at room temperature was followed by washing for 10 min in TBS pH 7.6. Nuclei were counterstained with haematoxylin. Endogenous peroxidase and alkaline phosphatase activities were blocked by 0.17% NaN3 and 1 mm levamisole, respectively.
Cell analysis
The degree of inflammation was confirmed by haematoxylin–eosin (H–E)-stained slides. Areas of significant round cell infiltrate in the connective tissues, which contained both T cells and B cells subjacent to the pocket epithelium as determined on CD3/CD19 double-stained slides, were selected. One to three areas per specimen were selected depending on the degree of cell infiltration. Positive cells were counted for these selected foci with an ocular grid (0.04 mm2) at a magnification of ×400. The area selected for counting was re-located on the seven serial sections from each specimen using an ocular grid and histological landmarks. In each area, CD28+, CTLA-4+ and CD40L+ fractions in the number of CD3+ cells and CD80+, CD86+ and CD40+ fractions in the number of CD19+ cells were calculated by dividing the number of costimulatory molecule-positive cells by the number of CD3+ or CD19+ cells.
Peripheral blood mononuclear cell culture
In vitro kinetics of the expression of costimulatory molecules was examined for peripheral blood mononuclear cells (PBMC). PBMC were separated from three healthy adult volunteers by centrifugation of heparinized venous blood over Ficoll–Paque (Pharmacia Fine Chemicals, Piscataway, NJ). Cells were seeded in a 24-well culture plate at a concentration of 2 × 106 cells/well under a final volume of 1 ml and were cultured in RPMI 1640 supplemented with 10% human AB serum, 20 mm HEPES, 100 U/ml penicillin, 100 μg/ml streptomycin, 2 mm glutamine and 5 × 10−5m 2-mercaptoethanol (2-ME) at 37°C in a humidified atmosphere of 5% CO2 and 95% air for varying periods of time. Phorbol myristate acetate (PMA; 10 ng/ml) and calcium-ionophore A23187 (A23187; 250 ng/ml) were added to the cultures for T cell stimulation. IL-4 (200 U/ml) plus anti-CD40 MoAb (0.1 μg/ml) or anti-CD40 MoAb alone were added to the cultures for B cell stimulation.
Flow cytometric analysis
Expression of CD28, CTLA-4 or CD40L on CD3+ T cells and CD80 or CD86 on CD19+ B cells was determined by two-colour staining. At the indicated time points, cells were taken, washed and resuspended at a concentration of 1 × 106 cells/50 ml in PBS containing 1% fetal calf serum (FCS; Gibco, Grand Island, NY) and 0.1% NaN3. The cells were incubated with saturating amounts of MoAb for anti-CD28 (Nichirei), anti-CTLA-4 (Pharmingen), and anti-CD40L (Ancell) for 20 min at 4°C, washed twice, then incubated with FITC-conjugated rabbit anti-mouse IgG antibody (Dako). After blocking with mouse serum (Dako), the cells were incubated with PE-conjugated anti-CD3 (Dako). For analysis of CD80 or CD86 expression on CD19+ B cells, FITC-conjugated anti-CD80 or CD86 (Pharmingen) and PE-conjugated anti-CD19 (Dako) were used for two-colour staining. The cells were analysed using a FACScan flow cytometer (Becton Dickinson, San Jose, CA) equipped with Consort 30 software (Hewlett-Packard, CA). The lymphocyte fraction was gated using their forward and side scatter characteristics, as described elsewhere. Mouse IgG1 or IgG2a (Becton Dickinson) were used to control non-specific bindings.
Statistical analysis
The difference between the expressions of each costimulatory molecule was analysed using unpaired t-test. The statistical significance risk rate was set at P < 0.05.
RESULTS
General histological findings of the tissues
The degree of cell infiltration was confirmed by H–E-stained sections. Moderate to widespread intense infiltration was observed in connective tissues subjacent to the pocket epithelium. The areas containing both T cells and B cells, confirmed on CD3/CD19 double-stained sections, were selected for analysis of the expression of costimulatory molecules in the periodontitis lesion. In CD3/CD19 double-stained sections, CD19+ cells were the dominant infiltrating cell type. CD3+ cells formed clusters or were scattered beneath the pocket epithelium (Fig. 1a).
Fig. 1.
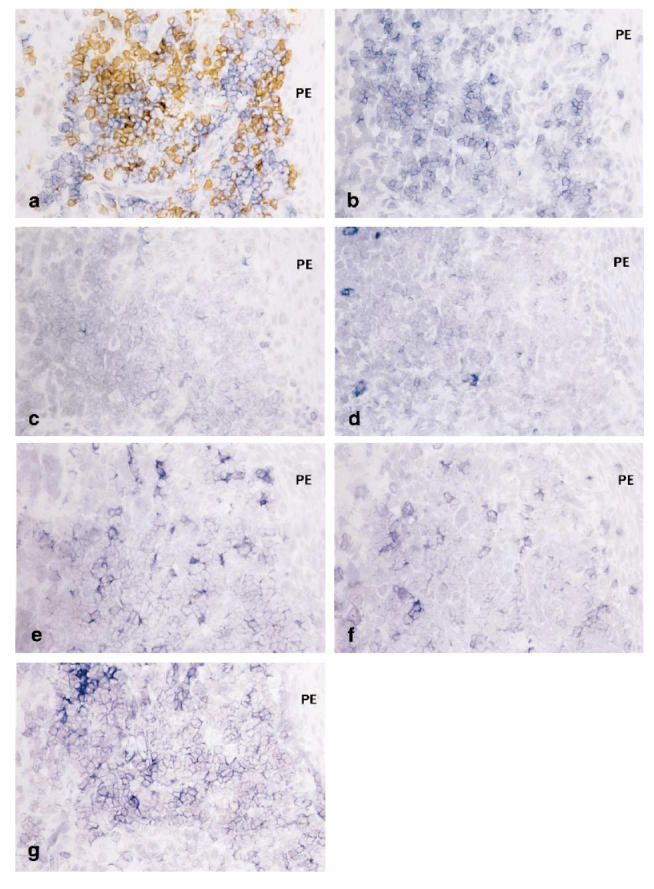
Immunoreactivity for costimulatory molecules in the periodontitis lesion. Double staining for CD3/CD19 (a) and single staining for CD28 (b), CTLA-4 (c), CD40L (d), CD80 (e), CD86 (f) and CD40 (g) was performed on sequential sections. Costimulatory molecule immunoreactivity was visualized using alkaline-phosphatase anti-alkaline-phosphatase (APAAP) and Substrate kit III (Vector). For double staining, CD3 was immunolabelled with peroxidase, whereas CD19 was immunlabelled with alkaline phosphatase (×100). PE, Pocket epithelium.
There was no correlation between the clinical parameters of the biopsy sites and the degree of inflammatory cell infiltrates or the areas of cell infiltration in the sections.
Immunohistochemical detection of CD28 and CD80/CD86 in periodontitis lesions
In most specimens, the distribution of CD28+ cells was consistent with that of CD3+ cells (Fig. 1a,b). The mean percentage of CD28+ cells within CD3+ cells was nearly 100% (Fig. 3). CD80 and CD86 were extensively expressed on the infiltrating cells in the connective tissues beneath the pocket epithelium. The distribution of CD80 and CD86 was consistent with that of CD19+ B cells, as evidenced by the findings on sequential sections of CD80/CD86 single staining and CD3/CD19 double staining (Fig. 1a,e,f).
Fig. 3.
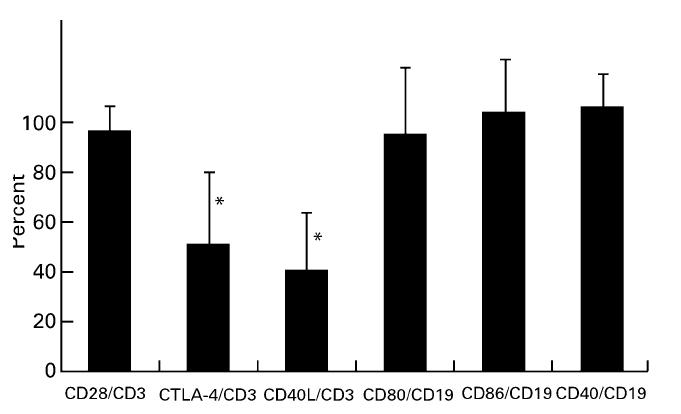
Expression of each costimulatory molecule. All positive cells for each MoAb in the selected areas were counted. CD28, CTLA-4 and CD40L were expressed as a percentage of the number of CD3+ T cells. CD80, CD86 and CD40 were expressed as a percentage of the number of CD19+ B cells (n = 16). Data are expressed as mean ± s.d. *CTLA-4 and CD40L expression was significantly lower than CD28 expression (P < 0.01).
Although the mean percentage of CD80+ and CD86+ fractions for the CD19+ subset was high, slight variations were found between specimens (65% to almost 100%) (Fig. 3). In order to detect cell–cell interaction via CD28 and CD80/CD86, double staining was carried out. CD28+ T cells and CD80/CD86+ cells were in close proximity and direct cell–cell contact of CD28+ and CD80/CD86+ cells was sometimes observed (Fig. 2a,b).
Fig. 2.
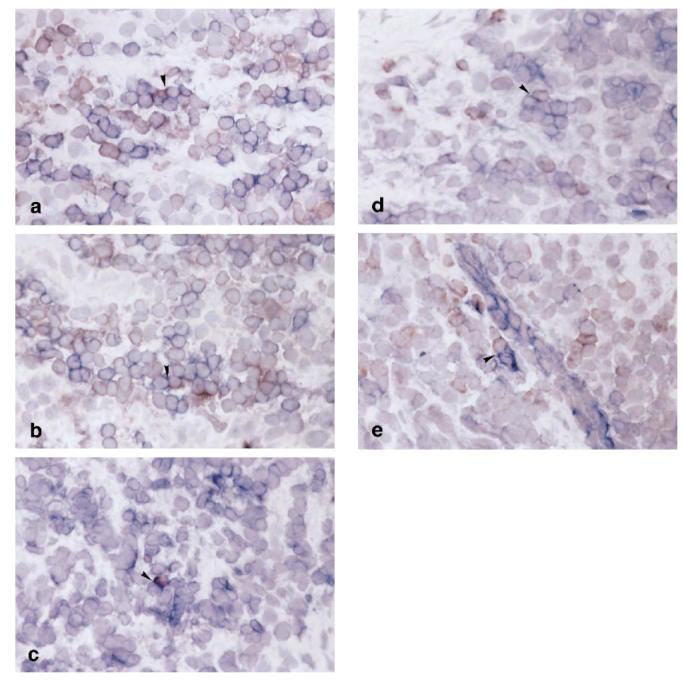
Double staining for CD28/CD80 (a), CD28/CD86 (b), CTLA-4/CD80 (c), CTLA-4/CD86 (d) and CD40L/CD40 (e) in the periodontitis lesion. In CD28/CD80 and CD28/CD86 double staining, CD80 and CD86 were immunolabelled with peroxidase, whereas CD28 was immunolabelled with alkaline phosphatase. In CTLA-4/CD80, CTLA-4/CD86 and CD40L/CD40 double staining, CTLA-4 and CD40L were immunolabelled with peroxidase, whereas CD80, CD86 and CD40 were immunolabelled with alkaline phosphatase (×200). Cognate interaction between receptors and their ligands is seen (arrowheads).
There was no correlation between percentages of CD80- and CD86-expressing cells within CD19+ cells.
Immunohistochemical detection of CTLA-4 and CD80/CD86 in periodontitis lesions
The number of CTLA-4+ cells was much lower than that of CD28+ cells (Fig. 3; P < 0.01). The distribution of CTLA-4+ cells was somewhat scattered even in the cluster of CD3+ cells (Fig. 1c). Furthermore, the proportion of CTLA-4+ cells within the CD3+ cells varied greatly among patients, from around 10% to > 90% (Fig. 4). In double-stained sections of CTLA-4 and CD80/CD86, CTLA-4+ cells were also observed in infiltrates of CD80/CD86+ cells (Fig. 2c,d).
Fig. 4.
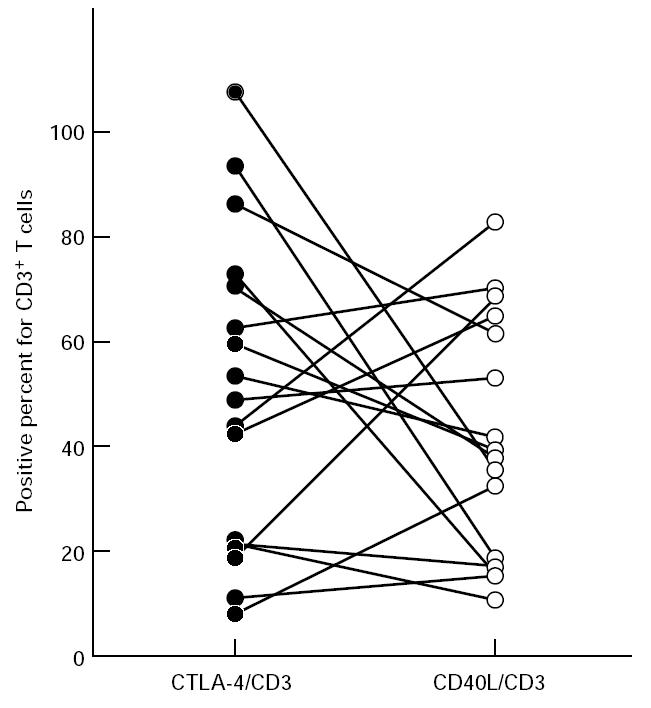
Frequency of CTLA-4- (•) and CD40L-producing cells (○) for CD3+ T cells in periodontitis lesions (n = 16). The plots with the connecting lines represent the individual tissue samples studied.
Correlations between the proportion of CTLA-4+ cells and other costimulatory molecule expressions were not found.
Immunohistochemical detection of CD40L and CD40 in periodontitis lesions
CD40L+ cells were also scattered in the same areas as CD3+ cells (Fig. 1d). The mean percentage of CD40L+ cells within the CD3+ cells was approx. 40% and was significantly lower than that of CD28 (Fig. 3; P < 0.01). Like CTLA-4, the expression of CD40L varied greatly between specimens. Frequency of CTLA-4+ and CD40L+ cells within CD3+ cells in the same specimen tended to show reciprocal expression (Fig. 4).
The distribution of CD40+ cells was similar to that of CD19+ cells (Fig. 1g). In addition, fibroblast-like cells, endothelial cells (data not shown) and the basal epithelium (Fig. 1g) were also positive for CD40. The percentage of CD40+ cells in the CD19+ cells was nearly 100% (Fig. 3). CD40L+ cells were found in close proximity to the CD40+ lymphocytes and the basal epithelium, as shown in double staining of CD40L and CD40 (Fig. 2e).
Flow cytometric detection of costimulatory molecule expression
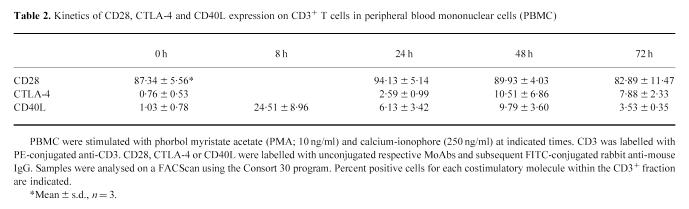
As shown in Table 2, transient up-regulation of expression was observed for CTLA-4 and CD40L upon stimulation. However, their kinetics were slightly different. Whereas CTLA-4 peaked at 48 h, CD40L peaked at 8 h.
Table 2.
Kinetics of CD28, CTLA-4 and CD40L expression on CD3+ T cells in peripheral blood mononuclear cells (PBMC)
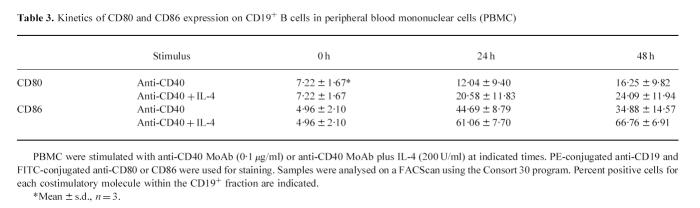
The stimulatory effect of anti-CD40 MoAb was more prominent for the expression of CD86 than that of CD80 and the effect was further enhanced by adding IL-4 in the culture (Table 3).
Table 3.
Kinetics of CD80 and CD86 expression on CD19+ B cells in peripheral blood mononuclear cells (PBMC)
DISCUSSION
In the present study, we demonstrated that the proportions of costimulatory molecule-expressing cells were high in the gingival tissues of periodontitis patients. We examined CD40 and its counter receptor, CD40L, and CD28 and CTLA-4 and their counter receptors, CD80 and CD86, by means of immunohistochemistry and flow cytometry. These costimulatory molecules have been reported to play important roles in humoral immune response and cell function regulation [13,18]. B cell activation and subsequent immunoglobulin production in periodontitis lesions is one of the immunopathological features of periodontitis [1,19]. A series of our studies has demonstrated variable stages of B cell activation in periodontitis lesions [4]. Differential expressions of cytokines [6,7,20] in these lesions led us to investigate costimulatory molecule expression.
CD40L expression was highly variable from specimen to specimen. As the CD40L molecule is expressed transiently after stimulation [21] and is expressed on activated mature T cells but not on resting T cells, variability in the expression of CD40L strongly suggests that the activation stages of infiltrating T cells differ among specimens obtained from clinically similar diseased sites. CD40L and its counter receptor CD40 have reportedly been involved in cognate T and B cell interactions which, in turn, are important for humoral immunity [22]. The results of CD40L expression in the present study suggest that in active periodontitis lesions, CD40L-expressing activated T cells stimulate B cells by cross-linking CD40 molecules to proliferate and differentiate to plasma cells and subsequent immunoglobulin production. Variable proportions of CD23-expressing B cells in periodontitis lesions [4] may also indicate an important role of CD40L in the regulation of B cell activation in periodontitis.
CD40, the counter receptor for CD40L, seems to be expressed mainly on B cells in periodontitis lesions, as evidenced by the staining of CD19 on sequential sections. However, it is obvious that some fibroblast-like cells, endothelial cells and the basal epithelium also expressed CD40. In some specimens, the CD40 count exceeded that of CD19. It is conceivable that CD40+ cells other than B cells were also counted in those specimens in spite of dominant lymphocytic infiltration of the analysed area. This is discussed further later. A recent report demonstrated that gingival fibroblasts expressed CD40 and the CD40 engagement resulted in up-regulation of IL-6 production [23]. Although we were unable to detect direct interaction between CD40L-expressing activated T cells and CD40-expressing gingival fibroblasts on double-stained sections, the finding of IL-6 production by gingival fibroblasts in situ [7] suggests that CD40–CD40L interaction could be involved in the regulation of gingival fibroblast function.
It is now well established that both CD40/CD40L [24] and CD28/B7 [25] signalling pathways play a critical role in B cell response. CD28 is expressed on the surface of most CD4+ T cells and approx. 50% of CD8+ T cells in humans [15]. In the present study, the mean proportion of CD28-expressing T cells was as high as 98% in the analysed areas, suggesting that CD4+ T cells were predominant in periodontitis lesions. This finding is supported by our previous studies [7].
Two well characterized counter receptors for CD28, CD80 and CD86, are shown to be induced by signalling through CD40, through either anti-CD40 MoAbs or activated T cells expressing CD40L [26,27]. In the present study, large numbers of CD80- and CD86-expressing cells were identified in periodontitis lesions. In addition to their expression on activated B cells, both molecules can also be detected on a variety of antigen-presenting cells (APC) including activated monocytes, dendritic cells and Langerhans cells, as well as on activated T cells [13]. CD28 and CD80 or CD86 were stained separately in most positive cells, but in some cells were simultaneously stained. We estimated the expression of CD80 and CD86 as percentages for CD19+ B cells, because the distribution of CD80 or CD86 coincided with that of CD19 on the serial sections. However, the possibility that these costimulatory molecules are expressed by macrophages and activated T cells can not be excluded, since periodontitis lesions contain both cell types, even though there are far fewer macrophages than B cells [28,29]. In fact, like CD40, the number of CD80 and CD86 exceeded that of CD19 in some specimens. Although the cell types expressing CD86 do not differ from CD80, each molecule is differentially expressed on populations of APC. On B cells, CD86 expression is rapid following activation, whereas CD80 expression appears significantly later [30]. While some specimens in the present study demonstrated similar proportions of CD80- and CD86-expressing cells, others showed higher proportions of CD86-expressing cells. Considering the differential expression of CD80 and CD86, the former indicate a later stage of activation while the latter reflect an earlier stage. In addition to their differential expressions of CD80 and CD86, they are functionally different. Freeman et al. [31] demonstrated that CD80 and CD86 equivalently costimulated IL-2 and interferon-gamma (IFN-γ) production and IL-2 receptor α- and γ-chain expression. CD86, however, induced significantly more IL-4 production than did CD80. Although CD86 induced significantly more IL-4 production than CD80, with the greatest difference seen in CD4+CD45RA+ naive T cells, CD86 also induced 3–11-fold more IL-4 than did CD80 in previously stimulated T cells or CD4+CD45RO+ T cells.
These results are quite supportive of our previous findings, in which a high proportion of IL-4-producing memory T cells existed in periodontitis lesions [6]. Furthermore, IL-4 is one of the most potent inducers of CD86 and, to a lesser extent, CD80 on B cells [32,33]. In fact, the addition of IL-4 to the cultures for B cell stimulation demonstrated further up-regulation of the expression of CD80 and CD86, with a much higher expression of CD86. Taking all data together, it is possible to assume that IL-4 production by memory T cells and the subsequent up-regulation of CD80 and CD86 induce further production of IL-4, resulting in aberrant B cell stimulation in periodontitis.
Another ligand for CD80 or CD86, CTLA-4 was expressed in different proportions from specimen to specimen. Like CD40L, CTLA-4 is not constitutively expressed on T cells and is up-regulated following T cell activation [16]. It is reported that in both human [16] and murine [17] systems, cell surface expression of CTLA-4 peaks 48 h after activation, returning to background levels by 96 h. Our in vitro stimulation experiment using peripheral blood T cells also confirmed transient expression of CTLA-4. Taking these facts together, the presence of CTLA-4-expressing T cells in periodontitis lesions suggests that T cells in the lesion are activated locally or that recently activated T cells migrate into the tissue.
In contrast to other costimulatory molecules, the major role of CTLA-4 in T cell activation is to deliver a negative signal [17]. Kearney et al. [34] have described how animals treated with either anti-CTLA-4 MoAb or Fab fragments of the anti-CTLA-4 MoAb showed augmented antigen-mediated clonal expansion using an adoptive transfer model. In this regard, expressions of CTLA-4 and CD40L seem to be reciprocal, although not statistically significant. As the expressions of CTLA-4 and CD40L were most dynamic in the lesion, these molecules might be markers indicative of periodontal disease activity.
Finally, the role of receptor–ligand interaction in the immune system is becoming more complex. Costimulatory molecules regulate the expression of each other, and there is increasing evidence that the functions of these molecules are more complicated than previously thought. In order to elucidate the precise role of costimulatory molecules and their relation to the disease stages of periodontitis, an in vitro system or animal model would be helpful, in which sequential change of expression could be investigated.
Acknowledgments
This work was supported by grants from the Ministry of Education, Science, Sports and Culture of Japan (07407054, 07457454) and a Fund for Scientific Promotion of Tanaka Industries Co., Ltd, Niigata, Japan.
REFERENCES
- 1.Mackler BF, Frostad KB, Robertson PB, Levy BM. Immunoglobulin bearing lymphocytes and plasma cells in human periodontal disease. J Periodont Res. 1977;12:37–45. doi: 10.1111/j.1600-0765.1977.tb00107.x. [DOI] [PubMed] [Google Scholar]
- 2.Seymour GJ, Greenspan JS. The phenotypic characterization of lymphocyte subpopulations in established human periodontal disease. J Periodont Res. 1979;14:39–46. doi: 10.1111/j.1600-0765.1979.tb00216.x. [DOI] [PubMed] [Google Scholar]
- 3.Gemmell E, Feldner B, Seymour GJ. CD45RA and CD45RO positive CD4 cells in human peripheral blood and periodontal disease tissue before and after stimulation with periodontopathic bacteria. Oral Microbiol Immunol. 1992;7:84–88. doi: 10.1111/j.1399-302x.1992.tb00514.x. [DOI] [PubMed] [Google Scholar]
- 4.Yamazaki K, Nakajima T, Aoyagi T, Hara K. Immunohistological analysis of memory T lymphocytes and activated B lymphocytes in tissues with periodontal disease. J Periodont Res. 1993;28:324–34. doi: 10.1111/j.1600-0765.1993.tb01076.x. [DOI] [PubMed] [Google Scholar]
- 5.Takeuchi Y, Yoshie H, Hara K. Expression of interleukin-2 receptor and HLA-DR on lymphocyte subsets of gingival crevicular fluid in patients with periodontitis. J Periodont Res. 1991;26:502–10. doi: 10.1111/j.1600-0765.1991.tb01802.x. [DOI] [PubMed] [Google Scholar]
- 6.Yamazaki K, Nakajima T, Gemmell E, Polak B, Seymour GJ, Hara K. IL-4- and IL-6-producing cells in human periodontal disease tissue. J Oral Pathol Med. 1994;23:347–53. doi: 10.1111/j.1600-0714.1994.tb00074.x. [DOI] [PubMed] [Google Scholar]
- 7.Yamazaki K, Nakajima T, Hara K. Immunohistological analysis of T cell functional subsets in chronic inflammatory periodontal disease. Clin Exp Immunol. 1995;99:384–91. doi: 10.1111/j.1365-2249.1995.tb05562.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tew J, Engel D, Mangan D. Polyclonal B-cell activation in periodontitis. J Periodont Res. 1989;24:225–41. doi: 10.1111/j.1600-0765.1989.tb01787.x. [DOI] [PubMed] [Google Scholar]
- 9.Carpenter AB, Sully EC, Ranney RR, Bick PH. T-cell regulation of polyclonal B-cell activation induced by extracts of oral bacteria associated with periodontal diseases. Infect Immun. 1984;43:326–36. doi: 10.1128/iai.43.1.326-336.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Springer TA. Adhesion receptors of the immune system. Nature. 1990;346:425–34. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- 11.Tohma S, Hirohata S, Lipsky PE. The role of CD11a/CD18–CD54 interactions in human T cell-dependent B cell activation. J Immunol. 1991;146:492–9. [PubMed] [Google Scholar]
- 12.Tohma S, Lipsky PE. Analysis of the mechanisms of T cell-dependent polyclonal activation of human B cells: induction of human B cell responses by fixed activated T cells. J Immunol. 1991;146:2544–52. [PubMed] [Google Scholar]
- 13.Lenschow DL, Walunas TL, Bluestone JA. CD28/B7 system of T cell costimulation. Ann Rev Immunol. 1996;14:233–58. doi: 10.1146/annurev.immunol.14.1.233. [DOI] [PubMed] [Google Scholar]
- 14.Banchereau J, Bazan F, Blanchard D, et al. The CD40 antigen and its ligand. Ann Rev Immunol. 1994;12:881–922. doi: 10.1146/annurev.iy.12.040194.004313. [DOI] [PubMed] [Google Scholar]
- 15.June CH, Ledbetter JA, Linsley PS, Thompson CB. Role of the CD28 receptor in T-cell activation. Immunol Today. 1990;11:211–6. doi: 10.1016/0167-5699(90)90085-n. [DOI] [PubMed] [Google Scholar]
- 16.Linsley PS, Greene JL, Tan P, et al. Coexpression and functional cooperation of CTLA-4 and CD28 on activated T lymphocytes. J Exp Med. 1992;176:1595–604. doi: 10.1084/jem.176.6.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walunas TL, Lenschow DJ, Bakker CY, et al. CTLA-4 can function as a negative regulator of T cell activation. Immunity. 1994;1:405–13. doi: 10.1016/1074-7613(94)90071-x. [DOI] [PubMed] [Google Scholar]
- 18.Foy TM, Aruffo A, Bajorath J, Buhlmann JE, Noelle RJ. Immune regulation by CD40 and its ligand gp39. Ann Rev Immunol. 1996;14:591–617. doi: 10.1146/annurev.immunol.14.1.591. [DOI] [PubMed] [Google Scholar]
- 19.Brandtzaeg P, Tolo K. Immunoglobulin systems of the gingiva. In: Lehner T, editor. The borderland between caries and periodontal disease. London: Academic Press; 1977. pp. 145–83. [Google Scholar]
- 20.Yamazaki K, Nakajima T, Kubota Y, Gemmell E, Seymour GJ, Hara K. Cytokine messenger RNA expression in chronic inflammatory periodontal disease. Oral Microbiol Immunol. 1997;12:281–7. doi: 10.1111/j.1399-302x.1997.tb00392.x. [DOI] [PubMed] [Google Scholar]
- 21.Lane P, Traunecker A, Hubele S, Inui S, Lanzavecchia A, Gray D. Activated human T cells express a ligand for the human B cell-associated antigen CD40 which participates in T cell-dependent activation of B lymphocytes. Eur J Immunol. 1992;22:2573–8. doi: 10.1002/eji.1830221016. [DOI] [PubMed] [Google Scholar]
- 22.Van den Eertwegh AJM, Noelle RJ, Roy M, et al. In vivo CD40–gp39 interactions are essential for thymus-dependent humoral immunity. I. In vivo expression of CD40 ligand, cytokines, and antibody production delineates sites of cognate T–B cell interactions. J Exp Med. 1993;178:1555–65. doi: 10.1084/jem.178.5.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sempowski GD, Chess PR, Moretti AJ, Padilla J, Phipps RP, Blieden TM. CD40 mediated activation of gingival and periodontal ligament fibroblasts. J Periodontol. 1997;68:284–92. doi: 10.1902/jop.1997.68.3.284. [DOI] [PubMed] [Google Scholar]
- 24.Nishioka Y, Lipsky PE. The role of CD40–CD40 ligand interaction in human T cell-B cell collaboration. J Immunol. 1994;153:1027–36. [PubMed] [Google Scholar]
- 25.de Boer M, Kasran A, Kwekkeboom J, Walter H, Vandenberghe P, Ceuppens JL. Ligation of B7 with CD28/CTLA-4 on T cells results in CD40 ligand expression, interleukin-4 secretion and efficient help for antibody production by B cells. Eur J Immunol. 1993;23:3120–5. doi: 10.1002/eji.1830231212. [DOI] [PubMed] [Google Scholar]
- 26.Ranheim EA, Kipps TJ. Activated T cells induce expression of B7/BB1 on normal or leukemic B cells through a CD40-dependent signal. J Exp Med. 1993;177:925–35. doi: 10.1084/jem.177.4.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roy M, Aruffo A, Ledbetter J, Linsley P, Kehry M, Noelle R. Studies on the interdependence of gp39 and B7 expression and function during antigen-specific immune responses. Eur J Immunol. 1995;25:596–603. doi: 10.1002/eji.1830250243. [DOI] [PubMed] [Google Scholar]
- 28.Johannessen AC, Nilsen R, Knudsen GE, Kristoffersen T. In situ characterization of mononuclear cells in human chronic marginal periodontitis using monoclonal antibodies. J Periodont Res. 1986;21:113–27. doi: 10.1111/j.1600-0765.1986.tb01444.x. [DOI] [PubMed] [Google Scholar]
- 29.Stoufi ED, Taubman MA, Ebersole JL, Smith DJ, Stashenko PP. Phenotypic analyses of mononuclear cells recovered from healthy and diseased human periodontal tissues. J Clin Immunol. 1987;7:235–45. doi: 10.1007/BF00915729. [DOI] [PubMed] [Google Scholar]
- 30.Lenschow DJ, Sperling AI, Cooke MP, et al. Differential up-regulation of the B7-1 and B7-2 costimulatory molecules after Ig receptor engagement by antigen. J Immunol. 1994;153:1990–7. [PubMed] [Google Scholar]
- 31.Freeman GJ, Boussiotis VA, Anumanthan A, et al. B7-1 and B7-2 do not deliver identical costimulatory signals, since B7-2 but not B7-1 preferentially costimulates the initial production of IL-4. Immunity. 1995;2:523–32. doi: 10.1016/1074-7613(95)90032-2. [DOI] [PubMed] [Google Scholar]
- 32.Vallé A, Aubry J-P, Durand I, Banchereau J. IL-4 and IL-2 upregulate the expression of antigen B7, the B cell counterstructure to T cell CD28: an amplification mechanism for T–B cell interactions. Int Immunol. 1991;3:229–35. doi: 10.1093/intimm/3.3.229. [DOI] [PubMed] [Google Scholar]
- 33.Jeannin P, Delneste Y, Lecoanet-Henchoz S, Gauchat J-F, Ellis J, Bonnefoy J-Y. CD86 (B7-2) on human B cells. J Biol Chem. 1997;272:15613–9. doi: 10.1074/jbc.272.25.15613. [DOI] [PubMed] [Google Scholar]
- 34.Kearney ER, Walunas TL, Karr RW, et al. Antigen-dependent clonal expansion of a trace population of antigen-specific CD4+ T cells in vivo is dependent on CD28 costimulation and inhibited by CTLA-4. J Immunol. 1995;155:1032–6. [PubMed] [Google Scholar]