Abstract
The aim of this study was to investigate the production of anti-Ro/SS-A and anti-La/SS-B antibodies in peripheral blood (PB) of patients with Sjögren's syndrome (SS). The ELISPOT method was performed to quantify the frequency of PB lymphocytes spontaneously secreting anti-Ro/SS-A and/or anti-La/SS-B antibodies. The total number of IgG-, IgA- and IgM-producing cells was also quantified. The recombinant Ro 52-kD, Ro 60-kD and La 48-kD proteins were used as target antigens. Three of 18 SS patients had PB lymphocytes secreting IgG antibodies against the recombinant Ro 52-kD protein. The same three patients had high serum titres of anti-Ro 52-kD antibodies. In addition, these patients were classified as having severe disease, and all three had focus scores of ≥ 8 in biopsies of the labial salivary glands (LSG). The correlation between the number of PB cells producing IgG antibodies against the recombinant Ro 52-kD protein and the focus score was significant (P < 0.01). The results indicate that only SS patients with severe disease and high degree of local inflammation in LSG have B cells producing anti-Ro/SS-A antibodies in PB. Thus, most of the spontaneous autoantibody production must take place in other body compartments, e.g. in exocrineglands and probably also in the lymphoid organs and/or other mucosal sites.
Keywords: Sjögren's syndrome, peripheral blood, Ro/SS-A, La/SS-B
INTRODUCTION
Sjögren's syndrome (SS) is a chronic autoimmune rheumatic disease characterized by severe dryness of the eyes and mouth, probably resulting from the lymphocytic infiltration of the lachrymal and salivary glands. In primary SS, patients have no associated connective tissue disease, while patients with secondary SS per definition also have another rheumatic disease, most commonly rheumatoid arthritis (RA) or systemic lupus erythematosus (SLE).
The presence of anti-Ro/SS-A or anti-La/SS-B autoantibodies is one of the classification criteria suggested by the European Community Study Group on Diagnostic Criteria for SS [1]. Anti-Ro/SS-A antibodies can be detected in about 70% of SS patients, while anti-La/SS-B antibodies are found in about 60% of the patients [2]. An association between these antibodies and certain subsets of the disease has been demonstrated [3,4].
The Ro/SS-A antigen is a RNP complex containing at least two proteins, Ro 52 kD and Ro 60 kD [5,6]. Four distinct but related human cytoplasmic RNAs (hY1, hY3, hY4 and hY5) have been identified as members of the complex. These RNAs are transcribed by RNA polymerase III, and range in size between 83 and 112 nucleotides [7]. The biological functions of the components of the Ro/SS-A complex are not known, but there is some evidence that the Ro 60-kD protein is involved in a discard pathway for defective 5S rRNA precursors [8]. The La/SS-B antigen consists of a 48-kD protein, which can bind transiently to the poly U tail of RNA polymerase III transcripts, including the hY RNAs [9]. A function for the La/SS-B protein as a transcription termination factor for RNA polymerase III transcripts has been reported [10].
In this study the enzyme-linked immunospot (ELISPOT) assay [11] was performed to assess the presence of anti-Ro/SS-A- and anti-La/SS-B-producing cells in peripheral blood (PB) of SS patients. The anti-Ro/SS-A and anti-La/SS-B antibodies have in previous studies been demonstrated in saliva of SS patients [12,13], indicating a local production of these autoantibodies. Anti-Ro 52-kD antibody-producing cells have also been found in labial salivary glands (LSG), spleen and lymph nodes of MRL/lpr mice [14]. We have in a recent study demonstrated anti-Ro/SS-A antibody-producing cells in LSG and PB of primary SS patients [15]. However, since PB was obtained from only two SS patients, we wanted to extend the study by including blood from a higher number of patients. The aim of this study was therefore to assess to what extent anti-Ro/SS-A and/or anti-La/SS-B antibodies are produced by PB lymphocytes of patients with SS.
PATIENTS AND METHODS
Patients
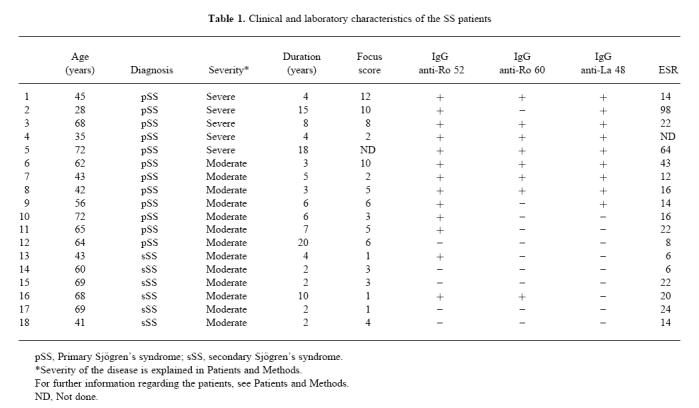
Peripheral blood was obtained from 18 female patients, aged 28–72 years (mean 56 years), with clinically definite SS [1] (Table 1). These patients were attending the Department of Rheumatology, Haukeland University Hospital (Bergen, Norway). Twelve patients had primary SS and six had secondary SS. Of those with secondary SS four had RA and two SLE. Of the 18 SS patients 13 were serologically positive for antibodies against Ro 52 kD, eight were positive for antibodies against Ro 60 kD and nine were positive for antibodies against La 48 kD as tested in ELISA [16]. All the Ro 60 kD- and La 48 kD-positive patients were also positive for Ro 52 kD. Seventeen patients had focus scores of ≥ 1 after histological evaluation of LSG biopsies [17]. The patients were divided into two groups, depending on the degree of disease severity. Five patients were classified as having severe disease [17–20], while 13 patients had moderate disease. Patients in the first group had severe clinical symptoms and extraglandular manifestations. They complained about extreme fatigue and long-lasting pain in joints and/or muscles. All five had high titres of IgG antibodies against Ro 52 kD and La 48 kD, and three had a focus score of ≥ 8. Eleven healthy females, aged 26–63 years (mean 40 years), served as controls.
Table 1.
Clinical and laboratory characteristics of the SS patients
The study was approved by the Committee of Ethics at the University of Bergen, and informed consent was obtained from all patients.
Recombinant proteins
cDNA encoding the Ro 52 kD, Ro 60 kD and La 48 kD were obtained as described previously [16,21,22]. For Ro 60 kD cDNA encoding amino acids 181–320 encompassing the immunodominant part of this protein was used [16].
Recombinant proteins were cloned into the pMAL vector (New England Biolabs, Beverly, MA) as described elsewhere [14] for expression of soluble recombinant fusion proteins with maltose binding protein (MaBP) as the vector-encoded fusion partner. The pMAL constructs were transformed into Escherichia coli TB-1 cells (New England Biolabs). The cultures were grown at 37°C. Expression of the recombinant protein was induced by addition of 0.3 mm isopropylthiogalactosidase (IPTG; Pharmacia, Uppsala, Sweden). Cell extracts were prepared and the recombinant proteins were purified using an amylose column according to manufacturer's instructions (New England Biolabs). Protein-containing fractions were pooled and the protein concentration was determined with the BioRad Protein assay (BioRad Labs, Richmond, CA). Purity of the proteins was estimated on coomassie-stained gel (Fig. 1).
Fig. 1.
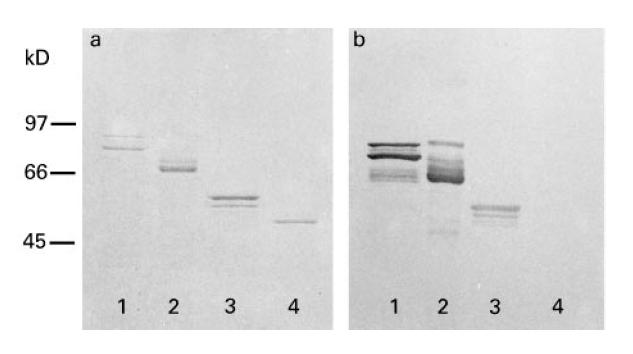
Purified recombinant antigens separated on 10% SDS–PAGE and analysed by (a) coomassie staining, and (b) immunoblotting of an identical gel, stained with serum from one of the included patients (patient 3) diluted 1:1000. Lane 1, La 48 kD; lane 2, Ro 52 kD; lane 3, Ro 60 kD; lane 4, maltose binding protein ( MaBP). Molecular weight markers are indicated to the left ( kD). Note that recombinant fusion proteins were used as described in Patients and Methods, with full-length clones for La 48 kD and Ro 52 kD. The Ro 60-kD clone corresponds to aa 181–320. Predicted molecular weights for the recombinant proteins were: La 48 kD, 92 kD; Ro 52 kD, 96 kD; Ro 60 kD, 60 kD; MaBP, 50 kD.
Immunoblotting
Purified proteins were boiled for 5 min in sample buffer containing 5% SDS and separated on 10% SDS–PAGE gels, transferred electrophoretically to nitrocellulose and probed for 2 h with sera diluted 1:1000. Visualization of bound IgG was performed using an ALP-conjugated affinity-purified rabbit anti-human IgG-specific antibody (Dakopatts, Glostrup, Denmark) diluted 1:2000. As substrate solution nitroblue tetrazolium and 5-bromo-4-chloro-3-indolyl phosphate (Sigma, St Louis, MO) were used. Between each step a 5-min wash with PBS–Tween was performed three times (Fig. 1).
Preparation of mononuclear cells from peripheral blood
Peripheral blood was collected in heparinized tubes and diluted with the same volume of PBS. The blood mononuclear cells (MNC) were separated by density gradient centrifugation (Lymphoprep, Nycomed A/S, Oslo, Norway) [23,24]. The cells from the interphase were carefully collected and washed three times with PBS, and resuspended in complete medium (RPMI 1640; Bio-Whittaker, Walkersville, MD) including l-glutamine, penicillin, gentamycin, streptomycin, fungizone and 5% heat-inactivated fetal calf serum (FCS). The MNC were counted and viability tested (> 90%), and the cell solution adjusted to the desired concentration.
ELISPOT assay
The ELISPOT assay was performed to detect single cells from PB secreting antibodies against the recombinant Ro 52-kD, Ro 60-kD and La 48-kD proteins [11]. The assay was performed using microtitre plates with 96 wells and nitrocellulose bottoms (Millititer-HA; Millipore Products Division, Bedford, MA). The plates were coated with recombinant Ro 52 kD, Ro 60 kD or La 48 kD. The proteins were dissolved in sterile PBS to a final concentration of 10 μg/ml, and 100 μl were added to each well overnight at 4°C. This antigen concentration was found to be optimal in preliminary experiments. In parallel, wells were also coated with 100-μl aliquots of diluted high-affinity purified goat anti-human heavy chain-specific IgG, IgA or IgM (Sigma) for enumeration of total IgG-, IgA- and IgM-producing cells in PB. Control wells were coated with PBS and MaBP. The plates were washed with sterile PBS to remove unabsorbed antibodies, and 200 μl of RPMI 1640 was applied per well at 37°C for at least 30 min to block non-specific binding sites. Individual wells were filled with 100-μl aliquots containing 2.54–105 blood MNC in RPMI 1640 and 5% FCS. All cultures were performed in triplicates or more. The cells were incubated 4 h at 37°C in a humid atmosphere containing 7% CO2. After incubation the plates were washed with PBS and PBS–Tween. Subsequently, 100 μl of catching antibody were added to each well. As catching antibodies we used peroxidase-conjugated goat anti-human IgG, IgA or IgM (Sigma) diluted 1:500 in PBS–Tween. The plates were incubated overnight at 4°C. After washing with PBS the plates were enzymatically developed with AEC substrate solution (10 mg 3-amino-9-ethylcarbazole in 1 ml dimethyl formamide, diluted to 30 ml with 0.1 m citrate acetate buffer of pH 5, followed by filtration through a 0.45-μm filter and addition of 15 μl 30% H2O2). The reaction was stopped by washing with tap water. Enzyme activity was visualized on the nitrocellulose membrane as red spots, which were counted in a stereomicroscope under ×40 magnification. No spots appeared in control wells where PBS or MaBP (fusion partner) was used instead of capture antibody, or in coated wells subjected to medium without cells. The presented data are expressed as numbers of spots/105 MNC. Numbers of spots exceeding 3 s.d. the mean value obtained from 11 negative controls (healthy females) were considered positive.
ELISA
The recombinant Ro 52-kD, Ro 60-kD, La 48-kD and MaBP proteins were used for coating ELISA plates (Costar, Cambridge, MA). The proteins were dissolved in 0.05 m sodium carbonate buffer pH 9.5, to the concentration of 10 μg/ml, and 100 μl were added to each well overnight at 4°C. This was found to be optimal in initial experiments [16]. Non-specific binding sites were blocked with 1% bovine serum albumin (BSA) in sodium carbonate buffer. Sera were diluted 1:1000 in PBS containing 0.5% BSA and 0.05% Tween, and 100 μl added to each well. After incubation for 2 h at room temperature, the plates were washed five times with PBS–Tween. One hundred microlitres of peroxidase-conjugated goat anti-human IgG (Southern Biotechnology Associates, Birmingham, AL) diluted 1:500 were added to each well. After another 2 h at room temperature, the plates were washed as above and 100 μl substrate solution added to the wells (four tablets of 2 mg OPD diluted in 12 ml 0.1 m citric acid–phosphate buffer and addition of 5 μl 30% H2O2). The reaction was stopped after 15 min at room temperature by adding 100 μl 2.0 m H2SO4, and the absorbance measured at 492 nm. Absorbance values 3 s.d. above mean values obtained from 27 negative controls (healthy individuals) were considered positive.
Statistical analysis
The Mann–Whitney test and Pearson's correlation coefficient were used for statistical evaluation.
RESULTS
Number of circulating B cells producing antibodies against the recombinant Ro 52-kD, Ro 60-kD or La 48-kD antigens in SS patients and healthy controls
The ELISPOT assay was used to estimate the frequencies of PB lymphocytes producing antibodies against the Ro/SS-A and La/SS-B antigens. For this purpose recombinant Ro 52-kD, Ro 60-kD and La 48-kD proteins were produced and purified. The purity of these antigens and MaBP is presented in Fig. 1. MaBP (fusion partner) and PBS were used as control antigens.
Three of 18 patients with SS had PB lymphocytes producing anti-Ro 52-kD antibodies of the IgG isotype, whereas one of 18 SS patients had PB cells secreting anti-Ro 60-kD antibodies of this isotype. No spots corresponding to cells producing IgA and IgM against the Ro 52-kD or Ro 60-kD antigens were detected in the blood of the investigated patients (Table 2). No patients or controls had PB cells producing antibodies against MaBP (fusion partner) or PBS.
Table 2.
Numbers of cells producing IgG, IgA and IgM and antigen-specific antibodies of three isotypes per 105 mononuclear cells from peripheral blood of patients with SS. Healthy individuals were used as controls.
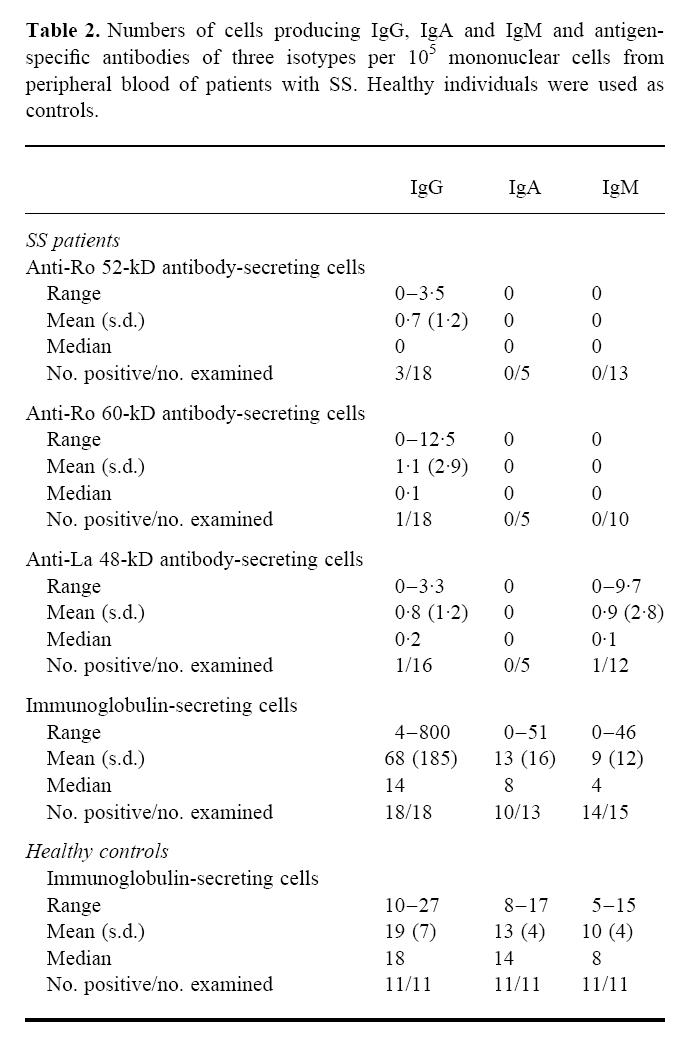
One of 16 SS patients had PB lymphocytes producing anti-La 48-kD antibodies of the IgG isotype. None of the investigated patients had PB cells producing anti-La 48-kD antibodies of the IgA isotype, while one patient had cells producing anti-La 48-kD antibodies of the IgM isotype (Table 2).
One of the SS patients had PB lymphocytes producing IgG antibodies against all three of the Ro 52-kD, Ro 60-kD and La 48-kD antigens, in addition to cells producing anti-La 48-kD antibodies of the IgM isotype.
None of the 11 healthy controls had PB lymphocytes producing significant levels of antibodies of the IgG, IgA or IgM isotypes against the Ro 52-kD, Ro 60-kD or La 48-kD antigens; all had < 0.2/105 MNC. Although a few patients had PB cells secreting antibodies to these antigens, there was no significant difference (P > 0.05) between the SS patients and the healthy controls regarding number of cells producing antibodies of the IgG, IgA and IgM isotypes against the Ro 52-kD, Ro 60-kD and La 48-kD antigens.
Total numbers of immunoglobulin-producing cells in PB of SS patients and healthy controls
All 18 SS patients had PB cells spontaneously producing IgG, but the number of IgG-producing cells varied greatly among the patients (Table 2). Ten of 13 patients had cells producing IgA, while 14 of 15 patients had IgM-producing PB cells. Also for IgA and IgM production there was a substantial variation among the patients. The 11 healthy controls all had PB cells producing IgG, IgA and IgM (Table 2). The number of cells producing IgG, IgA and IgM in the SS patients did not differ significantly from the controls (P > 0.05).
Levels of serum antibodies against the recombinant Ro 52-kD, Ro 60-kD and La 48-kD antigens in SS patients and healthy controls
For comparison with number of PB lymphocytes secreting antigen-specific antibodies, we used the ELISA assay to measure the levels of antibodies against the recombinant Ro 52-kD, Ro 60-kD and La 48-kD proteins in serum.
Thirteen of the 18 SS patients had IgG antibodies to Ro 52 kD in serum. Nine of the patients had IgG antibodies to Ro 60 kD, while 11 patients had IgG antibodies against the La 48-kD antigen. Twelve of the patients had IgA antibodies to Ro 52 kD in serum, one patient had IgA antibodies to Ro 60 kD, and six patients had IgA antibodies against the La 48-kD antigen. Nine of the SS patients had IgM antibodies to Ro 52 kD in serum, one patient had IgM antibodies to Ro 60 kD and six patients had such antibodies against the La 48-kD antigen.
The healthy controls all had very low levels of IgG, IgA or IgM antibodies against the Ro 52-kD, Ro 60-kD or La 48-kD antigens (optical density (OD) < 0.2).
Number of circulating B cells producing IgG antibodies against the Ro 52-kD antigen related to serological and clinical variables
The three SS patients with PB cells producing IgG antibodies against the Ro 52-kD antigen (patients 1, 2 and 3 in Table 1) all had very high titres of circulating IgG antibodies to Ro 52 kD (among the four highest OD values measured by ELISA). The same three patients also had high titres of circulating antibodies of the IgA and IgM isotypes against Ro 52 kD. Two of the three patients had high levels of IgG antibodies against Ro 60 kD, and all three had high levels of IgG antibodies against the La 48-kD antigen.
In addition, these three patients all had lip biopsy focus scores of ≥ 8. The correlation between the number of PB cells producing IgG antibodies against the Ro 52-kD protein and the focus score was significant (P < 0.01) (Fig. 2). These patients were classified as having primary SS and were among the five patients with severe disease as described in Patients and Methods.
Fig. 2.
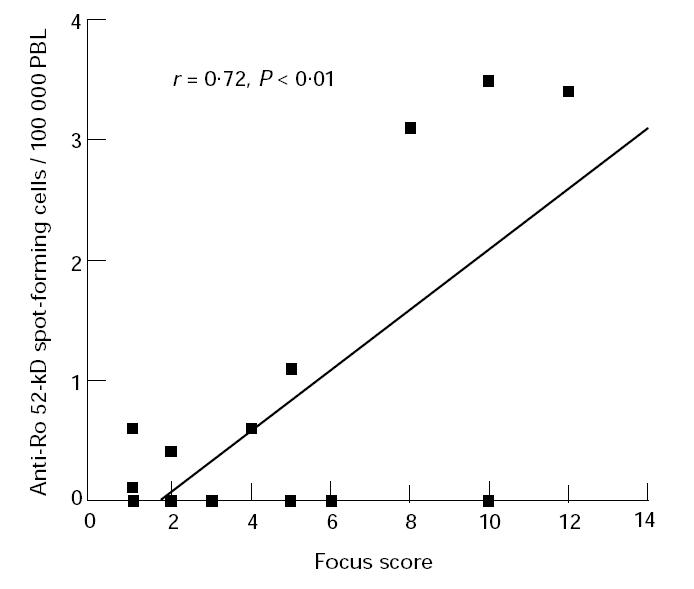
Correlation between the number of peripheral blood (PB) cells producing IgG antibodies against the recombinant Ro 52-kD protein and the focus score in the labial salivary glands. r = Pearson's correlation coefficient.
DISCUSSION
In this study we evaluated whether B cells specific for the Ro/SS-A and La/SS-B antigens are present in PB of SS patients. The total number of IgG-, IgA- and IgM-secreting cells in PB was also measured. In a recent study we presented two primary SS patients who had PB lymphocytes secreting antibodies against the bovine Ro 60-kD protein [15]. In the present follow-up study blood from 18 additional SS patients was investigated for B cells spontaneously producing antibodies against the recombinant Ro 52-kD, Ro 60-kD and La 48-kD proteins. We used the ELISPOT assay, which permits quantification of secreted products at the single-cell level. This method has previously been widely used to evaluate autoantibody production in PB and affected organs of different autoimmune diseases [25–29].
We found PB lymphocytes producing anti-Ro 52-kD antibodies of the IgG isotype in only three of 18 SS patients, and one of these patients also had B cells secreting anti-Ro 60-kD antibodies of the same isotype. The number of PB cells producing IgG against the recombinant Ro 52 kD was consistent with the number of PB cells producing IgG against the bovine Ro 60 kD in our previous study [15]. The three patients who had PB cells producing IgG antibodies against the Ro 52-kD protein also had high titres of anti-Ro 52-kD antibodies in serum. Moreover, these patients had high focus scores (between 8 and 12) and were classified as having severe disease [17–20]. This indicates that only patients with active disease have PB lymphocytes secreting antibodies against the Ro/SS-A antigen.
Previous studies have shown that anti-Ro 52-kD antibodies are mainly directed to the denatured protein [30,31]. This means that these antibodies may recognize linear epitopes that are not expressed on the surface of the native molecules. In contrast, anti-Ro 60-kD antibodies are mainly directed to the conformational epitopes on the native protein [30–32]. This means that by using the recombinant Ro 60-kD antigen, we were probably unable to detect the PB cells secreting antibodies to these conformational epitopes. We would probably have found more patients with PB cells secreting antibodies against the Ro 60-kD antigen if bovine Ro 60 kD had been used, but this protein was not available.
While the mechanism by which anti-Ro/SS-A and anti-La/SS-B antibodies arise is not clearly understood, knowledge about the pathogenic role of these autoantibodies is increasing. There is now substantial evidence that antibodies to the Ro/SS-A and La/SS-B proteins directly participate in the pathogenesis of SS and other connective tissue disease manifestations [33–35]. For example, children of anti-Ro/SS-A- and anti-La/SS-B-positive mothers are at risk of congenital heart block (CHB) and neonatal lupus dermatitis as a result of passage across the placenta of maternal IgG antibodies against Ro/SS-A and La/SS-B. Studies of hearts of babies with CHB have yielded evidence of deposition of maternal anti-La/SS-B antibodies at the site of the lesion in the conduction tissue [33]. Other studies have demonstrated anti-Ro/SS-A and anti-La/SS-B antibodies deposited in LSG of patients with SS [34,36], supporting the hypothesis that these autoantibodies are produced in LSG. To what extent the anti-Ro/SS-A and anti-La/SS-B antibodies are participating in the local inflammation of LSG is not known, but these autoantibodies might contribute to the exacerbation of the exocrinopathy seen in SS. The identification of Ro/SS-A-specific T cells in LSG of SS patients [37] and the accumulation of the La/SS-B protein found intracellularly and at the surface of acinar cells [38] indicate that the Ro/SS-A and/or La/SS-B proteins may be crucial in driving the local immune response.
Circulating B lymphocytes are normally in a resting state, and antibodies are thought to be produced mainly by non-circulating B cells. Nevertheless, we found PB lymphocytes secreting antibodies against the recombinant Ro 52-kD antigen in a small number of primary SS patients with active disease and high titres of anti-Ro/SS-A antibodies in serum. On the other hand, some patients had high titres of anti-Ro/SS-A and anti-La/SS-B antibodies in serum, but no autoantibody-producing cells were found in blood. This indicates that most of the autoantibodies are produced in other body compartments, e.g. in LSG, but probably also in the lymphoid organs and/or other mucosal sites. Future investigations should include evaluation of anti-Ro/SS-A and anti-La/SS-B antibody-producing cells and search for Ro/SS-A- and La/SS-B-specific T cells in the lymphoid organs of SS patients. This might further elucidate the site of autoantibody production and the possibility that Ro/SS-A and La/SS-B proteins are genuine autoantigens in SS.
Acknowledgments
The authors are grateful to the staff of the Department of Rheumatology, Haukeland University Hospital, in particular to Dr Hans-Jacob Haga, for providing most of the patients for this study. We also want to thank Marianne Eidsheim for technical assistance and Dr Christopher Robinson for linguistic help. The study was supported by Aslaug Andersen Foundation for Rheumatological Research in Bergen, Inger R. Haldorsen Foundation, the Research Council of Norway grant no. 115563/320 and the European BIOMED concerted action BMH4-CT96–0595.
REFERENCES
- 1.Vitali C, Bombardieri S, Moutsopoulos HM, et al. Preliminary criteria for the classification of Sjögren's syndrome. Arthritis Rheum. 1993;36:340–7. doi: 10.1002/art.1780360309. [DOI] [PubMed] [Google Scholar]
- 2.Tan EM. Antinuclear antibodies: diagnostic markers for autoimmune diseases and probes for cell biology. Adv Immunol. 1989;44:93–106. doi: 10.1016/s0065-2776(08)60641-0. [DOI] [PubMed] [Google Scholar]
- 3.Harley JB, Alexander EL, Bias WB, et al. Anti-Ro (SS-A) and anti-La (SS-B) in patients with Sjögren's syndrome. Arthritis Rheum. 1986;29:196–206. doi: 10.1002/art.1780290207. [DOI] [PubMed] [Google Scholar]
- 4.Pease CT, Charles PJ, Shattles W, Markwick J, Maini RN. Serological and immunogenetic markers of extraglandular primary Sjögren's syndrome. Br J Rheumatol. 1993;32:574–7. doi: 10.1093/rheumatology/32.7.574. [DOI] [PubMed] [Google Scholar]
- 5.Ben-Chetrit E, Chan EKL, Sullivan KF, Tan EM. A 52-kD protein is a novel component of the SS-A/Ro antigenic particle. J Exp Med. 1988;167:1560–71. doi: 10.1084/jem.167.5.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ben-Chetrit E, Gandy BJ, Tan EM, Sullivan KF. Isolation and characterization of a cDNA clone encoding the 60-kD component of the human SS-A/Ro ribonucleoprotein autoantigen. J Clin Invest. 1989;83:1284–92. doi: 10.1172/JCI114013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wolin SL, Steitz JA. The Ro small cytoplasmic ribonucleoproteins: identification of the antigenic protein and its binding site on the Ro RNAs. Proc Natl Acad Sci USA. 1984;81:1996–2000. doi: 10.1073/pnas.81.7.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O'Brien CA, Wolin SL. A possible role for the 60-kD Ro autoantigen in a discard pathway for defective 5S rRNA precursors. Genes Dev. 1994;8:2891–903. doi: 10.1101/gad.8.23.2891. [DOI] [PubMed] [Google Scholar]
- 9.Rinke J, Steitz JA. Precursor molecules of both human 5S ribosomal RNA and transfer RNAs are bound by a cellular protein reactive with anti-La lupus antibodies. Cell. 1982;29:149–59. doi: 10.1016/0092-8674(82)90099-x. [DOI] [PubMed] [Google Scholar]
- 10.Gottlieb E, Steitz JA. Function of the mammalian La protein: evidence for its action in transcription termination by RNA polymerase III. EMBO J. 1989;8:851–61. doi: 10.1002/j.1460-2075.1989.tb03446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Czerkinsky CC, Nilsson L-Å, Nygren H, Ouchterlony Ö, Tarkowski A. A solid-phase enzyme-linked immunospot (ELISPOT) assay for enumeration of specific antibody-secreting cells. J Immunol Methods. 1983;65:109–21. doi: 10.1016/0022-1759(83)90308-3. [DOI] [PubMed] [Google Scholar]
- 12.Horsfall AC, Rose LM, Maini RN. Autoantibody synthesis in salivary glands of Sjögren's syndrome patients. J Autoimmun. 1989;2:559–68. doi: 10.1016/0896-8411(89)90189-3. [DOI] [PubMed] [Google Scholar]
- 13.Ben-Chetrit E, Fischel R, Rubinow A. Anti-SSA/Ro and anti-SSB/La antibodies in serum and saliva of patients with Sjögren's syndrome. Clin Rheumatol. 1993;12:471–4. doi: 10.1007/BF02231773. [DOI] [PubMed] [Google Scholar]
- 14.Wahren M, Skarstein K, Blange I, Pettersson I, Jonsson R. MRL/lpr mice produce anti-Ro 52 000 MW antibodies: detection, analysis of specificity and site of production. Immunology. 1994;83:9–15. [PMC free article] [PubMed] [Google Scholar]
- 15.Halse A-K, Harley JB, Kroneld U, Jonsson R. Ro/SS-A reactive B lymphocytes in salivary glands and peripheral blood of patients with Sjögren's syndrome. Clin Exp Immunol. 1999;115:204–8. doi: 10.1046/j.1365-2249.1999.00778.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wahren M, Rudén U, Andersson B, Ringertz NR, Pettersson I. Identification of antigenic regions of the human Ro 60 kDa protein using recombinant antigen and synthetic peptides. J Autoimmun. 1992;5:319–32. doi: 10.1016/0896-8411(92)90146-h. [DOI] [PubMed] [Google Scholar]
- 17.Greenspan JS, Daniels TE, Talal N, Sylvester RA. The histopathology of Sjögren's syndrome in labial salivary gland biopsies. Oral Surg Oral Med Oral Pathol. 1974;37:217–29. doi: 10.1016/0030-4220(74)90417-4. [DOI] [PubMed] [Google Scholar]
- 18.Oxholm P. Primary Sjögren's syndrome—clinical and laboratory markers of disease activity. Semin Arthritis Rheum. 1992;22:114–26. doi: 10.1016/0049-0172(92)90005-x. [DOI] [PubMed] [Google Scholar]
- 19.Daniels TE. Labial salivary gland biopsy in Sjögren's syndrome. Assessment as a diagnostic criterion in 362 suspected cases. Arthritis Rheum. 1984;27:147–56. doi: 10.1002/art.1780270205. [DOI] [PubMed] [Google Scholar]
- 20.Daniels TE, Whitcher JP. Association of patterns of labial salivary gland inflammation with keratoconjunctivitis sicca. Analysis of 618 patients with suspected Sjögren's syndrome. Arthritis Rheum. 1994;37:869–77. doi: 10.1002/art.1780370615. [DOI] [PubMed] [Google Scholar]
- 21.Blange I, Ringertz NR, Pettersson I. Identification of antigenic regions of the human 52kD Ro/SS-A protein recognized by patient sera. J Autoimmun. 1994;7:263–74. doi: 10.1006/jaut.1994.1020. [DOI] [PubMed] [Google Scholar]
- 22.Nyman U, Ringertz NR, Pettersson I. Demonstration of an amino terminal La epitope recognized by human anti-La sera. Immunol Letters. 1989;22:65–72. doi: 10.1016/0165-2478(89)90144-2. [DOI] [PubMed] [Google Scholar]
- 23.Thorsby E, Bratlie A. A rapid method for preparation of pure lymphocyte suspensions. In: Terasaki PI, editor. Histocompatibility testing. Copenhagen: Munksgaard; 1970. pp. 655–6. [Google Scholar]
- 24.Ting A, Morris PJ. A technique for lymphocyte preparation from stored heparinized blood. Vox Sang. 1971;20:561–3. doi: 10.1111/j.1423-0410.1971.tb00469.x. [DOI] [PubMed] [Google Scholar]
- 25.Rönnelid J, Lysholm J, Engström-Laurent A, Klareskog L, Heyman B. Local anti-type II collagen antibody production in rheumatoid arthritis synovial fluid. Evidence for an HLA-DR4-restricted IgG response. Arthritis Rheum. 1994;37:1023–9. doi: 10.1002/art.1780370707. [DOI] [PubMed] [Google Scholar]
- 26.Tarkowski A, Klareskog L, Carlsten H, Herberts P, Koopman WJ. Secretion of antibodies to types I and II collagen by synovial tissue cells in patients with rheumatoid arthritis. Arthritis Rheum. 1989;32:1087–92. doi: 10.1002/anr.1780320906. [DOI] [PubMed] [Google Scholar]
- 27.Björkland A, Lööf L, Mendel-Hartvig I, Tötterman TH. Primary biliary cirrhosis. High proportions of B cells in blood and liver tissue produce anti-mitochondrial antibodies of several Ig classes. J Immunol. 1994;153:2750–7. [PubMed] [Google Scholar]
- 28.Olsson T, Baig S, Höjeberg B, Link H. Antimyelin basic protein and antimyelin antibody-producing cells in multiple sclerosis. Ann Neurol. 1990;27:132–6. doi: 10.1002/ana.410270207. [DOI] [PubMed] [Google Scholar]
- 29.Link H, Olsson O, Sun J, et al. Acetylcholine receptor-reactive T and B cells in myasthenia gravis and controls. J Clin Invest. 1991;87:2191–6. doi: 10.1172/JCI115253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Itoh Y, Reichlin M. Autoantibodies to the Ro/SSA antigen are conformation dependent. I. anti-60 kD antibodies are mainly directed to the native protein; anti-52 kD antibodies are mainly directed to the denatured protein. Autoimmunity. 1992;14:57–65. doi: 10.3109/08916939309077357. [DOI] [PubMed] [Google Scholar]
- 31.Tsuzaka K, Fujii T, Akizuki M, et al. Clinical significance of antibodies to native or denatured 60-kd or 52-kd Ro/SS-A proteins in Sjögren's syndrome. Arthritis Rheum. 1994;37:88–92. doi: 10.1002/art.1780370113. [DOI] [PubMed] [Google Scholar]
- 32.Boire G, Lopez-Longo F-J, Lapointe S, Ménard H-A. Sera from patients with autoimmune disease recognize conformational determinants on the 60-kd Ro/SS-A protein. Arthritis Rheum. 1991;34:722–30. doi: 10.1002/art.1780340613. [DOI] [PubMed] [Google Scholar]
- 33.Horsfall AC, Venables PJW, Taylor PV, Maini RN. Ro and La antigens and maternal anti-La idiotype on the surface of myocardial fibres in congenital heart block. J Autoimmun. 1991;4:165–76. doi: 10.1016/0896-8411(91)90015-5. [DOI] [PubMed] [Google Scholar]
- 34.Penner E, Reichlin M. Primary biliary cirrhosis associated with Sjögren's syndrome: evidence for circulating and tissue-deposited Ro/anti-Ro immune complexes. Arthritis Rheum. 1982;25:1250–3. doi: 10.1002/art.1780251014. [DOI] [PubMed] [Google Scholar]
- 35.Reichlin M. Molecular definition of the Ro (SSA) particle(s): a frequent target of autoimmunity in systemic lupus erythematosus and Sjögren's syndrome. Br J Rheumatol. 1991;30(Suppl. 1):58–62. [PubMed] [Google Scholar]
- 36.Tengnér P, Halse A-K, Haga H-J, Jonsson R, Wahren-Herlenius M. Detection of anti-Ro/SSA and anti-La/SSB autoantibody producing cells in salivary glands from patients with Sjögren's syndrome. Arthritis Rheum. 1998;41 doi: 10.1002/1529-0131(199812)41:12<2238::AID-ART20>3.0.CO;2-V. (in press) [DOI] [PubMed] [Google Scholar]
- 37.Namekawa T, Kuroda K, Kato T, et al. Identification of Ro (SSA) 52 kDa reactive T cells in labial salivary glands from patients with Sjögren's syndrome. J Rheumatol. 1995;22:2092–9. [PubMed] [Google Scholar]
- 38.de Wilde PCM, Kater L, Bodeutsch C, van den Hoogen FHJ, van de Putte LBA, van Venrooij WJ. Aberrant expression pattern of the SS-B/La antigen in the labial salivary glands of patients with Sjögren's syndrome. Arthritis Rheum. 1996;39:783–91. doi: 10.1002/art.1780390510. [DOI] [PubMed] [Google Scholar]


