Abstract
Modulation of the cytokine network may be of importance for the beneficial effects of therapy with IVIG seen in a wide range of immune-mediated disorders. In the present study we investigate the effect of IVIG administration in vivo on the IL-1 system in 12 patients with primary hypogammaglobulinaemia. Before IVIG infusion these patients had significantly elevated levels of IL-1α and IL-1β both in plasma and in supernatants from peripheral blood mononuclear cells (PBMC) compared with healthy controls. After one bolus infusion with IVIG (0.4 g/kg) we found a significant change in the profile of the components of the IL-1 system: a marked increase in levels of IL-1 receptor antagonist (IL-1Ra) and neutralizing antibodies against IL-1α, a moderate decrease in levels of IL-1α, IL-1β and soluble (s) IL-1 receptor type I and a significant increase in sIL-1 receptor type II levels. These changes were found both in plasma and in PBMC isolated after IVIG administration. Furthermore, pooled serum obtained after IVIG infusion suppressed lipopolysaccharide- and staphylococcal enterotoxin B-stimulated, but not phorbol myristate acetate-stimulated, release of IL-1α and IL-1β from PBMC isolated from healthy controls. Finally, these changes in circulating levels of various IL-1 modulators after IVIG infusion appeared to cause a significantly impaired ability of IL-1 to stimulate PBMC for tumour necrosis factor-alpha release. Our findings suggest that IVIG administration may not only down-regulate the activity in the IL-1 system, but also hamper IL-1 stimulation of PBMC.
Keywords: intravenous immunoglobulin, IL-1, tumour necrosis factor-alpha, primary hypogammaglobulinaemia
INTRODUCTION
Therapy with IVIG has been tried in a wide range of immune-mediated disorders, e.g. immune thrombocytopenic purpura, Kawasaki's syndrome, dermatomyositis, rheumatoid arthritis, asthma and multiple sclerosis [1–3]. Several modes of action have been proposed to explain the beneficial effects of IVIG in these disorders, such as neutralization of microbial antigens or superantigens, Fc-receptor blockade, accelerated immune complex removal, complement inactivation and neutralization of autoantibodies [1,4–6]. We and others have also demonstrated by both in vitro and in vivo studies that IVIG influences the levels of several cytokines and cytokine modulators and may directly interfere with cytokine production and release from various cells, e.g. monocytes and lymphocytes [7–10]. We have suggested that this capacity to modulate the cytokine network may be of importance for the beneficial effects of IVIG in several immune-mediated disorders.
IL-1 is a pluripotent cytokine which is involved in a variety of immunological and inflammatory processes [11,12]. While an adequate IL-1 response is of importance for host defence mechanisms, excessive IL-1 production seems to be involved in the pathogenesis of a range of immune-mediated disorders, e.g. rheumatoid arthritis (RA) and various infectious and haematological diseases [11,12]. Thus, therapeutical modalities which down-regulate the activity of the IL-1 system may be of potential interest in a variety of disorders. In vitro IVIG is a potent inducer of the IL-1 receptor antagonist (IL-1Ra) from monocytes [9,13], and this endogenous cytokine modulator can block the binding of IL-1 to its receptors on a number of cell types [11,12,14]. We have previously reported that IVIG administration in vivo leads to similar enhancing effect on IL-1Ra levels [10]. Furthermore, we have demonstrated that various IVIG preparations contain specific and neutralizing, high-affinity antibodies against IL-1α [15], and increased antibody binding of IL-1α was also found in serum from patients with autoimmune diseases after IVIG therapy [8]. However, the IL-1 system is physiologically unique in its complexity, involving not only two related cytokines (IL-1α and IL-1β), receptor antagonist (IL-1Ra) and neutralizing antibodies against IL-1α, but also one functional receptor (IL-1 receptor type I (IL-1RI)) and one ‘decoy’ receptor (IL-1RII) which can be shed from the cell surface, preventing the binding of IL-1 to IL-1RI [11,12]. Furthermore, while IL-1α and IL-1β bind poorly to soluble (s) IL-1RI, IL-1Ra binds with high affinity, leading indirectly to enhanced binding of IL-1 to membrane-bound IL-1RI [11,12,16]. Thus, to understand fully the effect of IVIG on the IL-1 system all these components will have to be evaluated.
In the present study we used different experimental approaches to investigate the effect of IVIG administration in vivo on the IL-1 system in patients with primary hypogammaglobulinaemia. We report not only the effect of IVIG infusion on plasma levels of several IL-1 parameters, but also the ability of peripheral blood mononuclear cells (PBMC) isolated from patients before and after IVIG infusion to release various IL-1 components of this cytokine system.
PATIENTS AND METHODS
Patients and control subjects
The study population has been described previously [10]. Twelve patients (four males and eight females; median age 39 years, range 20–60 years) with the diagnosis of primary hypogammaglobulinaemia based on established criteria [17,18] were included in the study. Ten of the patients were classified as common variable immunodeficiency (CVID) and two as congenital hypogammaglobulinaemia (CH) as previously described [19,20]. CH represents primary hypogammaglobulinaemia with onset of clinical manifestations before the age of 2 years, but with no known cases in the family suggesting an X-linked inheritance [19,20]. All patients had been treated with subcutaneous self-administered immunoglobulin for a minimum of 15 months and all had serum IgG levels > 5.0 g/l before the study. No patients had any signs of overt infection during the last month before blood collection. None was taking antibiotics or immunosuppressive drugs except for one patient who received tetracyclines because of rosacea. At the time of the study serum levels of alanine aminotransferase were < 55 U/l and serum creatinine levels < 100 μmol/l in all patients. Concentrations of IL-1 parameters in patients with primary hypogammaglobulinaemia before IVIG infusion were also compared with levels in 15 sex- and age-matched healthy blood donors (five males, 10 females; median age 42 years, range 22–64 years).
Immunoglobulin preparation
Octagam (Octapharma, Vienna, Austria) is a liquid virus-inactivated IVIG preparation (pH 4) produced from Norwegian fresh frozen plasma collected in Norwegian blood banks. The final product is dispensed in sterile water containing 10% maltose (final IgG concentration 50 g/l, IgA and IgM < 0.1 g/l). Each portion has been tested and found negative for antibodies to HIV-1, HIV-2, hepatitis B and C virus. The endotoxin level in the IVIG preparation was < 10 pg/ml (Quantitative chromogenic limulus amoebocyte lysate test; BioWhittaker, Inc., Walkersville, MD).
Study design
The study was part of an Octagam phase 1 non-placebo controlled tolerability study performed at the Section of Clinical Immunology and Infectious Diseases (Medical Department A, Rikshospitalet, Oslo, Norway), as previously described [10]. Briefly, all patients received a single infusion of Octagam (0.4 g/kg) using an infusion set with filter (median total infusion time 3.2 h, range 2.7–4 h). Blood samples were taken pre-infusion and post-infusion at 1, 3, 20 and 44 h. The study was approved by the Regional Ethical Committee and by the Norwegian Medicines Control Authority. Signed informed consent was obtained from each patient.
Blood sampling protocol
Blood was drawn into pyrogen-free blood collection tubes (Sarstedt, Numbrecht, Germany) with sodium heparin (15 U/ml blood) (plasma) or without any additives (serum). Tubes were immediately immersed in melting ice and centrifuged (400 g at 4°C for 10 min) within 20 min (plasma) or allowed to clot for 1 h before centrifugation (serum). After the first centrifugation, plasma was transferred to sterile Eppendorf tubes (Treff AG, Degersheim, Switzerland) and centrifuged at 10 000 g and 4°C for 5 min to obtain platelet-free plasma. Both serum and plasma were stored at −80°C until analysis. Samples were frozen and thawed only once. The endotoxin levels in blood collection tubes were < 10 pg/ml (Quantitative chromogenic limulus amoebocyte lysate test).
Isolation and stimulation of cells
PBMC were obtained from heparinized blood (sodium heparin; 15 U/ml blood) by Isopaque–Ficoll (Lymphoprep; Nycomed Pharma AS, Oslo, Norway) gradient centrifugation within 45 min after blood sampling [21]. Mononuclear cells were resuspended in RPMI 1640 (Gibco, Paisley, UK) with 2 mmol/ll-glutamine and 25 mmol/l HEPES buffer (Gibco) supplemented with gentamycin (40 μg/ml), hereafter referred to as culture medium, and 10% heat-inactivated pooled human AB+ serum. When studying levels of sIL-RI, sIL-1RII and IL-1Ra the cells were cultured in serum-free medium (X-Vivo 15; BioWhittaker) to avoid the influence of considerable levels of these molecules in AB+ serum. The fraction of monocytes (CD14+ cells) in PBMC was determined by immunomagnetic quantification [22]. The endotoxin levels in the serum-free and AB + serum containing medium were < 10 pg/ml (Quantitative chromogenic limulus amoebocyte lysate test). PBMC (3 × 106 cells/ml; 0.2 ml/well) obtained before and after IVIG infusion were incubated at 37°C in flat-bottomed 96-well trays (Costar, Cambridge, MA) with or without lipopolysaccharide (LPS) from Escherichia coli O26:B6 (Sigma Chemical Co., St Louis, MO; final concentration 10 ng/ml). After 20 h, cell-free supernatants were harvested and stored at −80°C until analysis. The cytokine levels were also analysed in PBMC lysates after lysing of the cell pellets in culture medium by freezing and thawing three times.
In separate experiments PBMC from healthy blood donors were cultured in medium alone or stimulated with LPS (final concentration 10 ng/ml), staphylococcal enterotoxin B (SEB; Sigma; final concentration 10 ng/ml), phorbol myristate acetate (PMA; Sigma; final concentration 100 ng/ml), recombinant (r) human IL-1α (R&D Systems, Minneapolis, MN; final concentration 10 ng/ml) or rIL-1β (R&D Systems; final concentration 10 ng/ml), with the addition of IVIG (Octagam; final IgG concentration 10 mg/ml) and different concentrations of pooled serum from controls or patients obtained before and 1 h and 20 h after IVIG infusion. IVIG and pooled serum were included in the culture medium throughout the culture period. Cell-free supernatants and cell lysates were harvested after 20 h as described above. For processing of pooled serum, 200 μl of serum from each patient (or control) were mixed immediately after thawing, and the mixed serum samples were added within 30 min to cell culture.
Enzyme immunoassays
Levels of IL-1α and IL-1β were determined by enzyme immunoassays (EIA; Immunotech, Marseille, France; detection limit for both assays 5 pg/ml) which recognize both precursor and mature form of these cytokines. In PBMC supernatants and lysates, the IL-1α levels were also determined by an EIA as described by Hansen et al. [23] using specific rabbit IgG to human IL-1α (detection limit 30 pg/ml). The measured IL-1α concentrations obtained by the two different EIAs were significantly correlated (data not shown). Levels of sIL-1RI were determined by EIA (detection limit 15 pg/ml) as previously described [24] using specific rabbit polyclonal IgG against human rIL-1RI. Levels of IL-1Ra (detection limit 20.0 pg/ml) and sIL-1RII (detection limit 10 pg/ml) were measured by EIA (R&D Systems) according to the manufacturer's instructions. Concentrations of tumour necrosis factor-alpha (TNF-α) were determined by EIA (Medgenix, Fleurus, Belgium) as described elsewhere [25]. The intra- and interassay coefficients of variation were < 15% for all EIAs. All samples from a given patient were analysed in the same microtitre plate to minimize run-to-run variability.
Measurement of IL-1α autoantibodies
The method has been described previously [26]. Briefly, duplicate plasma samples (25 μl) were diluted in 125 μl 0.02 m PBS, containing 0.05% gelatin and 0.1% Triton X-100, and co-incubated for 18 h at 4°C with 4000 ct/min 125I-labelled rIL-1α (specific activity 1.5 × 105 ct/min/ng), with or without increasing amounts of rIL-1α. The binding of 125I-rIL-1α to plasma was ascertained by affinity chromatography using protein G Sepharose CL-4B column (Pharmacia, Uppsala, Sweden), as described [26]. The results are given as maximal binding capacity in ng IL-1α per ml of undiluted serum.
Statistical analysis
For each parameter the post-infusion values were compared with pre-infusion values by using the non-parametric Wilcoxon matched pairs test. Coefficients of correlation were calculated by the Spearman rank test. Differences between two groups were calculated by the Mann–Whitney U-test (two-tailed). Data are given as medians and 25th to 75th percentiles if not otherwise stated. P values are two sided and considered significant when P < 0.05.
RESULTS
The IL-1 system in patients with primary hypogamma- globulinaemia
At baseline the patients with primary hypogammaglobulinaemia were characterized by abnormal levels of several components of the IL-1 system. While both IL-1α and IL-1β were undetectable in healthy controls, they were detected in eight (IL-1α) and six (IL-1β) of the patients (Table 1). Furthermore, primary hypogammaglobulinaemia patients also had significantly raised plasma levels of IL-1Ra, and in particular of neutralizing IL-1α antibodies and sIL-1RI compared with healthy controls (Table 1). In contrast, levels of sIL-1RII were significantly decreased in the patient group compared with control subjects (Table 1). Also, when analysing the release of IL-1α and IL-1β from unstimulated and LPS stimulated PBMC in vitro, patients with primary hypogammaglobulinaemia were characterized by enhanced IL-1 levels (Table 1). These significantly elevated IL-1 concentrations in PBMC supernatants were also found when IL-1 levels were calculated as concentration per 105 monocytes (data not shown). For all IL-1 components, the two patients with congenital hypogammaglobulinaemia were not significantly different from the 10 CVID patients (data not shown).
Table 1.
The IL-1 system in 12 patients with primary hypogammaglobulinaemia and in 15 healthy blood donors†
IVIG preparation
No detectable amounts of IL-1α, IL-1β, IL-1Ra, sIL-1RI or sIL-1RII were found in the IVIG product used in the present study. However, the IVIG preparation contained significant levels of neutralizing, high-affinity antibodies against IL-1α expressed as a maximal binding capacity of 0.14 ng IL-1α per mg IgG.
Effect of IVIG infusion in vivo on plasma levels of various IL-1 components
We have previously reported markedly raised plasma levels of IL-1Ra after IVIG infusion in these patients [10]. Here we demonstrate that IVIG infusion also significantly influenced plasma levels of other IL-1 components. In all patients with detectable concentrations of IL-1α and IL-1β at baseline, there was a fall in plasma levels of these cytokines and decreased levels persisted throughout the study period (Fig. 1). Also, in plasma concentration of sIL-1RI there was a slight decrease after IVIG infusion in all but four patients (Fig. 1). In contrast, IVIG infusion induced a moderate increase in the concentrations of sIL-1RII in all but one patient, with the highest levels at 20 h post-infusion (Fig. 1). The IVIG preparation contained significant binding capacity to IL-1α, and IVIG infusion resulted in a marked increase in anti-IL-1α binding capacity in plasma in all patients (Fig. 1). All the increased binding capacity (approx. 1.15 ng IL-1α/ml plasma) could be attributed to the increased plasma IgG levels afforded by the IVIG infusion (data not shown).
Fig. 1.
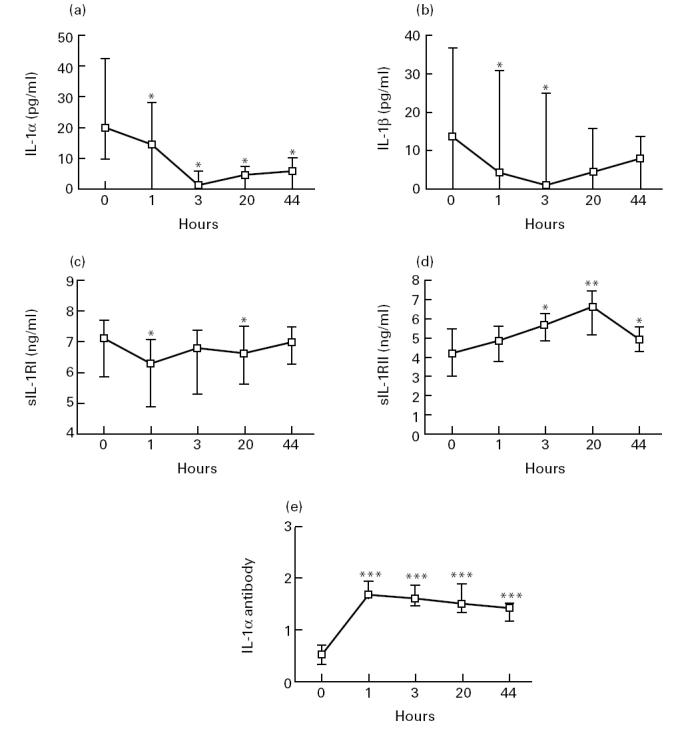
Plasma levels of IL-1α (a), IL-1β (b), sIL-1RI (c), sIL-1RII (d) and IL-1α antibodies (e) before and at various hours after IVIG infusion in vivo. The levels of neutralizing IL-1α antibodies are given as maximal binding capacity expressed as IgG-bound IL-1α (ng) per ml plasma (e). For plasma levels of IL-1α (a) and IL-1β (b), only patients with detectable levels at baseline (n = 8 and n = 6, respectively) are given in the figure. All patients with undetectable IL-1α and IL-1β levels before IVIG infusion had undetectable levels of these cytokines throughout the study period. Data are given as medians and 25th — 75th percentiles. *P < 0.05; **P < 0.01; ***P < 0.005 versus pre-infusion levels.
In vitro release of IL-1 components from PBMC after IVIG infusion in vivo
To analyse the effect of IVIG on the IL-1 system at the cellular level, we first performed in vitro studies in parallel with plasma analyses, examining the spontaneous and LPS-stimulated release of IL-1 components from PBMC isolated from seven patients before and 20 h after IVIG infusion. All of these were CVID patients, all had detectable plasma levels of IL-1α and all but one had detectable plasma levels of IL-1β. There was a significant decrease in the release of IL-1α, IL-1β, sIL-1RI and a significant increase in release of sIL-1RII and IL-1Ra in PBMC supernatants in vitro after IVIG infusion (Table 2). In fact, LPS-stimulated release of both IL-1α, IL-1β, sIL-1RI decreased by > 50% and unstimulated release of sIL-1RII and IL-1Ra increased by approx. 500% and 190%, respectively (Table 2). These changes were seen in all but one patient (data not shown). Similar results were found when calculating the levels of IL-1 parameters as concentration per 105 monocytes (data not shown).
Table 2.
In vitro release of various IL-1 parameters from unstimulated and lipopolysaccharide (LPS)-stimulated peripheral blood mononuclear cells (PBMC) isolated from seven patients with primary hypogammaglobulinaemia before and 20 h after IVIG infusion in vivo

Effect of serum obtained before and after IVIG infusion on IL-1 levels in PBMC
To examine further the influence of IVIG infusion on the IL-1 system, the effect of pooled serum taken from all patients before and 1 h and 20 h after IVIG infusion on stimulated IL-1 levels in PBMC from six healthy blood donors was studied. Thirty minutes before stimulation of PBMC with LPS, SEB and PMA, the cells were incubated in medium containing various concentrations of pooled serum (1–20%) obtained at different time points in the IVIG study, and the levels of IL-1α and IL-1β were analysed in both supernatants and cell lysates after culturing for 20 h. For comparison, the effect of pooled serum taken from six healthy blood donors was also studied. Pooled serum was included in the culture medium throughout the culture period. Several significant findings were revealed. First, compared with healthy control sera, sera obtained from patients with primary hypogammaglobulinaemia before IVIG infusion had enhanced effect on SEB- and particularly on LPS-stimulated levels of both IL-1α and IL-1β (Fig. 2). No such enhancing effect was seen on PMA-stimulated cells (Fig. 2). Second, compared with sera before IVIG infusion, pooled serum obtained 20 h, but not 1 h after IVIG infusion, significantly impaired SEB- and particularly LPS-stimulated levels of IL-1α and IL-1β (Fig. 2). This suppressive effect of ‘IVIG sera’ was found in a concentration-dependent fashion, with increasing suppressive effects along with increasing concentrations of pooled serum (data not shown). No such impairment was found for PMA-stimulated IL-1 levels (Fig. 2). Finally, the inhibitory effect of pooled serum obtained after IVIG infusion was most pronounced when analysing the IL-1 levels in cell lysates compared with those in cell-free supernatants (Fig. 2).
Fig. 2.
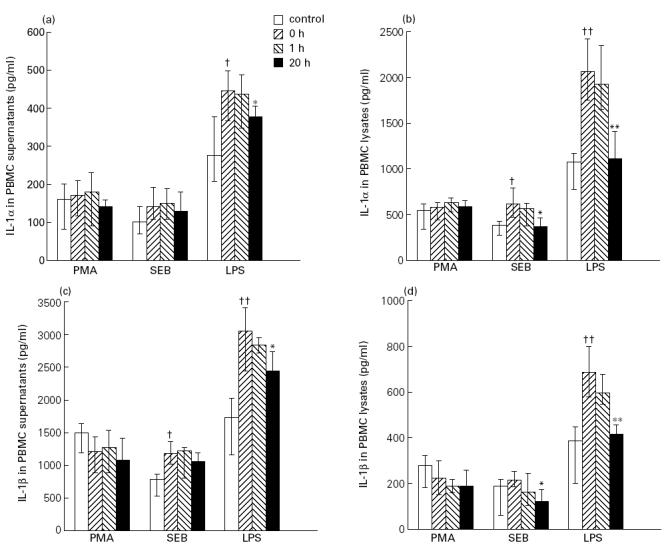
The effect of pooled serum obtained before and after IVIG infusion on stimulated IL-1 levels in peripheral blood mononuclear cells (PBMC). The figure presents data analysing the effect of medium containing 20% of pooled serum from six healthy blood donors (control) and 20% of pooled serum from patients with primary hypogammaglobulinaemia obtained before (0 h) and 1 h and 20 h after IVIG infusion in vivo on phorbol myristate acetate (PMA; 100 ng/ml), staphylococcal enterotoxin B (SEB; 10 ng/ml) and lipopolysaccharide (LPS; 10 ng/ml) stimulated levels of IL-1α (supernatants (a) and cell lysates (b)) and IL-1β (supernatants (c) and cell lysates (d)) in PBMC from six healthy blood donors after culturing for 20 h in vitro. Data are given as medians and 25th — 75th percentiles. *P < 0.05; **P < 0.01 versus pooled serum obtained before IVIG infusion (0 h); †P < 0.5; ††P < 0.01 versus pooled serum from healthy controls.
Effect of serum obtained before and after IVIG infusion on IL-1-stimulated levels of TNF-α in PBMC supernatants
The raised levels of IL-1Ra, IL-1α antibodies and sIL-1RII after IVIG infusion might impair the ability of IL-1 to bind and stimulate the IL-1 receptors on various cells. Since IL-1 can increase the levels of other inflammatory cytokines such as TNF-α by direct stimulation [11,12], we studied the effect of IVIG and sera obtained before and 1 h and 20 h after IVIG infusion on IL-1-stimulated release of TNF-α from PBMC. PBMC from six healthy blood donors were stimulated with IL-1α and IL-1β, and medium containing different concentrations of pooled serum (1–20%) obtained at various time points in the IVIG study or the IVIG preparation (final concentration 10 mg/ml) was added to the cells in vitro 2 h before, together with, or 2 h after IL-1 stimulation. IVIG and pooled serum was included in the culture medium throughout the culture period. As shown in Fig. 3, sera obtained from patients with primary hypogammaglobulinaemia before IVIG infusion and which were added to cell cultures 2 h before stimulation, significantly enhanced the IL-1β-, but not the IL-1α-stimulated release of TNF-α compared with the effect of AB+ serum. In contrast, sera obtained 1 h and 20 h after IVIG infusion impaired the IL-1α- and IL-1β-stimulated release of TNF-α in a concentration-dependent fashion (data not shown), compared with both AB+ serum and serum obtained from patients before IVIG infusion (Fig. 3). In fact, the suppressive effect of sera after IVIG infusion was even more pronounced than the suppressive effect of IVIG itself when added directly to cell culture in vitro, in particular on the IL-1β-stimulated levels (Fig. 3). Similar patterns were found when pooled serum and IVIG were added together or 2 h after IL-1 stimulation, although the differences when these reagents were added 2 h after stimulation did not reach statistical significance (data not shown).
Fig. 3.
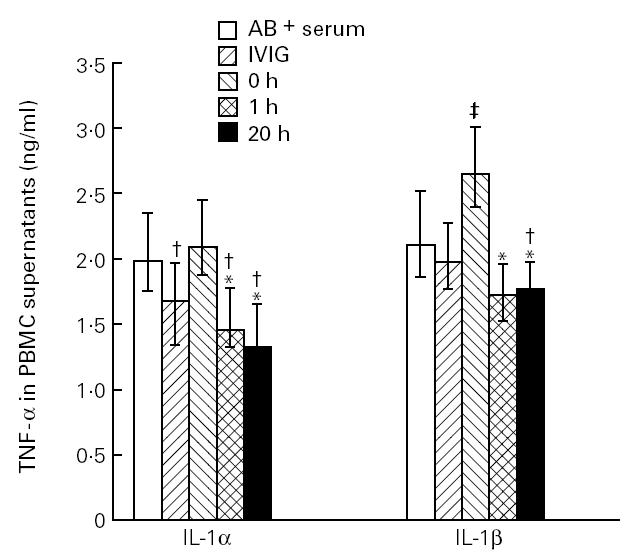
The effect of medium containing 20% of the IVIG preparation (final concentration 10 mg/ml) and 20% of pooled serum obtained before (0 h) and 1 h and 20 h after IVIG infusion in vivo in patients with primary hypogammaglobulinaemia on IL-1α- and IL-1β-stimulated release of tumour necrosis factor-alpha (TNF-α) in vitro from peripheral blood mononuclear cells (PBMC) in six healthy controls when added to cell cultures 2 h before IL-1 stimulation. For comparison, PBMC cultured in medium containing 20% AB+ serum are also shown. Data are given as medians and 25th — 75th percentiles. *P < 0.05 versus pooled serum obtained before IVIG infusion (0 h); †significantly decreased (P < 0.05) and ‡significantly increased (P < 0.05) versus AB+ serum.
DISCUSSION
The present study demonstrates that IVIG administration in vivo results in a significant modulation of several components of the IL-1 system, most probably reflecting down-regulation of IL-1 activity. IVIG infusion induced a marked increase in circulating levels of IL-1Ra and neutralizing antibodies against IL-1α, and in addition there was a moderate decrease in the levels of IL-1α, IL-1β and sIL-1RI as well as an increase in sIL-1RII levels. Notably, these changes in components of the IL-1 system after IVIG administration were found not only in plasma, but also in PBMC isolated after IVIG infusion. Furthermore, our findings in the present study suggest that IVIG administration in vivo is not only a potent down-regulator of IL-1 levels, but may also hamper IL-1 stimulation of PBMC.
We found decreased plasma levels of IL-1β and IL-1α after IVIG administration, which may seem in conflict with our previous report of a slight increase in plasma levels of IL-1β after IVIG infusion in the same study population [10]. This discrepancy probably reflects methodological differences in the two studies. In contrast to the previous report, in the present study we used an EIA which in addition to the mature IL-1 molecule also detects its precursor, the level of which may be important in evaluating the complete activity of the IL-1 system [12,27–29]. We cannot exclude that the decreased plasma levels of IL-1α and IL-1β after IVIG infusion at least partly reflect increased formation of IL-1α- and IL-1β-containing complexes as a consequence of enhanced binding of these cytokines to high-affinity IL-1α antibodies and sIL-1RII, respectively. However, the decrease in IL-1 levels was also found in PBMC isolated after IVIG infusion. Furthermore, sera obtained from patients after IVIG infusion markedly impaired the in vitro stimulatory release of IL-1α and IL-1β from PBMC obtained from control subjects. These findings clearly suggest that the decrease in IL-1 levels after IVIG infusion is not only a reflection of methodology correlated with the capacity of the actual EIA to detect relevant IL-1 metabolites. Our results indicate that this decrease in IL-1 also occurs at the cellular level.
PBMC isolated after IVIG infusion spontaneously released markedly elevated IL-1Ra levels, in agreement with the increased plasma levels of IL-1Ra after IVIG administration in vivo [10]. These findings support data from previous reports indicating that IVIG is a potent inducer of IL-1Ra release [9,12–14]. This increase in IL-1Ra levels, and in particular the increase in concentrations of neutralizing antibodies against IL-1α, may markedly impair IL-1 activity after IVIG infusion [8,11,12]. Also, the increase in sIL-1RII and the decrease in sIL-1RI levels found after IVIG administration may possibly impair IL-1 activity. Soluble IL-1RII can nearly irreversibly bind IL-1β with high affinity, thereby preventing its binding to cellular IL-1RI [11,12,30,31]. Furthermore, while both IL-1α and IL-1β bind weakly to native sIL-1RI, IL-1Ra binds more avidly [16,24]. As a consequence, decreased concentrations of sIL-1RI may lead to decreased ‘trapping’ of IL-1Ra, indirectly leading to decreased binding of the agonist to cellular IL-1RI. Thus, it appears that the relative amounts of the two sIL-1 receptors are of importance for modulation of the activity in the IL-1 system [11,12,28], and a rise in sIL-1RII and a fall in sIL-1RI levels, as found after IVIG infusion in the present study, may down-regulate IL-1 activity.
In addition to suppressive effects on IL-1 concentrations, we also found suppressive effects of sera obtained after IVIG infusion on IL-1 activity as assessed by the IL-1-mediated release of TNF-α from PBMC. High-avidity autoantibodies to cytokines appear to be of major importance as modulators of cytokine activity [32], and the markedly increased levels of neutralizing IL-1α antibodies after IVIG infusion are probably of major importance for the inhibitory effect of ‘IVIG sera’ on IL-1-stimulated TNF-α release. However, additional factors such as IL-1Ra and sIL-1RII, and possibly neutralizing antibodies against IL-1β [32], may also be involved as well. Thus, pooled serum obtained after IVIG infusion impaired not only the IL-1α stimulatory, but also the IL-1β stimulatory effect on PBMC. Moreover, the in vitro inhibitory effect of sera obtained after IVIG infusion was even more pronounced than the inhibitory effect of IVIG itself, suggesting that factors in addition to neutralizing IL-1α antibodies or other factors present in the IVIG preparation (e.g. other cytokine antibodies) are involved in the inhibitory effect of ‘IVIG sera’ on IL-1 stimulation.
It has been suggested that the immunomodulating effect of IVIG is at least partly caused by the presence of superantigen antibodies with neutralizing activity in the IVIG preparation [33,34]. In this regard, we found that the down-regulation of IL-1 levels in PBMC after IVIG infusion was not restricted to cells stimulated with superantigen, but was even more pronounced after LPS stimulation. Abe et al. [35] have recently suggested that the mechanism for inhibitory effects of IVIG on monocyte activation involves interference with intracellular pathways upstream of protein kinase C (PKC) activation. In accordance with this, we found that when PBMC were activated by PMA through direct PKC activation, sera obtained after IVIG infusion had no inhibitory effect on IL-1 release. Whatever the mechanism(s), if the demonstrated refractory state of PBMC after IVIG infusion also occurs when these cells are naturally stimulated in vivo, it may contribute to anti-inflammatory effects of IVIG.
Most studies analysing the in vitro effect of IVIG have been performed on cells from healthy individuals. However, the immunomodulating effect of IVIG may differ in different patient groups with variable pre-existing immunological abnormalities [9,36]. The present study was performed on patients with primary hypogammaglobulinaemia. In addition to recurrent bacterial infections, these patients are characterized by increased occurrence of autoimmune disorders, lymphoid hyperplasia and non-caseating granulomata [17,18]. Furthermore, in addition to abnormal B cell function, many patients present with T cell deficiency and persistent immune activation in vivo, particularly in monocytes/macrophages [19,37,38]. The present study indicates increased activation of the IL-1 system in these patients, further suggesting that persistent activation of inflammatory cytokines is a distinctive feature in a considerable proportion of patients with this form of primary immunodeficiency. Finally, it is conceivable that the immunological effects of IVIG infusion found in these patients may have relevance to the clinical and immunological effects of IVIG in other groups of patients with autoimmune and other inflammatory disorders known to benefit from IVIG therapy.
The results of the present study indicate that IVIG administration in vivo down-regulates IL-1 activity, reflected in both decreased IL-1 levels and impairment of IL-1 stimulatory effects. We have previously demonstrated that IVIG may down-regulate enhanced TNF-α activity [10,36], and this combined suppressive effect on these two important inflammatory cytokine systems which may operate in a positive feedback loop [11,12,39], may be of importance for the immunomodulating effect of IVIG in several disorders.
Acknowledgments
We thank Bodil Lunden, Lisbeth Wikeby and Marianne Thomsen for excellent technical assistance. This work was supported by Octapharma, Hurdal, Norway; the Research Council of Norway; the Norwegian Cancer Society; Anders Jahre's Foundation; Medinnova Foundation; Odd Kåre Rabben's Memorial Fund for AIDS Research; and the Danish Biotechnology Program.
REFERENCES
- 1.Dwyer JM. Manipulating the immune system with immune globulin. N Engl J Med. 1992;326:107–16. doi: 10.1056/NEJM199201093260206. [DOI] [PubMed] [Google Scholar]
- 2.Mobini N, Sarela A, Ahmed AR. Intravenous immunoglobulins in the therapy of autoimmune and systemic inflammatory disorders. Ann Allergy Asthma Immunol. 1995;74:119–28. [PubMed] [Google Scholar]
- 3.Dalakas MC. Intravenous immune globulin therapy for neurologic disease. Ann Intern Med. 1997;126:721–30. doi: 10.7326/0003-4819-126-9-199705010-00008. [DOI] [PubMed] [Google Scholar]
- 4.Wolf HM, Eibl MM. Immunomodulatory effect of immunoglobulins. Clin Exp Rheumatol. 1996;14(Suppl. 15):S17–S25. [PubMed] [Google Scholar]
- 5.Ballow M. Mechanisms of action of intravenous immune serum globulin in autoimmune and inflammatory diseases. J Allergy Clin Immunol. 1997;100:151–7. doi: 10.1016/s0091-6749(97)70217-3. [DOI] [PubMed] [Google Scholar]
- 6.Smiley JD, Talbert MG. High-dose intravenous gamma globulin therapy: how does it work? Am J Med Sci. 1995;309:295–303. doi: 10.1097/00000441-199530950-00010. [DOI] [PubMed] [Google Scholar]
- 7.Andersson U, Björk L, Skansén-Saphir U, Andersson J. Pooled human IgG modulates cytokine production in lymphocytes and monocytes. Immunol Rev. 1994;139:21–42. doi: 10.1111/j.1600-065x.1994.tb00855.x. [DOI] [PubMed] [Google Scholar]
- 8.Ross C, Svenson M, Nielsen H, Lundsgaard C, Hansen MB, Bendtzen K. Increased in vivo antibody activity against interferon α, interleukin-1α and interleukin-6 after high-dose Ig therapy. Blood. 1997;90:2376–80. [PubMed] [Google Scholar]
- 9.de Souza VR, Carreno M-P, Kaveri SV, et al. Selective induction of interleukin-1 receptor antagonist and interleukin-8 in human monocytes by normal polyspecific IgG (intravenous immunoglobulin) Eur J Immunol. 1995;25:1267–73. doi: 10.1002/eji.1830250521. [DOI] [PubMed] [Google Scholar]
- 10.Aukrust P, Frøland SS, Liabakk N-B, Müller F, Nordøy I, Haug C, Espevik T. Release of cytokines, soluble cytokine receptors and interleukin-1 receptor antagonist after intravenous immunoglobulin administration in vivo. Blood. 1994;84:2136–43. [PubMed] [Google Scholar]
- 11.Bendtzen K. Cytokines and natural regulators of cytokines. Immunol Letters. 1994;43:111–23. doi: 10.1016/0165-2478(94)00153-7. [DOI] [PubMed] [Google Scholar]
- 12.Dinarello CA. Biologic basis for interleukin-1 in disease. Blood. 1996;87:2095–147. [PubMed] [Google Scholar]
- 13.Arend WP, Leung DYM. IgG induction of IL-1 receptor antagonist production by human monocytes. Immunol Rev. 1994;139:71–78. doi: 10.1111/j.1600-065x.1994.tb00857.x. [DOI] [PubMed] [Google Scholar]
- 14.Dinarello CA, Thompson RC. Blocking IL-1: interleukin 1 receptor antagonist in vivo and in vitro. Immunol Today. 1991;12:404–10. doi: 10.1016/0167-5699(91)90142-G. [DOI] [PubMed] [Google Scholar]
- 15.Svenson M, Hansen MB, Bendtzen K. Binding of cytokines to pharmaceutically prepared human immunoglobulin. J Clin Invest. 1993;92:2533–9. doi: 10.1172/JCI116862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Svenson M, Nedergaard S, Heegaard PMH, Whisenand TD, Arend WP, Bendtzen K. Differential binding of human interleukin-1 (IL-1) receptor antagonist to natural and recombinant soluble and cellular type I receptors. Eur J Immunol. 1995;25:2842–50. doi: 10.1002/eji.1830251020. [DOI] [PubMed] [Google Scholar]
- 17.Spickett GP, Misbah SA, Chapel HM. Primary antibody deficiency in adults. Lancet. 1991;337:281–4. doi: 10.1016/0140-6736(91)90882-p. [DOI] [PubMed] [Google Scholar]
- 18.WHO Scientific Group Report. Primary immunodeficiency diseases. Clin Exp Immunol. 1997;97(Suppl. 1):1–24. [PubMed] [Google Scholar]
- 19.Aukrust P, Frøland SS, Müller F. Raised serum neopterin levels in patients with primary hypogammaglobulinaemia; correlation to other immunological parameters and to clinical and histological features. Clin Exp Immunol. 1992;89:211–6. doi: 10.1111/j.1365-2249.1992.tb06934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aukrust P, Müller F, Frøland SS. Elevated serum levels of interleukin-4 and interleukin-6 in patients with common variable immunodeficiency are associated with chronic immune activation and low numbers of CD4+ lymphocytes. Clin Immunol Immunopathol. 1994;70:217–24. doi: 10.1006/clin.1994.1032. [DOI] [PubMed] [Google Scholar]
- 21.Müller F, Rollag H, Gaudernack G, Frøland SS. Impaired in vitro survival of monocytes from patients with HIV infection. Clin Exp Immunol. 1990;81:25–30. doi: 10.1111/j.1365-2249.1990.tb05286.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gaudernack G, Lundin KE. Rapid immunomagnetic phenotyping of cells. J Immunogenet. 1989;16:169–75. doi: 10.1111/j.1744-313x.1989.tb00459.x. [DOI] [PubMed] [Google Scholar]
- 23.Hansen MB, Svenson M, Bendtzen K. Human anti-interleukin 1α antibodies. Immunol Letters. 1991;30:133–40. doi: 10.1016/0165-2478(91)90102-g. [DOI] [PubMed] [Google Scholar]
- 24.Svenson M, Hansen MB, Heegaard P, Abell K, Bendtzen K. Specific binding of interleukin-1 (IL-1) β and IL-1 receptor antagonist (IL-1ra) to human serum, high-affinity binding of IL-1ra to soluble IL-1 receptor type I. Cytokine. 1993;5:427–35. doi: 10.1016/1043-4666(93)90032-z. [DOI] [PubMed] [Google Scholar]
- 25.Aukrust P, Svardal AM, Müller FM, Lunden B, Berge RK, Frøland SS. Decreased levels of total and reduced glutathione in CD4+ lymphocytes in common variable immunodeficiency are associated with activation of the tumor necrosis factor α system. Possible immunopathogenic role of oxidative stress. Blood. 1995;86:1383–91. [PubMed] [Google Scholar]
- 26.Svenson M, Hansen MB, Bendtzen K. Distribution and characterization of autoantibodies to interleukin-1α in normal human sera. Scand J Immunol. 1990;32:695–701. doi: 10.1111/j.1365-3083.1990.tb03212.x. [DOI] [PubMed] [Google Scholar]
- 27.Cannon JG, Nerad JL, Poutsiaka DD, Dinarello CA. Measuring circulating cytokines. J Appl Physiol. 1993;75:1897–902. doi: 10.1152/jappl.1993.75.4.1897. [DOI] [PubMed] [Google Scholar]
- 28.Higgins GC, Foster JL, Postlethwaite AE. Interleukin-1β propeptide is derived intracellularly and extracellularly when human monocytes are stimulated with LPS in vitro. J Exp Med. 1994;180:607–14. doi: 10.1084/jem.180.2.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Herzyk DJ, Berger AE, Allen JN, Wewers MD. Sandwich ELISA formats designed to detect 17kDa IL-1β significantly underestimate 35 kDa IL-1β. J Immunol Methods. 1992;48:243–54. doi: 10.1016/0022-1759(92)90178-v. [DOI] [PubMed] [Google Scholar]
- 30.Arend WP, Malyak N, Smith MF, Jr, et al. Binding of IL-1α, IL-1β, and IL-1 receptor antagonist by soluble IL-1 receptors and levels of soluble IL-1 receptors in synovial fluids. J Immunol. 1994;153:4766–74. [PubMed] [Google Scholar]
- 31.Giri JG, Wells J, Dower SK, et al. Elevated levels of shed type II IL-1 receptor in sepsis. J Immunol. 1994;53:5802–9. [PubMed] [Google Scholar]
- 32.Bendtzen K, Hansen MB, Ross C, Svenson M. High-avidity autoantibodies to cytokines. Immunol Today. 1998;19:209–11. doi: 10.1016/s0167-5699(98)01252-3. [DOI] [PubMed] [Google Scholar]
- 33.Takei S, Arora YK, Walker SM. Intravenous immunoglobulin contains specific antibodies inhibitory to the action of T cells by staphylococcal toxin superantigens. J Clin Invest. 1993;91:602–7. doi: 10.1172/JCI116240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Norrby-Teglund A, Kaul R, Low DE, et al. Plasma from patients with severe group A streptococcal infections treated with normal polyspecific IgG inhibits streptococcal superantigen-induced T-cell proliferation and cytokine production. J Immunol. 1996;156:3057–64. [PubMed] [Google Scholar]
- 35.Abe Y, Horiuchi A, Miyake M, Kimura S. Anti-cytokine nature of human immunoglobulin: one possible mechanism of the clinical effects of intravenous immunoglobulin therapy. Immunol Rev. 1994;139:6–14. doi: 10.1111/j.1600-065x.1994.tb00854.x. [DOI] [PubMed] [Google Scholar]
- 36.Aukrust P, Hestdal K, Lien E, et al. Effects of intravenous immunoglobulin in vivo on abnormally increased tumor necrosis factor α activity in patients with human immunodeficiency virus type 1 infection. J Infect Dis. 1997;176:913–23. doi: 10.1086/516510. [DOI] [PubMed] [Google Scholar]
- 37.Aukrust P, Lien E, Kristoffersen AK, Müller F, Haug CJ, Espevik T, Frøland SS. Persistent activation of the tumor necrosis factor system in a subgroup of patients with common variable immunodeficiency—possible immunological and clinical consequences. Blood. 1996;87:674–81. [PubMed] [Google Scholar]
- 38.Spickett GP, Farrant J, North M, Zhang J-G, Morgan L, Webster ADB. Common variable immunodeficiency: how many diseases? Immunol Today. 1997;18:325–8. doi: 10.1016/s0167-5699(97)01086-4. [DOI] [PubMed] [Google Scholar]
- 39.Granowitz EV, Vannier E, Poutsiaka DB, Dinarello CA. Effect of interleukin-1 (IL-1) blockade on cytokine synthesis: II. IL-1 receptor antagonist inhibits lipopolysaccharide-induced cytokine synthesis by human monocytes. Blood. 1992;79:2364–9. [PubMed] [Google Scholar]


