Abstract
We investigated the production of IL-2, IFN-γ, IL-10 and IL-4 by PBMC from 24 patients with SLE and 10 healthy individuals. Basal and mitogen-stimulated (lipopolysaccharide and phytohaemagglutinin (LPS + PHA)) cytokine production was determined in a whole blood assay (WBA). Supernatants were collected and assayed with specific ELISAs. Although the IL-2 and IFN-γ contents did not differ significantly between patients and controls under both conditions, statistically significant correlations were found between each cytokine and disease activity (SLAM index) after stimulation (respectively, r = 0.501, P = 0.01 and r = 0.631, P = 0.001). PBMC IL-10 production was significantly higher for patients than controls (P = 0.05), but no correlation between IL-10 levels and the SLAM index was obtained. IL-4 production was not statistically different between SLE patients and controls. For stimulated WBAs, the IL-10/IL-2 and IL-10/IFN-γ ratios were significantly correlated with disease severity (P = 0.02; P = 0.001, respectively). Overall, our data suggest that SLE is characterized by an elevated production of IL-10, reflecting the basal state of activation of the immune system. During exacerbation of SLE, IL-2 and IFN-γ are synthesized in larger amounts and may cause the tissue damage observed.
Keywords: IL-2, IL-4, IL-10, interferon-gamma, systemic lupus erythematosus
INTRODUCTION
SLE is an autoimmune disease characterized by polyclonal activation of B cells, resulting in the production of a wide range of autoantibodies [1]. The aberrant immune regulation in SLE which is thought to underlie the production of these autoantibodies might be ascribed to a decreased suppressive function or increased production of B cell-activating functions. In this respect, soluble mediators of inflammation and immune responses such as cytokines are prime candidates for the pathogenic factors responsible for this systemic disease.
In the past few years, reports have shown that both murine and human CD4+ T-helper (Th) cells represent a functionally heterogeneous population, comprising at least three distinct subsets termed Th1, Th2, and Th0 cells, defined by their cytokine secretion profiles [2–6]. Th1 cells produce IL-2, IFN-γ and tumour necrosis factor-beta (TNF-β), and promote both macrophage activation resulting in DTH, and production of complement-fixing and -opsonizing antibodies. Th2 cells, which synthesize IL-4, IL-5, IL-6, IL-10 and IL-13, provide optimal help for antibody production, and promote both mast cell growth and eosinophil differentiation and activation resulting in humoral or allergic responses.
In the absence of orienting conditions leading to T cell clone commitment and stereotyped Th1 or Th2 patterns, CD4+ T cells secreting both Th1- and Th2-type cytokines (Th0 cells) usually arise and mediate intermediate effects, depending on the cytokines predominating in the surrounding medium and the nature of the responding cells [6]. However, if this situation holds true for the cytokines secreted only by T lymphocytes (IFN-γ, IL-2, and IL-4), it is not the case for others, such as IL-10 or IL-6, which are primarily synthesized by monocytes. We have therefore used the terms Th1 and Th2 in this study with this restriction in mind.
The respective contributions of the Th1 and Th2 cytokines to the pathogenesis of SLE are still a matter of debate. Because several Th2 cytokines, e.g. IL-6 and IL-10, are known to promote antibody production by B cells [7], it has been advanced that Th2 cells might play an active role in the development of autoantibody-mediated autoimmune diseases such as SLE [8]. However, immune responses involving the Th1 phenotype have also been found in the murine model of lupus. For example, exacerbation of SLE by repeated injections of recombinant IFN-γ, and inhibition by anti-IFN-γ antibodies in (NZB × NZW)F1 hybrid mice, suggest the possible involvement of Th1-type cells in the spontaneous development and progression of SLE [9].
The respective roles of IL-2, IFN-γ, IL-4 and IL-10 cytokines in the pathogenesis of SLE were investigated by examining the spontaneous and mitogen-induced synthesis of these cytokines by PBMC from patients with active SLE with a whole blood assay (WBA), which appears to preserve better the natural environment and constitutes an appropriate milieu in which to study in vitro cytokine production [10–14].
PATIENTS AND METHODS
Patients
The present study included 10 healthy controls and 24 SLE patients, none of whom was taking corticosteroids, immunosuppressive drugs or non-steroidal antinflammatory drugs at the time of the study. SLE patients fulfilled at least four of the American Rheumatism Association 1982 revised criteria for SLE [15]. Some patients also had an antiphospholipid syndrome (n = 2), defined by the presence of positive tests for the lupus anticoagulant or anti-cardiolipin antibodies, and more than one of the following features: thrombosis (arterial, venous or both), recurrent fetal losses (with or without accompanying thrombocytopenia) [16].
Clinical disease activity was assessed by applying the systemic lupus activity measure (SLAM) [17].
Blood collection and WBA protocol
Blood samples were collected in sterile Vacutainer tubes (Becton Dickinson, Grenoble, France) containing 100 U/ml of heparin (Choay, Paris, France). After a maximum storage period of 1 h at room temperature, blood was diluted 1:1 in RPMI 1640 (Gibco, Les Ullis, France), and 1-ml aliquots were deposited in 2-ml wells of a 24-well plate (Nunc, Roskilde, Denmark). Basal and mitogen-stimulated (phytohaemagglutinin (PHA; Sigma, St Louis, MO), final concentration of 5 μg/ml; and lipopolysaccharide (LPS, from Salmonella enteritidis; Sigma), final concentration 25 μg/ml) conditions were evaluated. The plates were incubated at 37°C in a 5% CO2 atmosphere. After culture periods of 24 h and 48 h the contents of the wells were harvested, spun at 2000 g for 2 min and the supernatants were collected and stored frozen at −80°C until use.
Cytokine assays
Culture supernatants were collected after 24 h to measure the IL-2, IL-4 and IFN-γ contents and after 48 h to evaluate IL-10. Supernatant cytokine concentrations were determined by ELISA (Immunotech, Marseille, France). The positivity thresholds were 10 pg/ml for IL-2 (Ref. 1116; Immunotech), 0.08 U/ml for IFN-γ (Ref. 1743; Immunotech), 1.5 pg/ml for IL-4 (Ref. 1631; Immunotech) and 3 pg/ml for IL-10 (Ref. 1634; Immunotech). Results are adjusted to 106 PBMC as determined with an automatic haemocytometer for all samples (H2; Bayer Diagnostics, Darmstadt, Germany).
The potential interference of soluble receptors in IL-2, IL-4 and IL-10 ELISAs was tested in vitro. Addition of recombinant soluble p55 IL-2 receptor chain from 800 pm to 62.5 pm (equivalent to 1 μg/ml) of recombinant IL-2 did not interfere in the IL-2 assay. Similarly, soluble recombinant IL-4 (428 pm) or soluble recombinant IL-10 (417 pm) receptor chains added, respectively, to IL-4 (55.6 pm, equivalent to 1 μg/ml) or IL-10 (105 pm, equivalent to 2 μg/ml) had no effect in these tests. Comparable experiments could not be conducted for IFN-γ because the soluble receptor is not yet commercially available. The results of all the ELISAs used in this study were not affected by either the presence of natural soluble receptors, as tested by adding their recombinant soluble counterparts to the analyte, or by any unknown component present in lupus patients' sera susceptible to alter significantly the measurement of a known amount of recombinant cytokine used to spike it prior to the standard assay.
To calculate the IL-10/IFN-γ ratio, we first converted IFN-γ units into pg/ml on the basis of 1 U = 50 pg (manufacturer's recommendation).
Statistical analysis
The distributions of cytokine concentrations are reported as their median values, first and third quartiles, and ranges as recommended in [18]. Comparisons between two sample populations were made with the non-parametric Mann–Whitney U-test, with the level of significance set at 0.05. Tests were performed with the statistical software STATISTICA (Statsoft, Guyancourt, France). Correlations were determined by linear regression and Spearman's rank correlation.
RESULTS
Patients
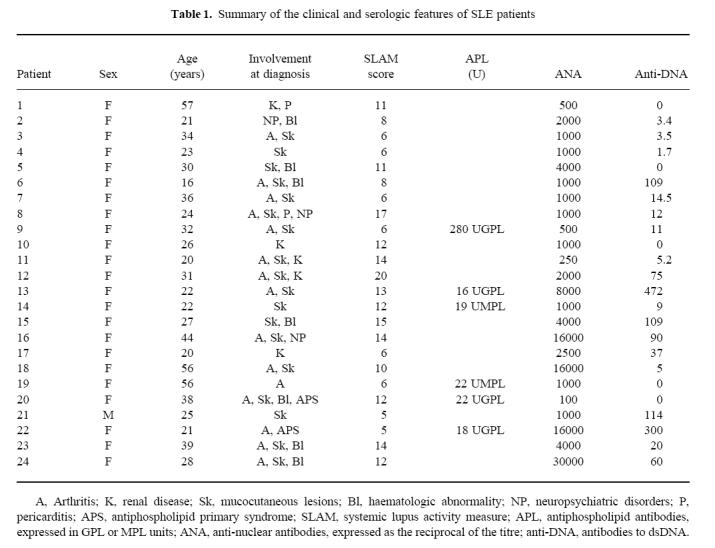
Ten healthy volunteers (age 21–60 years) served as controls. Twenty-four patients (mean age 31.16 years) fulfilled at least four of the revised 1982 American Rheumatism Association criteria for SLE (Table 1). SLE had been diagnosed 3 months to 12 years earlier (mean 5.8 years). The major clinical manifestations, in order of decreasing frequency were: 75% mucocutaneous lesions, 62.5% non-erosive polyarthritis, 20.8% nephropathy, 12.5% neuropsychiatric disorder, 8.3% each pericarditis and antiphospholipid syndrome. Biological signs were headed by 91.7% antinuclear antibodies ≥ 1/500, followed by 54.2% anti-dsDNA antibodies ≥ 10 U, 29.16% thrombopenia ≤ 110 000/mm3, and 25% IgG or IgM antiphospholipid antibodies. Serum levels of complement components were low in all but two patients (1 and 4).
Table 1.
Summary of the clinical and serologic features of SLE patients
Cytokine production
Th1 cytokines
In the absence of exogenous stimuli, the spontaneous production of IL-2 was comparable for patients and controls (Table 2). After 24 h in the presence of LPS + PHA, IL-2 levels were higher in supernatants from both populations, with SLE patients' IL-2 concentrations being higher than those of controls, but not significantly so (Table 2). However, mitogens were able to induce very high IL-2 synthesis by the PBMC from some patients, especially those with high SLAM scores. Indeed, basal and induced IL-2 levels were significantly correlated with the clinical SLAM index (Fig. 1a).
Table 2.
IL-2, IFN-γ, IL-4, and IL-10 cytokine production by PBMC from healthy adults and SLE patients
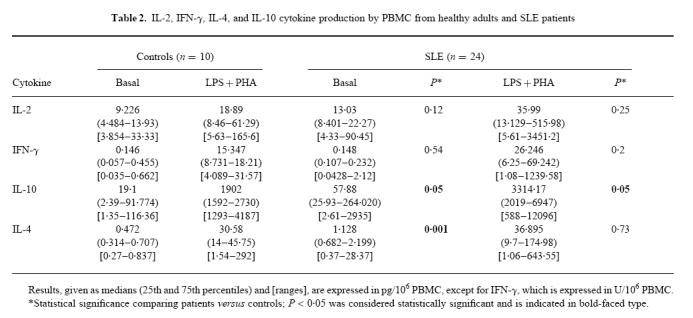
Fig. 1.
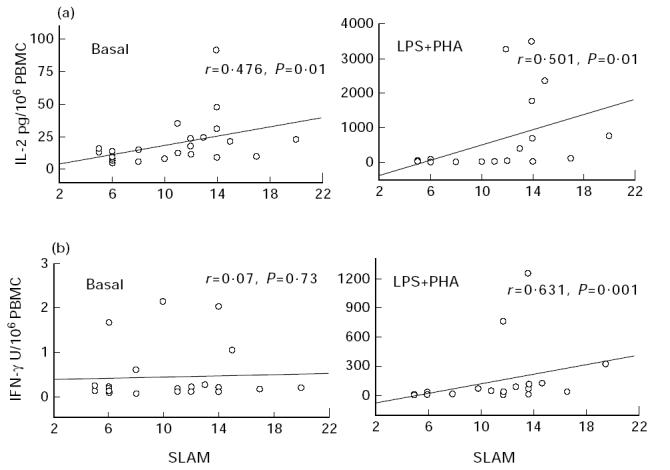
Correlations between IL-2 (a) and IFN-γ (b) cytokine production after 24 h of whole blood culture in patients with SLE and systemic lupus activity measure (SLAM) values. Basal, unstimulated culture conditions; LPS + PHA, mitogen-stimulated culture conditions. Results are expressed in pg/106 PBMC as estimated on the haemogram for IL-2 and in U/106 PBMC for IFN-γ. Correlations were determined by linear regression and Spearman's rank correlation.
As for IL-2, basal IFN-γ production was comparable for both populations, and LPS + PHA stimulation increased these concentrations, and more so for patients (Table 2). It should be noted that these latter values were statistically significantly correlated with the SLAM index (Fig. 1b), indicating that patients with high disease activity also had elevated IFN-γ production. No such correlation was found under basal conditions.
It is noteworthy that a significant correlation between IL-2 and IFN-γ production was established only when patients' PBMC were stimulated (r = 0.58, P = 0.0003).
Th2 cytokines
Induced IL-10 production was significantly higher than basal synthesis by control and patients' PBMC (control versus patients, P = 0.01). Significantly higher amounts of IL-10 were detected in samples from patients compared with controls under all culture conditions (Table 2), but no correlation was found between IL-10 levels and disease activity (Fig. 2a).
Fig. 2.
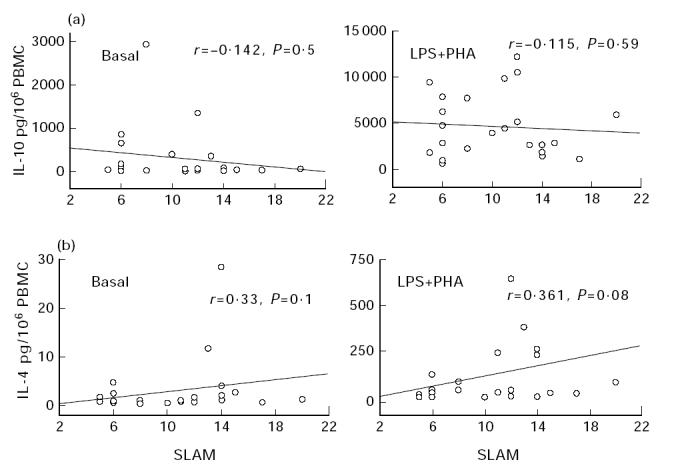
Correlations between IL-10 (a) and IL-4 (b) cytokine production after 24 h (IL-4) or 48 h (IL-10) of whole blood culture in patients with SLE and systemic lupus activity measure (SLAM) values. Basal, unstimulated culture conditions; LPS + PHA, mitogen-stimulated culture conditions. Results are expressed in pg/106 PBMC as estimated on the haemogram. Correlations were determined by linear regression and Spearman's rank correlation.
Spontaneous IL-4 production differed significantly between SLE patients and healthy individuals (Table 2), but because these values were close to the positivity threshold, this difference was not taken into consideration. Mitogen-activated PBMC from both populations generated enhanced IL-4 concentrations, but no statistical difference between groups was observed (Table 2), although some patients' stimulated PBMC produced high amounts of IL-4. A weak correlation between IL-4 amounts and disease activity was noted only under LPS + PHA stimulation (Fig. 2b).
Correlations between disease activity and the IL-10/IFN-γ or IL-10/IL-2 ratio
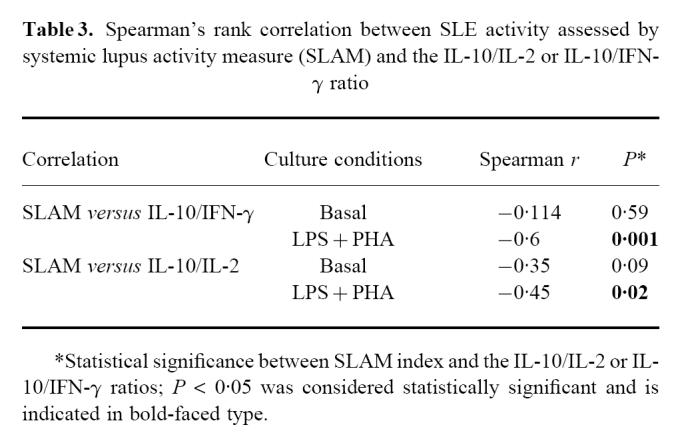
As shown above, significantly positive correlations were established between SLE activity, assessed by the SLAM score, and the IL-2 or IFN-γ concentration. In contrast, no correlation was observed between disease activity and IL-10 production, thus suggesting that the increased IL-10 production seen in SLE patients was independent of clinical disease severity. We therefore examined the IL-10/IL-2 and IL-10/IFN-γ ratios and tried to correlate them independently to disease activity. Under stimulatory conditions, the IL-10/IL-2 and IL-10/IFN-γ ratios were inversely and negatively significantly correlated with the SLAM index (Table 3).
Table 3.
Spearman's rank correlation between SLE activity assessed by systemic lupus activity measure (SLAM) and the IL-10/IL-2 or IL-10/IFN-γ ratio
DISCUSSION
The concentrations of IL-2, IFN-γ, IL-4 or IL-10 secreted in a WBA by PBMC from SLE patients and healthy individuals were determined and compared. Until now, cytokine production by immunocompetent cells has been investigated either by detection of the cytokine in the peripheral blood or by using multistep procedures to isolate blood cells. The latter procedure may lead to uncontrolled cell activation, a change in the ratio between lymphocytes and monocytes, and could deprive cells of exposure to essential cellular and molecular components present in whole blood. Indeed, the PBMC isolation procedure has been reported to reduce the accuracy and the reproducibility of cytokine synthesis compared with whole blood stimulation [10].
In whole blood, not only are natural cell–cell interactions preserved but also circulating stimulatory and inhibitory mediators, such as soluble receptors, are present at their physiological concentrations. Recently, this method was applied to measure TNF-α and IL-1 production in SLE [19] and other autoimmune diseases [11,12,20].
Our results, obtained from a homogeneous Caucasian population receiving no treatment, confirmed that SLE is a disease characterized by an abnormally high production of IL-10, and are in agreement with the data obtained from untreated Mexican SLE patients who exhibited an increased spontaneous synthesis of IL-10 by PBMC compared with healthy controls [21]. Those authors demonstrated that the high IL-10 secretion by monocytes and lymphocytes was responsible for the heightened immunoglobulin production [22]. IL-10 production was also found to be enhanced in patients with rheumatoid arthritis or Sjögren's syndrome, two disorders characterized by prominent B lymphocyte hyperactivity which results in increased production of immunoglobulins and the synthesis of autoantibodies [23]. Alternatively, this over-expression may also reflect an ongoing negative loop, as IL-10 is known to inhibit the production of IFN-γ by Th1 CD4+ T cell clones [24,25] via its ability to abrogate production of macrophage/monocyte cytokines, such as TNF-α, IL-1 and IL-12 [26–28]. Several studies demonstrated that IL-10 down-regulates the development of a cell-mediated immune response [29,30]. However, because IL-10 is also a potent stimulator of B lymphocytes [31], it probably plays a major role in the polyclonal B lymphocyte hyperactivity associated with these diseases and in the development of autoimmunity.
IL-10 alterations can not explain the clinical exacerbation of the lupus, since IL-10 abnormalities are equally present in SLE patients regardless of the degree of disease activity. Therefore, the disease exacerbation might be the result of the failure of the negative regulatory circuits of which IL-10 is a part.
The most salient point of our study is the significant correlation between the severity of SLE and the amounts of IL-2 or IFN-γ secreted by SLE patients' PBMC in response to LPS + PHA. This pattern was previously noted in recently developed murine SLE models dealing with MRL-lpr/lpr and MRL.Yaa mice [32]. The development of the lupus-like autoimmune disease in these mice was correlated with the enhanced expression of IFN-γ mRNA but not a further increase of IL-10 mRNA in CD4+ T cells, which was already high. The role of IFN-γ in the development of SLE was previously documented by the earlier appearance of the disease after repeated injections of recombinant IFN-γ into (NZB × NZW)F1 hybrid mice [9]. Treatment of these mice when young with anti-IFN-γ antibodies was beneficial, inhibiting the progression toward SLE. Investigations measuring the IFN-γ levels in SLE patients' sera found them to be enhanced in association with lymphadenopathy or nephrotic syndrome [33], whereas others found no such increase in this context [34]. This discrepancy might be explained by the likely influence of the quality of renal function on systemic cytokine clearance, because impaired renal function could lead to increased serum cytokine levels. However, the extent of IFN-γ production by PBMC from our SLE patients was not correlated with renal dysfunction. In this respect, the WBA is, by definition, independent of renal IFN-γ catabolism and probably reflects more accurately the IFN-γ secretory capacity of the patients' PBMC.
Previous studies concluded that IL-2 synthesis was decreased or normal in SLE patients [35–38]. We found that patients with high SLAM indices had IL-2 concentrations higher than those of controls. This discrepancy may be explained by the methodology used here, as IL-2 is produced and consumed by T cells which also express membrane high-affinity IL-2 receptors (IL-2R) upon antigenic activation [39]. IL-2 determination would be more accurate if an anti-p55 antibody, able to block its binding to the α-chain of the receptor and subsequent internalization, was added, thereby preventing IL-2 removal from the supernatant, as previously described [40,41].
Although some patients had high levels of mitogen-stimulated IL-4 production, no correlation with SLAM was found [42], suggesting that the role of IL-4 in this disease remains to be defined. Because in vivo constitutive expression of IL-4 has been reported to induce autoimmune-type disorders in mice [43] and others have shown that IL-4 might protect against a genetically linked lupus-like autoimmune syndrome [44], further studies on IL-4's role in SLE are warranted. For example, a new T lymphocyte subpopulation carrying Vα24+ invariant TCR α-chain homologous to murine NK1.1 cells has recently been found to potentially regulate immune responses, as it produces high amounts of IL-4 able to commit the immune response towards a Th2 imbalance [45,46].
Finally, we examined the IL-10/IL-2 and IL-10/IFN-γ ratios. Enhanced ratios attributable to high IL-10 production were found for all patients, regardless of SLE severity, and might trigger the B lymphocyte hyperactivity. Conversely, low ratios reflecting elevated IL-10 and IL-2 or IFN-γ levels could be taken as a marker of inflammation and tissue injury observed in the clinical exacerbation of SLE. In patients with minor or few tissue injuries, these ratios were high, whereas they were low in those with tissue lesions, i.e. with active disease. This relationship was previously investigated by examining the number of cells secreting IL-10 and IFN-γ with an ELISPOT assay [47]; disease severity was associated with an increased ratio of IL-10/IFN-γ cytokine-producing cells, which resulted from a combination of fewer T cells producing IFN-γ and more cells secreting IL-10. Our results do not support their findings, but their ratio was established from density gradient-separated unstimulated PBMC in a different experimental setting, thus precluding any direct comparison between the two studies.
In conclusion, we confirmed that SLE is characterized by the increased secretion of IL-10 and demonstrated that this high cytokine synthesis was independent of disease severity and could be required to contain the over-production of IL-2 or IFN-γ pathogenic for the host. When this protective immune response is overwhelmed, a full-blown cell-mediated immune response characterized by enhanced IL-2 and IFN-γ production leads to the tissue damage responsible for clinical symptoms.
REFERENCES
- 1.Klinman DM, Steinberg AD. Systemic autoimmune disease arises from polyclonal B cell activation. J Exp Med. 1987;165:1755–60. doi: 10.1084/jem.165.6.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cherwinski HM, Schumacher JH, Brown KD, Mosmann TR. Two types of mouse helper T cell clone. III. Further differences in lymphokine synthesis between Th1 and Th2 clones revealed by RNA hybridization, functionally monospecific bioassays, and monoclonal antibodies. J Exp Med. 1987;166:1229–44. doi: 10.1084/jem.166.5.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mosmann TR, Coffman RL. Th1 and Th2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–73. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 4.Fowell D, McKnight AJ, Powrie F, Dyke R, Mason D. Subsets of CD4+ T cells and their role in the induction and prevention of autoimmunity. Immunol Rev. 1991;123:37–64. doi: 10.1111/j.1600-065x.1991.tb00605.x. [DOI] [PubMed] [Google Scholar]
- 5.Romagnani S. Human TH1 and TH2 subsets: doubt no more. Immunol Today. 1991;12:256–7. doi: 10.1016/0167-5699(91)90120-I. [DOI] [PubMed] [Google Scholar]
- 6.Romagnani S. Lymphokine production by human T cells in disease states. Annu Rev Immunol. 1994;12:227–57. doi: 10.1146/annurev.iy.12.040194.001303. [DOI] [PubMed] [Google Scholar]
- 7.Snapper CM, Mond JJ. Towards a comprehensive view of immunoglobulin class switching. Immunol Today. 1993;14:15–17. doi: 10.1016/0167-5699(93)90318-F. [DOI] [PubMed] [Google Scholar]
- 8.Goldman M, Druet P, Gleichmann E. Th2 cells in systemic autoimmunity: insights from allogeneic diseases and chemically-induced autoimmunity. Immunol Today. 1991;12:223–7. doi: 10.1016/0167-5699(91)90034-Q. [DOI] [PubMed] [Google Scholar]
- 9.Jacob CO, van der Meide PH, McDevitt HO. In vivo treatment of (NZB X NZW) F1 lupus-like nephritis with monoclonal antibody to γ interferon. J Exp Med. 1987;166:798–803. doi: 10.1084/jem.166.3.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Groote D, Zangerle PF, Gevaert Y, et al. Direct stimulation of cytokines (IL-1 beta, TNF-alpha, IL-6, IL-2, IFN-gamma and GM-CSF) in whole blood. Comparison with isolated PBMC stimulation. Cytokine. 1992;4:239–48. doi: 10.1016/1043-4666(92)90062-v. [DOI] [PubMed] [Google Scholar]
- 11.Zangerle PF, De Groote D, Lopez M, et al. Direct stimulation of cytokines (IL-1 beta, TNF-alpha, IL-6, IL-2, IFN-gamma and GM-CSF) in whole blood: II. Application to rheumatoid arthritis and osteoarthritis. Cytokine. 1992;4:568–75. doi: 10.1016/1043-4666(92)90021-i. [DOI] [PubMed] [Google Scholar]
- 12.Chernoff AE, Granowitz EV, Shapiro L, et al. A randomized, controlled trial of IL-10 in humans. Inhibition of inflammatory cytokine production and immune responses. J Immunol. 1995;154:5492–9. [PubMed] [Google Scholar]
- 13.Petrovsky N, Harrison LC. Cytokine-based human whole blood assay for the detection of antigen-reactive T cells. J Immunol Methods. 1995;186:37–46. doi: 10.1016/0022-1759(95)00127-v. [DOI] [PubMed] [Google Scholar]
- 14.Johnson K, Aarden L, Choi Y, De Groot E, Creasey A. The proinflammatory cytokine response to coagulation and endotoxin in whole blood. Blood. 1996;87:5051–60. [PubMed] [Google Scholar]
- 15.Tan EM, Cohen AS, Fries JF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25:1271–7. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 16.Asherson RA. A ‘primary’ antiphospholipid syndrome? J Rheumatol. 1988;15:1742–6. [PubMed] [Google Scholar]
- 17.Liang MH, Socher SA, Larson MG, Schur PH. Reliability and validity of six systems for the clinical assessment of disease activity in systemic lupus erythematosus. Arthritis Rheum. 1989;32:1107–18. doi: 10.1002/anr.1780320909. [DOI] [PubMed] [Google Scholar]
- 18.O'Brien PC, Shampo MA, Dyck PJ. Statistical analysis in clinical laboratory medicine: fundamentals and common uses. Crit Rev Lab Sci. 1989;27:319–40. doi: 10.3109/10408368909105718. [DOI] [PubMed] [Google Scholar]
- 19.Swaak AJG, van den Brink HG, Aarden LA. Cytokine production (IL-6 and TNFα) in whole blood cell cultures of patients with systemic lupus erythematosus. Scand J Rheumatol. 1996;25:233–8. doi: 10.3109/03009749609069992. [DOI] [PubMed] [Google Scholar]
- 20.Kallmann BA, Hâuter M, Tubes M, Feldkamp J, Bertrams J, Gries FA, Lampeter EF, Kolb H. Systemic bias of cytokine production toward cell-mediated immune regulation in IDDM and toward humoral immunity in Grave's disease. Diabetes. 1997;46:237–43. doi: 10.2337/diab.46.2.237. [DOI] [PubMed] [Google Scholar]
- 21.Llorente L, Richaud-Patin Y, Wijdenes J, et al. Spontaneous production of interleukin-10 by B lymphocytes and monocytes in systemic lupus erythematosus. Eur Cytokine Netw. 1993;4:421–7. [PubMed] [Google Scholar]
- 22.Llorente L, Zou W, Levy Y, et al. Role of interleukin-10 in the B lymphocyte hyperactivity and autoantibody production of human systemic lupus erythematosus. J Exp Med. 1995;181:839–44. doi: 10.1084/jem.181.3.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Llorente L, Richaud-Patin Y, Fior R, Alcocer-Varela J, Wijdenes J, Morel Fourrier B, Galanaud P, Emilie D. In vivo production of interleukin-10 by non-T cells in rheumatoid arthritis, Sjögren's syndrome, and systemic lupus erythematosus. Arthritis Rheum. 1994;37:1647–55. doi: 10.1002/art.1780371114. [DOI] [PubMed] [Google Scholar]
- 24.Moore KW, O'Garra A, de Waal Malefyt R, Vieira P, Mossman TR. Interleukin-10. Annu Rev Immunol. 1993;11:165–90. doi: 10.1146/annurev.iy.11.040193.001121. [DOI] [PubMed] [Google Scholar]
- 25.Moore KW, Vieira P, Fiorentino DF, Trounstine ML, Khan TA, Mossman TR. Homology of cytokine synthesis inhibitory factor (IL-10) to the Epstein–Barr virus gene BCRFI. Science. 1990;248:1230–4. doi: 10.1126/science.2161559. [DOI] [PubMed] [Google Scholar]
- 26.Fiorentino DF, Zlotnik A, Mossman TR, Howard M, O'Garra A. IL-10 inhibits cytokine production by activated macrophages. J Immunol. 1991;146:3815–22. [PubMed] [Google Scholar]
- 27.Fiorentino DF, Zlotnik A, Vieira P, Mossman TR, Howard M, Moore KW, O'Garra A. IL-10 acts on the antigen-presenting cells to inhibit cytokine production by Th1 cells. J Immunol. 1991;146:3444–51. [PubMed] [Google Scholar]
- 28.Rennick D, Berg D, Holland G. Interleukin-10: an overview. Prog Growth Factor Res. 1992;4:207–27. doi: 10.1016/0955-2235(92)90020-i. [DOI] [PubMed] [Google Scholar]
- 29.Ding L, Linsley PS, Huang LY, Germain RN, Shevach EM. IL-10 inhibits macrophage costimulatory activity by selectively inhibiting the up-regulation of B7 expression. J Immunol. 1993;151:1224–34. [PubMed] [Google Scholar]
- 30.Gazzinelli RT, Oswald IP, James SL, Sher A. IL-10 inhibits parasite killing and nitrogen oxide production by IFN-gamma-activated macrophages. J Immunol. 1992;148:1792–6. [PubMed] [Google Scholar]
- 31.Rousset F, Garcia E, Defrance T, Peronne C, Hsu DH, Kastelein R, Moore KW, Banchereau J. Interleukin-10 is a potent growth and differentiation factor for activated human B lymphocytes. Proc Natl Acad Sci USA. 1992;89:1890–3. doi: 10.1073/pnas.89.5.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takahashi S, Fossati L, Iwamoto M, Mérino R, Motta R, Kobayakawa T, Izui S. Imbalance towards Th1 predominance is associated with acceleration of lupus-like autoimmune syndrome in MRL mice. J Clin Invest. 1996;97:1597–604. doi: 10.1172/JCI118584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Al-Janadi M, Al-Balla S, Al-Dalaan A, Raziuddin S. Cytokine profile in systemic lupus erythematosus, rheumatoid arthritis, and other rheumatic diseases. J Clin Immunol. 1993;13:58–67. doi: 10.1007/BF00920636. [DOI] [PubMed] [Google Scholar]
- 34.Tsokos GC, Boumpas DT, Smith PL. Deficient gamma-interferon production in patients with systemic lupus erythematosus. Arthritis Rheum. 1986;29:1210–5. doi: 10.1002/art.1780291005. [DOI] [PubMed] [Google Scholar]
- 35.Alcocer-Varela J, Alarcon-Segovia D. Decreased production of and response to interleukin-2 by cultured lymphocytes from patients with systemic lupus erythematosus. J Clin Invest. 1982;69:1388–92. doi: 10.1172/JCI110579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Linker-Israeli M, Bakke AC, Kitridou RC, Gendler S, Gillis S, Horwitz DA. Defective production of interleukin-1 and interleukin-2 in patients with systemic lupus erythematosus. J Immunol. 1983;130:2651–5. [PubMed] [Google Scholar]
- 37.de Faucal P, Godard A, Peyrat MA, Moreau JF, Soulillou JP. Impaired IL2 production by lymphocytes of patients with systemic lupus erythematosus. Ann Immunol (Inst Pasteur) 1984;135D:161–72. doi: 10.1016/s0769-2625(84)81108-3. [DOI] [PubMed] [Google Scholar]
- 38.Draeger AM, Swaak AJ, van der Brink HG, Aarden LA. T cell function in systemic lupus erythematosus: normal production of and responsiveness to interleukin-2. Clin Exp Immunol. 1986;64:80–87. [PMC free article] [PubMed] [Google Scholar]
- 39.Kaplan D. Autocrine secretion and the physiological concentration of cytokines. Immunol Today. 1996;17:303–4. doi: 10.1016/0167-5699(96)30017-0. [DOI] [PubMed] [Google Scholar]
- 40.Hallet MM, Peyrat MA, Moisan JP, Soulillou JP, Moreau JF. Proliferation dependence and production of IL-2 in human alloreactive T-cell clones. Cell Immunol. 1989;124:95–106. doi: 10.1016/0008-8749(89)90114-7. [DOI] [PubMed] [Google Scholar]
- 41.Claret E, Renversez JC, Zheng X, Bonnefoix T, Sotto JJ. Valid estimation of IL-2 secretion by PHA-stimulated T-cell clones absolutely requires the use of anti-CD25 monoclonal antibody to prevent IL-2 consumption. Immunol Letters. 1992;33:179–86. doi: 10.1016/0165-2478(92)90045-p. [DOI] [PubMed] [Google Scholar]
- 42.Seder RA, Le Gros G, Ben-Sasson SZ, Urban J, Jr, Finkelman FD, Paul WE. Increased frequency of IL-4 producing T cells as a result of polyclonal priming. Use of a single-cell assay to detect IL-4 producing cells. Eur J Immunol. 1991;21:1241–7. doi: 10.1002/eji.1830210522. [DOI] [PubMed] [Google Scholar]
- 43.Erb KJ, Ruger B, von Brevern M, Ryffel B, Schimpl A, Rivett K. Constitutive expression of interleukin (IL)-4 in vivo causes autoimmune-type disorders in mice. J Exp Med. 1997;185:329–39. doi: 10.1084/jem.185.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Santiago ML, Fossati L, Jacquet C, Müller W, Izui S, Reininger L. Interleukin-4 protects against a genetically linked lupus-like autoimmune syndrome. J Exp Med. 1997;185:65–70. doi: 10.1084/jem.185.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yoshimoto T, Bendelac A, Watson C, Hu-Li J, Paul WE. Role of NK1.1+ T cells in a Th2 response and in immunoglobulin E production. Science. 1995;270:1845–7. doi: 10.1126/science.270.5243.1845. [DOI] [PubMed] [Google Scholar]
- 46.Davodeau F, Peyrat MA, Necker A, et al. Close phenotypic and functional similarities between human and murine αβ T cells expressing invariant TCR α-chains. J Immunol. 1997;58:5603–11. [PubMed] [Google Scholar]
- 47.Hagiwara E, Gourley MF, Lee S, Klinman DM. Disease severity in patients with systemic lupus erythematosus correlates with an increased ratio of interleukin-10: interferon-γ-secreting cells in the peripheral blood. Arthritis Rheum. 1996;39:379–85. doi: 10.1002/art.1780390305. [DOI] [PubMed] [Google Scholar]


