Abstract
We report here the expression of β2-GPI mRNA by cell types involved in the pathophysiology of the anti-phospholipid syndrome (APS), i.e. endothelial cells as a target of autoantibodies in the APS, astrocytes and neurones involved in APS of the central nervous system (CNS). Lymphocytes were also included in the study, as it has been demonstrated that patients with systemic lupus erythematosus-associated CNS diseases have serum anti-lymphocyte antibodies cross-reacting with brain antigens, and intrathecally synthesized anti-neurone antibodies. Reverse transcriptase-polymerase chain reaction followed by restriction enzyme digestion of the product obtained demonstrated the presence of β2-GPI mRNA in all cell types here tested, cultured both in presence and absence of fetal calf serum. In both culture conditions, the same cell types were immunoreactive to an anti-β2-GPI MoAb, as determined by indirect immunofluorescence technique. Taken together, these results indicate a direct cell synthesis of β2-GPI, suggesting an antigenic function of β2-GPI in the APS, including the CNS disease that occurs in this syndrome.
Keywords: β2-glycoprotein I, anti-phospholipid syndrome, endothelium, central nervous system
INTRODUCTION
β2-GPI is an apolipoprotein involved in lipid metabolism and classified among members of the complement control protein superfamily [1]. The β2-GPI sequence, determined by protein and cDNA analysis, is strongly homologous among species [2,3]. The attention of the immunologists has recently focused on β2-GPI because of its requirement for the formation of the antigenic epitopes of some anti-phospholipid autoantibodies (aPL) [4–6]. In fact, clinical and experimental reports suggest that β2-GPI is one of the main target antigens for aPL in the anti-phospholipid syndrome (APS), either primary (PAPS) or secondary to systemic lupus erythematosus (SLE) [7–10]. APS is characterized by fetal loss and a wide spectrum of clinical manifestations, including neurological symptoms such as focal cerebral and ocular ischaemia, the myelopathy of lupoid sclerosis and Degos' disease, and, less frequently, Guillan–Barré polyradiculoneuritis, migraine, chorea and seizures [11,12]. Because of the frequent association with aPL, the clinical relevance of anti-β2-GPI autoantibodies (a β2-GPI) in APS has been long debated [13–16]. A direct pathogenic role of the aβ2-GPI in APS has been recently suggested by the identification of these antibodies as a population distinct from anti-cardiolipin antibodies (aCL) [17] and by a series of clinical reports. Thus, aβ2-GPI have been associated with thrombosis and thrombocytopenia occurring in the APS more significantly than aPL [8,18–21]. Moreover, APS has been recently reported in patients having aβ2-GPI but not aPL. For this reason, to describe this primary variant of the APS, some authors coined the term ‘aPL-cofactor syndrome’ [22,23]. Interestingly, central nervous system (CNS) involvement is frequent in these conditions. The binding of aβ2-GPI to several cell elements, such as macrophages, apoptotic thymocytes, trophoblast cells, activated platelets and, in particular, endotheliocytes has been previously reported [24–31]. More recently, we demonstrated that aβ2-GPI bind cerebrovascular endothelium, astrocytes and neurones [32]. The aβ2-GPI immunoreactivity indicates that β2-GPI is present in cell types other than hepatocytes, which represent the main site of synthesis in the organism.
The immunoreactivity by aβ2-GPI has been particularly investigated in the endothelial cells because of their relevant involvement in APS pathophysiology. Whether aβ2-GPI immunoreactivity of endotheliocytes is due to the uptake of extracellular β2-GPI molecules (i.e. of serum origin) or to direct intracellular synthesis of the glycoprotein is still a matter of debate. Some authors described the disappearance of aβ2-GPI immunoreactivity in endotheliocytes grown in serum-free medium [31], whereas others reported its lasting presence even after serum depletion [33–36]. However, the direct evidence of β2-GPI synthesis by cell types involved in APS pathophysiology is provided by the demonstration of β2-GPI mRNA in human fetal astrocytes as well as in human cells of intestine and placenta, as determined by reverse transcriptase-polymerase chain reaction (RT-PCR) [37–39]. In the present study, we performed RT-PCR to determine β2-GPI mRNA expression by endothelial cells, a known target of autoantibodies in APS, astrocytes and neurones, being cell populations involved in the CNS disease of the APS. We also investigated lymphocytes because this cell type has been a known target of a wide spectrum of antibodies detected in sera of SLE patients, frequently with evidence of CNS disease. These antibodies share some degree of cross-reactivity with neuronal and glial antigens [40]. Moreover, intrathecal synthesis of anti-neurone antibodies has been recently demonstrated in SLE patients with CNS disease [41].
MATERIALS AND METHODS
Cells
The following human cells were used: LAN5 (neuroblastoma line), human umbilical vein endothelial cells (HUVEC), two established human glial cell lines T67 and T70, respectively derived from a III WHO gemistocytic astrocytoma and from a glioblastoma, as previously described [32,42] (kindly provided by Professor G. Lauro, Department of Biology, III University of Rome, Italy), HEpGL2 hepatoma line used as positive control, according to Averna et al. and Chamley et al. [38,39] (kindly provided by R. Nicotra, Istituto Regina Elena, Roma, Italy), and normal skin fibroblasts as negative controls. Cell lines were cultured in their usual medium supplemented by 5–20% fetal calf serum (FCS) and gentamycin (0.05 mg/ml) (Gibco BRL, Paisley, UK). After 3 days, one sample of each cell line was repeatedly washed in PBS pH 7.3 to remove the culture medium and then analysed; a second sample was cultured for a further 3 days in FCS-free medium before analysis. Peripheral blood lymphocytes from three healthy donors were separated by Ficoll (Nycomed, Oslo, Norway) gradient centrifugation followed by hypotonic lysis of the erythrocytes and washed three times in PBS pH 7.3.
Detection of β2-GPI mRNA by RT-PCR
Total RNAs, extracted from 5–10 × 106 cells of each line by Ultraspec RNA isolation system (Biotecx, Houston, TX) according to the manufacturer's instructions, were treated with DNase I RNase-free (Gibco BRL) and then converted to first-strand cDNA copies by random primers of 4 μg of total RNA with Super Script H−RNase RT, as suggested by the supplier (Gibco BRL). Oligonucleotide primers designed for PCR amplification of the human β2-GPI and β-actin mRNAs were checked by Genebank. Based on the coding sequences [2,43], the following primers were used: β2-GPI-F 5′ – TCTGCCATGCCAAGTTGTAAAG − 3′ (784–805); β2-GPI-R 5′ – CATCGGATGCATCAGTTTTCCA − 3′ (1045−1024); β-actin-F 5′ – AAGAGAGGCATCCTCACCCT − 3′ (222–241); β-actin-R 5′ – TACATGGCTGGGGTGTTGAA − 3′ (439−420) [44].
One quarter of cDNA synthesis reaction volume was combined for PCR amplification in a 100-μl final volume containing each primer and Taq polymerase (Gibco BRL). PCR was performed for either 35 (β2-GPI) or 25 (β-actin) cycles, each cycle consisting of denaturation at 94°C (45 s), annealing at 60°C (30 s), extension at 72°C (30 s), after predenaturation at 95°C (2 min), and final extension at 72°C (10 min). RT-PCR products (15 μl) were electrophoresed on 2% agarose gels in TAE buffer. To rule out the possibility of amplification of contaminating genomic DNA, RNA samples treated with DNase were submitted to PCR amplification without RT. The optical density (OD) measurements of the lanes on the agarose gels were performed by means of a computer-assisted image analysis system (MCID, Imaging Research, St Catherine's, Ontario, Canada) using dedicated software. The system was calibrated using a set of OD standards (Kodak, Rochester, NY). Non-linear distance calibration was performed using the molecular length marker (50-bp DNA ladder; Gibco BRL). Background subtraction was automatically performed before analysis. In order to compare the results obtained from each cell line cultured in presence or absence of FCS, a semiquantitative approach was used by comparing in the same cell line samples the OD value of β2-GPI lane to that of β-actin. The RT-PCR products were digested with the restriction enzyme Alu I (GIBCO BRL) and electrophoresed on 2% agarose gels in TAE buffer.
Detection of β2-GPI by indirect immunofluorescence
Cells were cultured for 3 days on cover slides in medium containing FCS, then repeatedly washed in PBS to remove the culture medium. For each cell line, some samples were immediately fixed in PBS 4% formaldehyde (v/v) (1 h at room temperature); other samples were grown for another 3 days in FCS-free medium before fixation. The presence of β2-GPI was detected by indirect immunofluorescence (IIF) using the antibody anti-human β2-GPI affinity-purified mouse monoclonal (aβ2-GPI MoAb 1A4, isotype IgG2, 1:10 diluted in PBS) prepared as previously reported [17,32,45]. The immune reaction was revealed by goat anti-mouse IgG conjugated with FITC (GAM-FITC; Sigma, St Louis, MO; 30 min at room temperature) and observed under a fluorescence microscope with an FITC outfit (Leitz, Wetzlar, Germany), as described in our previous study [32]. The aβ2-GPI MoAb 1A4 immunoreactivity with the lymphocytes was detected on fresh cells spotted on slides and fixed in PBS 4% formaldehyde (v/v). Negative controls were performed on each cell line replacing the a β2-GPI MoAb 1A4 with mouse non-immune IgG.
RESULTS
The expression of mRNA for β2-GPI and β-actin in the different cell lines cultured in medium containing FCS for 3 days and in fresh lymphocytes is shown in Fig. 1. The β2-GPI mRNA was identified from the HEpGL2, HUVEC, LAN5, T67, T70 cell lines and from lymphocytes, whereas no detectable signal was obtained from the fibroblasts. The RT-PCR of RNAs resulted in amplification of the expected bands, i.e. 262 bp for β2-GPI and 218 bp for β-actin. Similar results were obtained from RT-PCR of RNAs extracted from the cell lines grown for another 3 days in FCS-free medium (data not shown). When RT-PCR products were digested with Alu I restriction enzyme and subjected to electrophoresis analysis, two bands of the expected length (i.e. 101 and 161 bp) were observed, as shown in Fig. 2. The possibility of amplification of contaminating genomic DNA was excluded, since no products were obtained from the RNA samples subjected to PCR without RT. Moreover, the β-actin-specific primer pairs were selected from two exons separated by one intronic sequence [44]. As shown in Fig. 1, the RT-PCR product of β-actin mRNA (218 bp), but no gene fragment (659 bp) was observed. The immunoreactivity of the aβ2-GPI MoAb 1A4, as detected by IIF, was observed with HEpGL2, HUVEC, T67, T70 and LAN5 cell lines both in presence and absence of FCS in the culture medium, and with fresh PBL, but never with the fibroblasts. The aβ2-GPI MoAb 1A4 immunoreactivity with HUVEC, T67 and T70 glial lines, LAN5 neuroblastoma line and PBL in serum-free condition is shown in Fig. 3 (left panels). The fluorescent pattern was homogeneous and localized within the cytoplasm and/or on the cell membrane. The intensity of fluorescence, as well as the pattern, were similar to those obtained in cells cultured in medium containing FCS (data not shown). Technique controls performed on each cell type replacing the aβ2-GPI MoAb 1A4 with non-immune IgG were negative (Fig. 3, right panels).
Fig. 1.
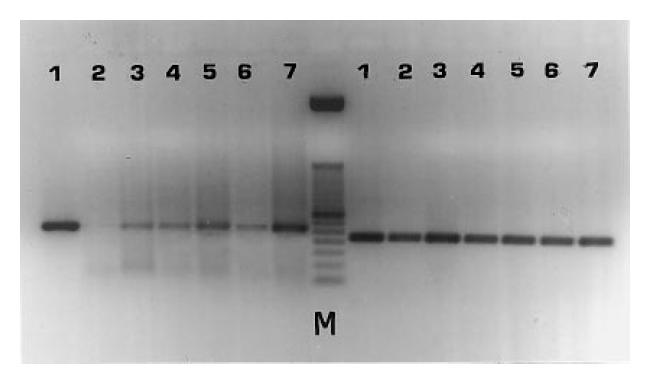
Shown are reverse transcriptase-polymerase chain reaction (RT-PCR) products of mRNAs for β2-GPI (left lanes) and for β-actin (right lanes) from HEpGL2 (1), fibroblasts (2), human umbilical vein endothelial cells (HUVEC) (3), T67 (4), T70 (5), LAN5 (6), and lymphocytes (7). The RT-PCR of RNAs results in amplification of the expected bands: 262 bp for β2-GPI, 218 bp for β-actin. M, 50-bp DNA ladder.
Fig. 2.
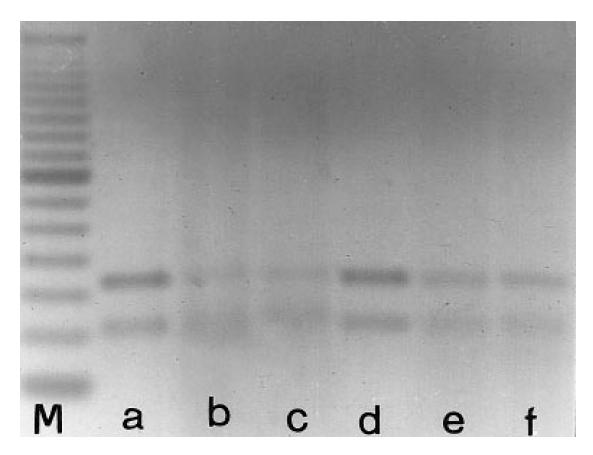
Shown are the fragments obtained by digestion with the Alu I restriction enzyme of the reverse transcriptase-polymerase chain reaction (RT-PCR) products of β2-GPI mRNA from HEpGL2 (a), human umbilical vein endothelial cells (HUVEC) (b), T67 (c), T70 (d), LAN5 (e), lymphocytes (f). M, 50-bp DNA ladder. The two fragments are of the expected molecular length (101 and 161 bp), confirming the specificity of the primers used.
Fig. 3.
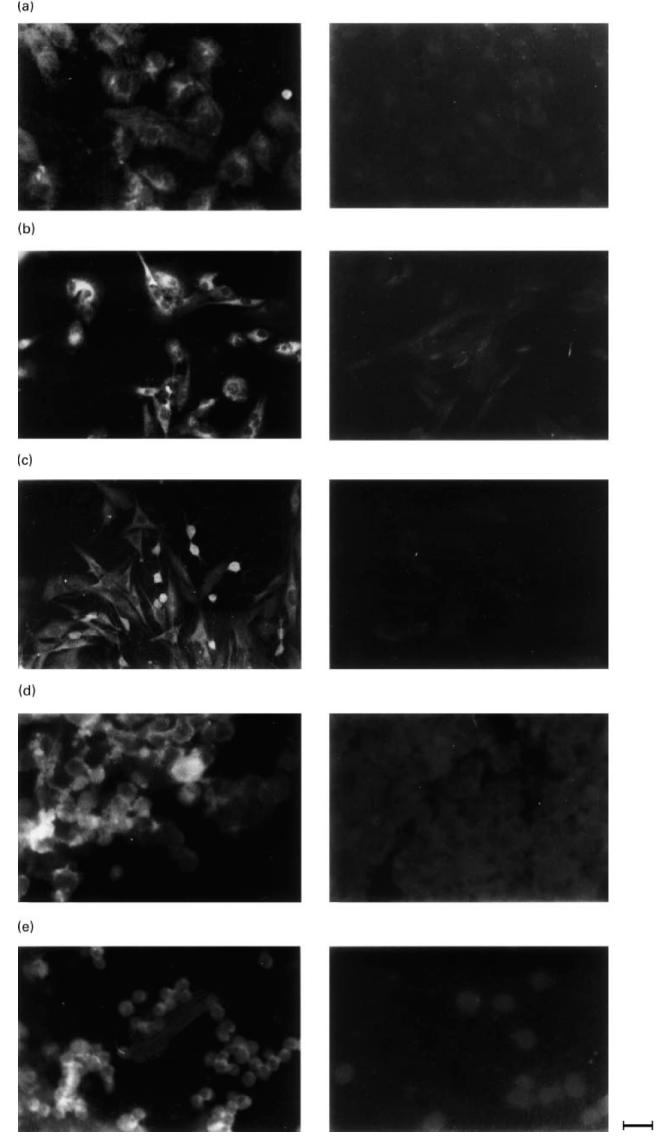
Shown is the immunoreactivity by the aβ2-GPI MoAb 1A4 with endothelial cells (human umbilical vein endothelial cells (HUVEC)) (a) astrocytoma line T67 (b), glioma line T70 (c), neuroblastoma line LAN5 (d), all cultured in serum-depleted medium for 3 days, and lymphocytes (e). The aβ2-GPI binding was revealed by indirect immunofluorescence using a goat anti-mouse conjugated with FITC (left panels). Control techniques were performed on each cell type replacing the aβ2-GPI with non-immune IgG (right panels). Calibration bar = 25 μm.
DISCUSSION
The results of the present study demonstrate β2-GPI mRNA expression by endothelial cells, astrocytes, neurones and lymphocytes, thus indicating that these cell types synthesize β2-GPI. The RT-PCR product of total RNA from these cell types resulted in amplification of the expected band for β2-GPI mRNA, i.e. 262 bp. The same band was also obtained from the HEpGL2 hepatocyte cell line used as positive control for β2-GPI mRNA expression both in this study and in previous reports [38,39].
After digestion with Alu I restriction enzyme we observed the two expected bands (101 and 161 bp). This confirms that the RT-PCR product resulted from the amplification of the 262 bp sequence belonging to the β2-GPI mRNA. The results' viability is supported by the observations that no RT-PCR product was obtained from RNA of fibroblasts, used as negative control, and that PCR performed without RT on each RNA excluded the possibility of amplifying the contaminating genomic DNA. This latter possibility was further excluded since we observed the RT-PCR product of β-actin mRNA (218 bp), but no gene fragment (659 bp), as previously demonstrated [44].
The demonstration of β2-GPI mRNA in endotheliocytes, CNS cells and lymphocytes, by RT-PCR, extends the knowledge that the liver is not the unique site of synthesis of β2-GPI. In this respect, β2-GPI mRNA has been previously demonstrated using the same technique in fetal astrocytes, cells of intestine and placenta [37–39]. The question whether β2-GPI is synthesized by endotheliocytes is particularly relevant, since these cells are targeted by aPL and aβ2-GPI in APS and aPL cofactor syndrome. In view of this, the present results of aβ2-GPI immunoreactivity of endothelial cells cultured for 3 days in FCS-free conditions, and in particular aβ2-GPI immunoreactivity localized within the cytoplasm, provide evidence in favour of the presence of endogenous β2-GPI in the endothelial cells. These results are consistent with previous reports of the persistence of aβ2-GPI immunoreactivity in endotheliocytes cultured in β2-GPI-deficient medium [33–36].
Since translocation of extracellular β2-GPI within the cytoplasm is unknown, the intracellular localization of aβ2-GPI immunoreactivity suggests β2-GPI synthesis by these cells. The β2-GPI molecules, synthesized in the cytoplasm, might be successively carried to the cell surface, as routinely occurs for numerous molecules expressed on the cell membrane. Thus, the aβ2-GPI immunoreactivity observed on the cell membrane in serum-free cultured cells, as reported here and in our previous study [32], might be due, at least in part, to this latter mechanism. Other authors [31] reported that aβ2-GPI binding to endothelial cell surface, as determined by ELISA, disappears after 5 h of culture in serum-free conditions and is re-established after addition of serum purified β2-GPI. The lack of aβ2-GPI binding in serum-free cultured endotheliocytes reported by these authors [31] might be due to the short period of culture in serum-free medium. It is conceivable that cells require longer than 5 h to synthesize and carry β2-GPI to the cell surface. However, we found β2-GPI mRNA in cells cultured both in the absence and presence of serum. Moreover, the β2-GPI mRNA amount was similar in both culture conditions.
These results provide evidence that endothelial cells constitutively express β2-GPI mRNA, since the absence of serum β2-GPI does not affect, at least for a 3-day period, β2-GPI mRNA transcription in the endothelial cells. The same result was obtained on astrocytes and neurones. The role of β2-GPI in the growth and long-term survival of endothelial cells has been demonstrated by purification and characterization of an endothelial cell viability-maintaining factor from fetal bovine serum identified as β2-GPI [46]. Therefore, β2-GPI synthesis by cells in serum-free medium might represent a mechanism of protection from the absence of extracellular β2-GPI. In other words, the cell synthesis of β2-GPI might assure endothelial cell survival even in disadvantageous conditions.
The demonstration of β2-GPI synthesis by lymphocytes suggests that cells of the immune system are another potential target of aβ2-GPI. With regard to previous reports that anti-lymphocyte antibodies detected in serum of SLE patients with neurological disease cross-react with brain tissue antigens [47,48], the β2-GPI expression by lymphocytes appears of particular interest. In vitro studies demonstrated that anti-lymphocyte antibodies are cytotoxic for neurones and astrocytes [48]. These antibodies, detected also in cerebrospinal fluid of SLE patients with evidence of intrathecal synthesis, were shown to correlate with CNS lupus disease activity [41,49]. The demonstration that astrocytes and neurones synthesize β2-GPI suggests the direct antigenic function of this molecule within the CNS and, consequently, the putative role of APS-associated autoantibodies in the CNS damage. aβ2-GPI might contribute to the CNS pathologies by interaction with brain cytotypes, besides the interaction with cerebral vessel endothelium [32].
Further studies are advised on a larger variety of cell types and tissues in order to check the specificity of β2-GPI expression. However, the present results clearly indicate that β2-GPI is expressed by different cell types that are supposed to be involved in immune-mediated tissue lesions in APS.
Acknowledgments
Banca di Roma, M.U.R.S.T. ex 60% and M.U.R.S.T. 40% supported this work.
REFERENCES
- 1.Kandiah DA, Krilis SA. Beta-2-glycoprotein I. Lupus. 1994;3:207–12. doi: 10.1177/096120339400300401. [DOI] [PubMed] [Google Scholar]
- 2.Steinkasserer A, Estaller C, Weiss EH, et al. Complete nucleotide and deduced amino acid sequence of human beta 2-glycoprotein. Biochem J. 1991;277:387–91. doi: 10.1042/bj2770387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matsuura E, Igarashi M, Igarashi Y, et al. Molecular definition of human β2-glycoprotein I (β2-GPI) by cDNA cloning and inter-species differences of β2-GPI in alternation of anticardiolipin binding. Int Immunol. 1991;3:1217–21. doi: 10.1093/intimm/3.12.1217. [DOI] [PubMed] [Google Scholar]
- 4.McNeil HP, Simpson RJ, Chesterman CN, et al. Anti-phospholipid antibodies are directed against a complex antigen that includes a lipid-binding inhibitor of coagulation: β2-glycoprotein I (apolipoprotein H) Proc Natl Acad Sci USA. 1990;87:4120–4. doi: 10.1073/pnas.87.11.4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Galli M, Comfurius P, Massau C, et al. Anticardiolipin antibodies (ACA) directed not to cardiolipin but to a plasma cofactor. Lancet. 1990;355:1544–7. doi: 10.1016/0140-6736(90)91374-j. [DOI] [PubMed] [Google Scholar]
- 6.Rauch J, Janoff AS. The nature of anti-phospholipid antibodies. J Rheumatol. 1992;19:1782–5. [PubMed] [Google Scholar]
- 7.Valesini G, Shoenfelf Y. A new player in the antiphospholipid syndrome: the β2GPI cofactor. Autoimmun. 1992;14:105–10. doi: 10.3109/08916939209083128. [DOI] [PubMed] [Google Scholar]
- 8.McNally T, Mackie IJ, Machin SJ, et al. Increased levels of beta 2 glycoprotein I antigen and beta 2 glycoprotein I binding antibodies are associated with a history of thromboembolic complications in patients with SLE and primary antiphospholipid syndrome. Br J Rheumatol. 1995;34:1031–6. doi: 10.1093/rheumatology/34.11.1031. [DOI] [PubMed] [Google Scholar]
- 9.Kandiah DA, Sheng YH, Krilis SA. Beta 2 glycoprotein I: target for autoantibodies in the ‘antiphospholipid syndrome’. Lupus. 1996;5:381–5. doi: 10.1177/096120339600500509. [DOI] [PubMed] [Google Scholar]
- 10.Koike T, Matsuura E. Anti-beta 2-glycoprotein I antibody: specificity and clinical significance. Lupus. 1996;5:378–80. doi: 10.1177/096120339600500508. [DOI] [PubMed] [Google Scholar]
- 11.Levine SR, Welch MA. The spectrum of neurologic disease associated with antiphospholipid antibodies. Arch Neurol. 1987;44:876–83. doi: 10.1001/archneur.1987.00520200078024. [DOI] [PubMed] [Google Scholar]
- 12.Hughes GVR. The antiphospholipid syndrome: ten years on. Lancet. 1993;342:341–4. doi: 10.1016/0140-6736(93)91477-4. [DOI] [PubMed] [Google Scholar]
- 13.Arvieux J, Roussel B, Jacob MC, et al. Measurement of anti-phospholipid antibodies by ELISA using beta-2-glycoprotein I as an antigen. J Immunol Methods. 1991;193:223–9. doi: 10.1016/0022-1759(91)90047-j. [DOI] [PubMed] [Google Scholar]
- 14.Viard JP, Amoura Z, Bach JF. Association of anti-β2-glycoprotein I antibodies with lupus-type circulating anticoagulant and thrombosis in systemic lupus erythematosus. Am J Med. 1992;93:181–6. doi: 10.1016/0002-9343(92)90049-h. [DOI] [PubMed] [Google Scholar]
- 15.Cabral AR, Rodriguez M, Cabiedes J, et al. Antibodies to β2-glycoprotein I associate with antiphospholipid syndrome whether primary (PAPS) or secondary to systemic lupus erythematosus. Arthritis Rheum. 1993;6(Suppl.):S243. [Google Scholar]
- 16.Gharavi AE, Harris EN, Sammaritano LR. Do patients with antiphospholipid syndrome have autoantibodies to beta (2) glycoprotein-1? J Lab Clin Med. 1993;122:426–31. [PubMed] [Google Scholar]
- 17.Sorice M, Circella A, Griggi T, et al. Anticardiolipin and anti-β2-GPI are two distinct populations of autoantibodies. Thromb Haemost. 1996;75:303–8. [PubMed] [Google Scholar]
- 18.Balestrieri G, Tincani A, Spatola L, et al. Anti-beta2-glycoprotein I antibodies: a marker of antiphospholipid syndrome? Lupus. 1995;4:122–30. doi: 10.1177/096120339500400208. [DOI] [PubMed] [Google Scholar]
- 19.Puurunen M, Vaarala O, Julkunen H, et al. Antibodies to phospholipid-binding plasma proteins and occurrence of thrombosis in patients with systemic lupus erythematosus. Clin Immunol Immunopathol. 1996;80:16–22. doi: 10.1006/clin.1996.0089. [DOI] [PubMed] [Google Scholar]
- 20.Tsusumi A, Matsuura E, Ichikawa K, et al. Antibodies to beta 2-glycoprotein I and clinical manifestation in patients with systemic lupus erythematosus. Arthritis Rheum. 1996;39:1466–74. doi: 10.1002/art.1780390905. [DOI] [PubMed] [Google Scholar]
- 21.Pengo V, Biasiolo A, Brocco T, et al. Autoantibodies to phospholipid-plasma proteins in patients with thrombosis and phospholipid-reactive antibodies. Thromb Haemost. 1996;75:721–4. [PubMed] [Google Scholar]
- 22.Cabral AR, Amigo MC, Cabiedes J, et al. The antiphospholipid syndromes: a primary variant with antibodies to glycoprotein-I but no antibodies detectable in standard antiphospholipid assays. Am J Med. 1996;101:472–81. doi: 10.1016/s0002-9343(96)00254-9. [DOI] [PubMed] [Google Scholar]
- 23.Alarcon-Segovia D, Cabral AR. The concept and classification of antiphospholipid/cofactor syndromes. Lupus. 1996;5:364–7. doi: 10.1177/096120339600500505. [DOI] [PubMed] [Google Scholar]
- 24.Hasunuma Y, Matsuura E, Makita Z, et al. Involvement of beta 2-glycoprotein I and anticardiolipin antibodies in oxidatively modified low-density lipoprotein uptake by macrophages. Clin Exp Immunol. 1997;107:569–73. doi: 10.1046/j.1365-2249.1997.d01-948.x. [DOI] [PubMed] [Google Scholar]
- 25.Price BE, Rauch J, Shia MA, et al. Anti-phospholipid autoantibodies bind to apoptotic, but not viable, thymocytes in a beta 2-glycoprotein I-dependent manner. J Immunol. 1996;157:2201–8. [PubMed] [Google Scholar]
- 26.La Rosa L, Meroni PL, Tincani A, et al. Beta 2 glycoprotein I and placental anticoagulant protein I in placenta from patients with antiphospholipid syndrome. J Rheumatol. 1994;21:1684–93. [PubMed] [Google Scholar]
- 27.Pierangeli SS, Dean J, Golsmith GH, et al. Studies on interaction of placental anticoagulant protein I, beta 2 glycoprotein I, and antiphospholipid antibodies in the prothrombokinase reaction and in the solid phase anticardiolipin assay. J Lab Clin Med. 1996;128:194–201. doi: 10.1016/s0022-2143(96)90011-6. [DOI] [PubMed] [Google Scholar]
- 28.Vasquez-Mellado J, Llorente P, Alarcon-Segovia D. Exposure of anionic phospholipids upon platelet activation permits binding of β2 glycoprotein I and through it IgG antiphospholipid antibodies. Studies in platelets from patients with antiphospholipid syndrome and normal subjects. J Autoimmun. 1994;12:122–6. doi: 10.1006/jaut.1994.1024. [DOI] [PubMed] [Google Scholar]
- 29.Del Papa N, Guidali L, Spatola L, et al. Relationship between anti-phospholipid and anti-endothelial cell antibodies III: beta 2 glycoprotein mediates the antibody binding to endothelial membranes and induces the expression of adhesion molecules. Clin Exp Rheumatol. 1995;13:179–85. [PubMed] [Google Scholar]
- 30.Meroni PL, Del Papa N, Beltrami B, et al. Modulation of endothelial cell function by antiphospholipid antibodies. Lupus. 1996;5:448–50. doi: 10.1177/096120339600500523. [DOI] [PubMed] [Google Scholar]
- 31.Simantov R, Lo SK, Gharavi A, et al. Antiphospholipid antibodies activate vascular endothelial cells. Lupus. 1996;5:440–1. doi: 10.1177/096120339600500521. [DOI] [PubMed] [Google Scholar]
- 32.Del Papa N, Guidali L, Sala A, et al. Endothelial cells as target for antiphospholipid antibodies. Human polyclonal and monoclonal anti-beta 2-glycoprotein I antibodies react in vitro with endothelial cells through adherent beta 2-glycoprotein I and induce endothelial activation. Arthritis Rheum. 1997;40:551–61. doi: 10.1002/art.1780400322. [DOI] [PubMed] [Google Scholar]
- 33.Caronti B, Pittoni V, Palladini G, et al. Anti-β2-glycoprotein I antibodies bind to central nervous system. J Neurol Sci. 1998;156:211–9. doi: 10.1016/s0022-510x(98)00027-6. [DOI] [PubMed] [Google Scholar]
- 34.Oosting JD. In vitro studies of antiphospholipid antibodies and its cofactor β2-glycoprotein I, show no effect on endothelial cell mediated protein C activation. Thromb Haemost. 1991;66:666–71. [PubMed] [Google Scholar]
- 35.Oosting JD, Derksen RHWM, Entjes HT, et al. Lupus anticoagulant activity is frequently dependent on the presence of β2-glycoprotein I. Thromb Haemost. 1992;67:499–502. [PubMed] [Google Scholar]
- 36.Oosting JD, Derksen RHWM, Entjes HT, et al. Antiphospholipid antibody positive sera enhance endothelial cell procoagulant activity: studies in a thrombosis model. Thromb Haemost. 1992;68:278–84. [PubMed] [Google Scholar]
- 37.Derksen RHWM, De Groot PG, Kater L. Antiphospholipid antibodies and vascular pathology. Neth J Med. 1994;45:257–61. [PubMed] [Google Scholar]
- 38.Avery VM, Adrian DL, Gordon DL. Detection of mosaic protein mRNA in human astrocytes. Immunol Cell Biol. 1993;71:215–9. doi: 10.1038/icb.1993.24. [DOI] [PubMed] [Google Scholar]
- 39.Chamley LW, Allen JL, Johnson PM. Synthesis of β2 glycoprotein I by the human placenta. Placenta. 1997;18:403–10. doi: 10.1016/s0143-4004(97)80040-9. [DOI] [PubMed] [Google Scholar]
- 40.Averna M, Paravizzini G, Marino G, et al. Liver is not the unique site of synthesis of β2-glycoprotein I (apolipoprotein H): evidence for an intestinal localization. Int J Clin Lab Res. 1997;27:207–12. doi: 10.1007/BF02912460. [DOI] [PubMed] [Google Scholar]
- 41.Searles RP, Williams RC. Lymphocyte-reactive antibodies in SLE. In: Hughes GRV, editor. Clinic and rheumatic diseases. Vol. 8. London, Philadelphia, Toronto: W.B. Saunders, Co. Ltd; 1982. pp. 77–90. [PubMed] [Google Scholar]
- 42.Mevorach D, Raz E, Steiner I. Evidence for intrathecal synthesis of autoantibodies in systemic lupus erythematosus in neurological involvement. Lupus. 1994;3:117–21. doi: 10.1177/096120339400300211. [DOI] [PubMed] [Google Scholar]
- 43.Cusimano G, Palladini G, Lauro GM. MHC class II expression by human glioma cells after in vitro incubation with soluble antigens. Acta Neurol Scand. 1990;81:215–22. doi: 10.1111/j.1600-0404.1990.tb00969.x. [DOI] [PubMed] [Google Scholar]
- 44.Ponte P, Ng SY, Engel J, et al. Evolutionary conservation in the untranslated regions of actin mRNAs: DNA sequence of human β-actin cDNA. Nucleic Acid Res. 1984;12:1687–96. doi: 10.1093/nar/12.3.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Caronti B, Calderaro C, Passarelli F, et al. Dopamine receptor mRNAs in the rat lymphocytes. Life Sci. 1998;62:1919–25. doi: 10.1016/s0024-3205(98)00160-x. [DOI] [PubMed] [Google Scholar]
- 46.Valesini G, Tincani A, Harris EN, et al. Use of monoclonal antibodies to identify shared idiotypes on anti-cardiolipin and anti-DNA antibodies in human sera. Clin Exp Immunol. 1987;70:18–25. [PMC free article] [PubMed] [Google Scholar]
- 47.Cai G, Satoh T, Hoshi H. Purification and characterization of an endothelial cell-viability maintaining factor from fetal bovine serum. Biochim Biophyis Acta. 1995;1269:13–18. doi: 10.1016/0167-4889(95)00091-6. [DOI] [PubMed] [Google Scholar]
- 48.Bluestein HG, Zvaifler NJ. Brain-reactive lymphocytotoxic antibodies in the serum of patients with systemic lupus erythematosus. J Clin Invest. 1976;57:509–16. doi: 10.1172/JCI108303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bluestein HG. Heterogeneous neurocytotoxic antibodies in systemic lupus erythematosus. Clin Exp Immunol. 1979;35:210–7. [PMC free article] [PubMed] [Google Scholar]
- 50.Bluestein HG, Williams GN, Steinberg AD. Cerebrospinal fluid antibodies to neuronal cells: association with neuropsychiatric manifestations of systemic lupus erythematosus. Am J Med. 1981;70:240–6. doi: 10.1016/0002-9343(81)90756-7. [DOI] [PubMed] [Google Scholar]


