Abstract
Based on the positive therapeutic results with ursodeoxycholic acid (UDCA) in patients with primary biliary cirrhosis, in whom we observed a clinical improvement in conjunction with the normalization of the low pretreatment dipeptidyl peptidase (DPIV, CD26) expression of peripheral blood lymphocytes (PBL), we hypothesized that the very low DPIV expression in AIDS patients could be positively influenced by UDCA. Four young male AIDS patients were therefore treated with 750 mg of UDCA for 4 months. The low CD26 expression (2–8% of the PBL versus 18–28% in healthy controls) at the beginning of the study rose to 10–16% after UDCA therapy. Simultaneously we observed a two-to-three-fold elevation of the absolute number of lymphocytes as well as a slight increase of CD4+ cells. These effects were similar in all examined patients. Further investigations should be conducted on this potentially beneficial effect of UDCA.
Keywords: ursodeoxycholic acid, AIDS, dipeptidyl peptidase, primary biliary cirrhosis, peripheral blood lymphocytes
INTRODUCTION
Infection of cells by HIV is initiated by an interaction of the virus surface envelope glycoprotein gp120 and the virus receptor CD4 on lymphocytes, monocytes and macrophages [1]. However, this interaction alone is not sufficient to produce the infectious process, since the CD4+ cells only bind the virus without definitely leading to their infection. Therefore, it was suggested that other host cell-specific factors would be necessary to interact with the HIV surface protein and activate the fusion domain of the viral gp41. Recent investigations [2–4] have proposed that dipeptidyl peptidase IV (CD26) could be one responsible molecule. CD26, an ectoenzyme in the cell membrane of human T lymphocytes, is an important constituent in the process of lymphocyte activation and proliferation, as well as a functional characteristic of IL-2-producing cells [5,6]. HIV-infected patients were shown to display a decreased expression of CD26 [7–9].
Ursodeoxycholic acid (UDCA), a hydrophilic bile acid, is widely used as an anti-cholestatic drug in primary biliary cirrhosis (PBC) [10]. The immunomodulatory effect of UDCA is proposed as one of the underlying mechanisms for its beneficial effects [11]. Thus, patients with PBC were found to reveal significantly decreased expression of CD26. Therapy with UDCA in these patients leads to normalization of this expression and increased production of IL-2 [10].
Therefore, we hypothesized that UDCA could also play an immunomodulatory role in AIDS patients and increase their reduced CD26 expression. To test this hypothesis we examined the effect of UDCA therapy on several immunological parameters, such as expression of CD26, CD4, CD8, IL-2 and interferon-gamma (IFN-γ) production in HIV-infected patients.
MATERIALS AND METHODS
We conducted a pilot study with UDCA therapy in four young men with AIDS. The patients were recruited from our AIDS clinic and included into the study after giving their written consent. The protocol of the study was approved by the local Ethical Committee of the University Clinic in Dresden. UDCA was applied orally in dosage 10 mg/kg body weight for 4 months. The patients were in a stable clinical phase. Two of them were under regular antiviral therapy with retrovir and videx for 2 years before and during the study. The other patients had not been treated with specific antiviral drugs. The CD4+ lymphocytes were in the range of 162–401 cells/μl at baseline. The expression of dipeptidyl peptidase (DPIV; CD26) of peripheral blood lymphocytes (PBL) was determined before, during and after UDCA therapy. PBL were isolated from blood by density gradient centrifugation [12]. Cells were collected at the interphase and washed twice. Cell numbers and viability were assessed by acridine orange staining. Viability was ≥ 95%. PBL were counted by light microscopy. Cytochemical staining for dipeptidyl peptidase IV-positive PBL (DPIV+) was performed after a modification of the method by Lojda [13]. DPIV+ lymphocytes were detected in a histochemical reaction by incubation with glycyl-proline-4-methoxy-β-naphthylamide. CD4+ and CD8+ T cells were detected by means of flow cytometry.
RESULTS
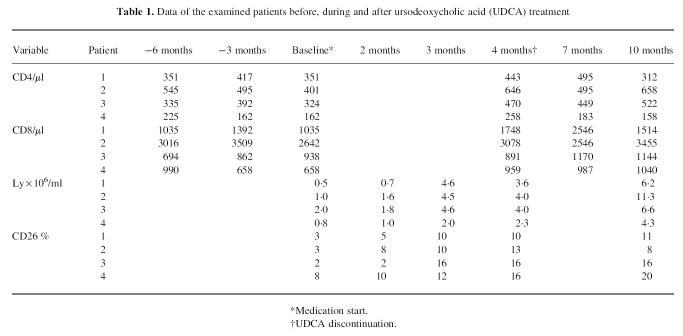
All patients showed very low expression of DPIV (between 2% and 8%) before UDCA therapy (Table 1). The healthy controls (n = 20) exhibited DPIV expression between 18% and 28%. The absolute number of lymphocytes in the examined patients was considerably lower in comparison with the healthy subjects (1.1 × 106versus 4.0 × 106).
Table 1.
Data of the examined patients before, during and after ursodeoxycholic acid (UDCA) treatment
Surprisingly the percentage of DPIV+ (CD26+) PBL increased after UDCA treatment, reaching 10–16% after 4 months. The absolute number of lymphocytes was also improved in all patients after UDCA treatment, with a complete normalization in three of the patients after 4 months therapy. The drug safety and tolerability appeared to be very good, with no side-effects observed. The CD4+ PBL after UDCA treatment were increased in comparison with pretreatment levels in all patients. CD26 expression 6 months after the end of the study remained increased in three patients and fell slightly in one patient, although remaining higher than baseline. The other laboratory parameters showed no significant alterations.
DISCUSSION
In a previous study we demonstrated an immunomodulatory effect of UDCA in 22 patients with PBC [11]. DPIV expression in PBL in every PBC patient was lower than the normal range in healthy controls, but after 4–8 weeks of UDCA treatment reached normal ranges. Simultaneously we observed a significant improvement in liver function. These findings suggest that UDCA exerts a normalizing effect on immunocompetent cells and positively influences their immunoregulatory capacity.
Bile acids were shown to completely inactivate HIV-1 in vitro and destroy all the cultured persistently HIV-1-infected T cells, as well as selectively inhibiting the replication of HIV-1 [14,15]. Chan et al. showed the positive effect of UDCA therapy on AIDS-associated cholangitis [16]. As UDCA is a well tolerated and safe drug we thought it reasonable to test its therapeutic effect in HIV-infected subjects who have decreased CD26 expression [7,8]. Our results suggest a beneficial effect of UDCA in AIDS, with elevation of DPIV (CD26) expression and of the absolute lymphocyte number. CD26 is a known marker of IL-2-producing lymphocytes. In addition, we observed an increase of the CD4+ PBL. Recently UDCA was found to suppress antigen-specific CD4+ T cell-mediated apoptosis of target cells [17], and apoptosis appears to be the cause of the HIV-associated T cell defect. This could partly explain the immunomodulatory effect of UDCA.
Our study provides preliminary data for a potentially beneficial effect of UDCA in the treatment of AIDS. However, our trial was conducted on a small number of subjects and no virological parameters were examined. Further investigations will be necessary to scrutinize the immunomodulating effect of UDCA in carefully designed clinical trials with larger numbers of patients.
REFERENCES
- 1.Sweet RW, Truneh A, Hendrikson WA. CD4: its structure, role in immune function and AIDS pathogenesis, and potential as a pharmacological target. Curr Opin Biotechnol. 1991;2:622–33. doi: 10.1016/0958-1669(91)90089-n. [DOI] [PubMed] [Google Scholar]
- 2.Callebaut C, Krust B, Jacotot E, Hovanessian AG. T cell activation antigen, CD26, as a cofactor for entry of HIV in CD4+ cells. Science. 1993;262:2045–50. doi: 10.1126/science.7903479. [DOI] [PubMed] [Google Scholar]
- 3.Lazaro I, Naniche D, Signoret N, et al. Factors involved in entry of the human immunodeficiency virus type 1 into permissive cells: lack of evidence of a role for CD26. J Virol. 1994;68:6535–46. doi: 10.1128/jvi.68.10.6535-6546.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gutheil WG, Subramanyam M, Flentke GR, Sanford DG, Munoz E, Huber B, Bachovchin WW. Human immunodeficiency virus 1 Tat binds to dipeptidyl aminopeptidase IV (CD26): a possible mechanism for Tat's immunosuppressive activity. Proc Natl Acad Sci USA. 1994;91:6594–8. doi: 10.1073/pnas.91.14.6594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cozzi M, Gloghini A, Volpe R, Carbone A. Immunohistocytochemical correlation of DAPIV–CD26 reactivity with immunologic markers of lymphocyte activation in human lymphoid tissues. Br J Haematol. 1990;75:325–32. doi: 10.1111/j.1365-2141.1990.tb04344.x. [DOI] [PubMed] [Google Scholar]
- 6.Schön E, Ansorge S. Dipeptidyl peptidase IV in the immune system. Cytofluorimetric evidence for induction of the enzyme on activated T lymphocytes. Biol Chem Hoppe Seyler. 1990;371:699–705. doi: 10.1515/bchm3.1990.371.2.699. [DOI] [PubMed] [Google Scholar]
- 7.Vanham G, Kestens L, De-Meester I, et al. Decreased expression of the memory marker CD26 on both CD4+ and CD8+ T lymphocytes of HIV-infected subjects. J Acquir Immune Defic Syndr. 1993;6:749–57. [PubMed] [Google Scholar]
- 8.Blazquez MV, Madueno JA, Gonzalez R, Jurado R, Bachovchin WW, Pena J, Munoz E. Selective decrease of CD26 expression in T cells from HIV-1-infected individuals. J Immunol. 1992;149:3073–7. [PubMed] [Google Scholar]
- 9.Chalmers AH, Hare C, Woolley G, Frazer IH. Lymphocyte ectoenzyme activity compared in healthy persons and patients seropositive to or at high risk of HIV infection. Immunol Cell Biol. 1990;68:81–85. doi: 10.1038/icb.1990.12. [DOI] [PubMed] [Google Scholar]
- 10.Leuschner U, Fischer H, Kurtz W, Guldutuna S, Hubner K, Hellstern A, Gatzen M, Leuschner M. Ursodeoxycholic acid in primary biliary cirrhosis: results of a controlled double blind trial. Gastroenterology. 1989;97:1268–74. doi: 10.1016/0016-5085(89)91698-3. [DOI] [PubMed] [Google Scholar]
- 11.Kürktschiev D, Subat S, Adler D, Schentke KU. Immunomodulating effect of ursodeoxycholic acid therapy in patients with primary biliary cirrhosis. J Hepatol. 1993;18:373–7. doi: 10.1016/s0168-8278(05)80284-3. [DOI] [PubMed] [Google Scholar]
- 12.Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- 13.Lojda Z. Studies on glycyl-proline naphthylamidase. Histochemistry. 1977;54:299–309. doi: 10.1007/BF00508273. [DOI] [PubMed] [Google Scholar]
- 14.Lloyd G, Atkinson T, Sutton PM. Effect of bile salts and of fusidic acid on HIV-1 infection of cultured cells. Lancet. 1988:1418–21. doi: 10.1016/s0140-6736(88)92236-2. [DOI] [PubMed] [Google Scholar]
- 15.Baba M, Schols D, Nakashima H, Pauwels R, Parmentier G, Meijer DK, De Clercq E. Selective activity of several cholic acid derivatives against human immunodeficiency virus replication in vitro. J Acquir Immune Defic Syndr. 1989;2:264–71. [PubMed] [Google Scholar]
- 16.Chan MF, Koch J, Cello JP. Ursodeoxycholic acid (URSO) for symptomatic AIDS-associated cholangiopathy. Gastrointest Endoscopy. 1994;40(2, Part 2) [Google Scholar]
- 17.Yoshikawa M, Matsui Y, Umemoto N, et al. Effects of ursodeoxycholic acid on target apoptosis induced by an antigen-specific CD4+ T cell line. Internat Hepatol Commun. 1996;4:268–76. [Google Scholar]